Abstract
By using three isotopes of diisopropyl-phosphofluoridate ([3H]-, [14C]-, and [32P]DFP) simultaneously, the life span of red cells from 20 patients with sickle cell anemia (Hb SS) has been studied after varying degrees of carbamylation in vitro with cyanate (NCO) and carbamyl phosphate (CP). The results are expressed in terms of the red cell mean life span (MLS).
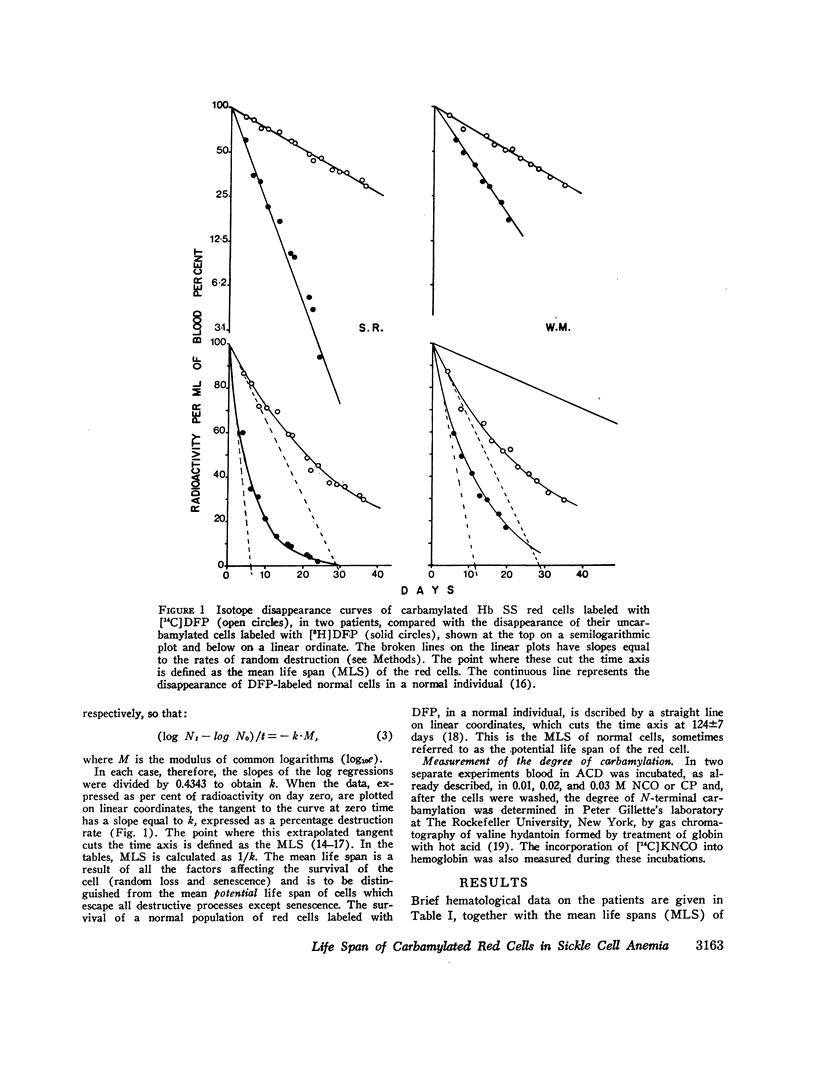
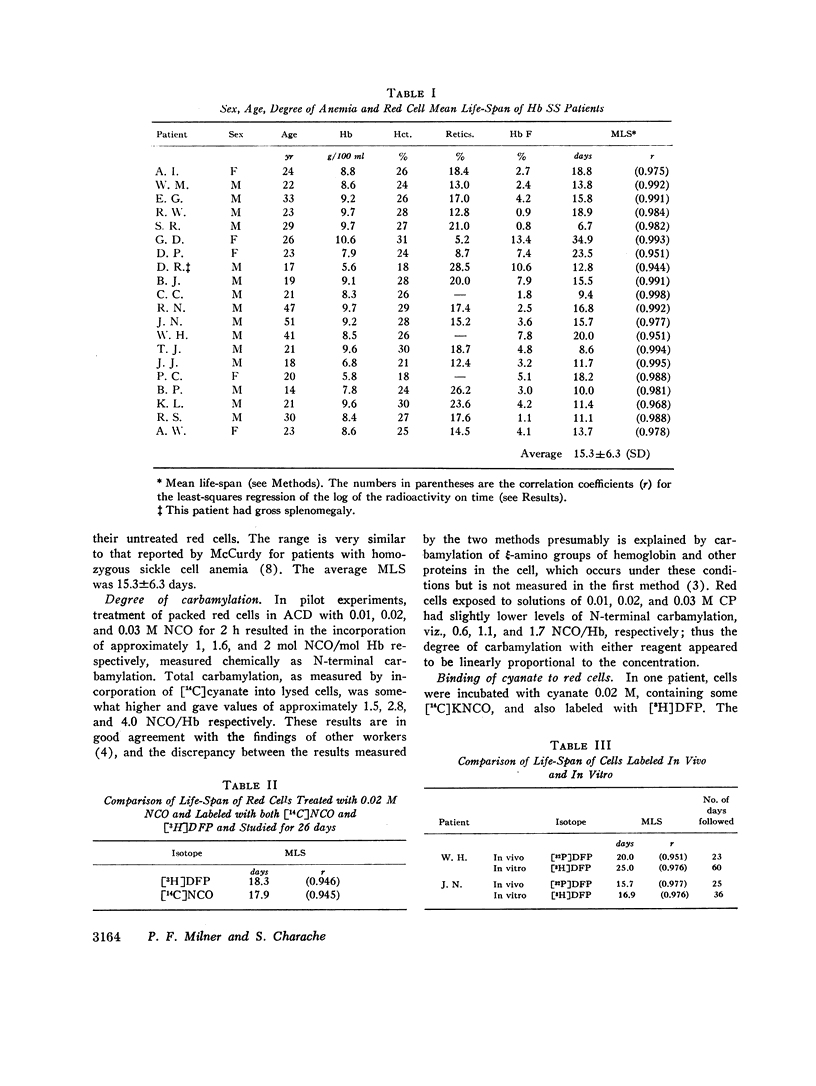
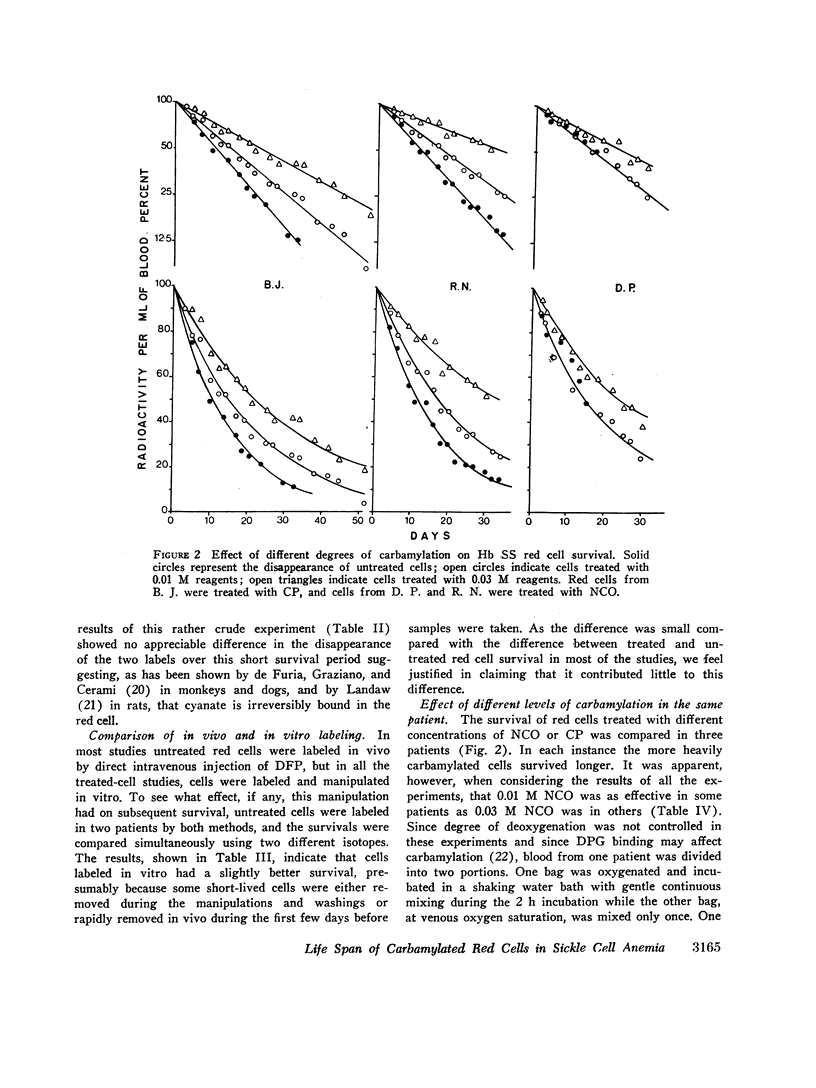
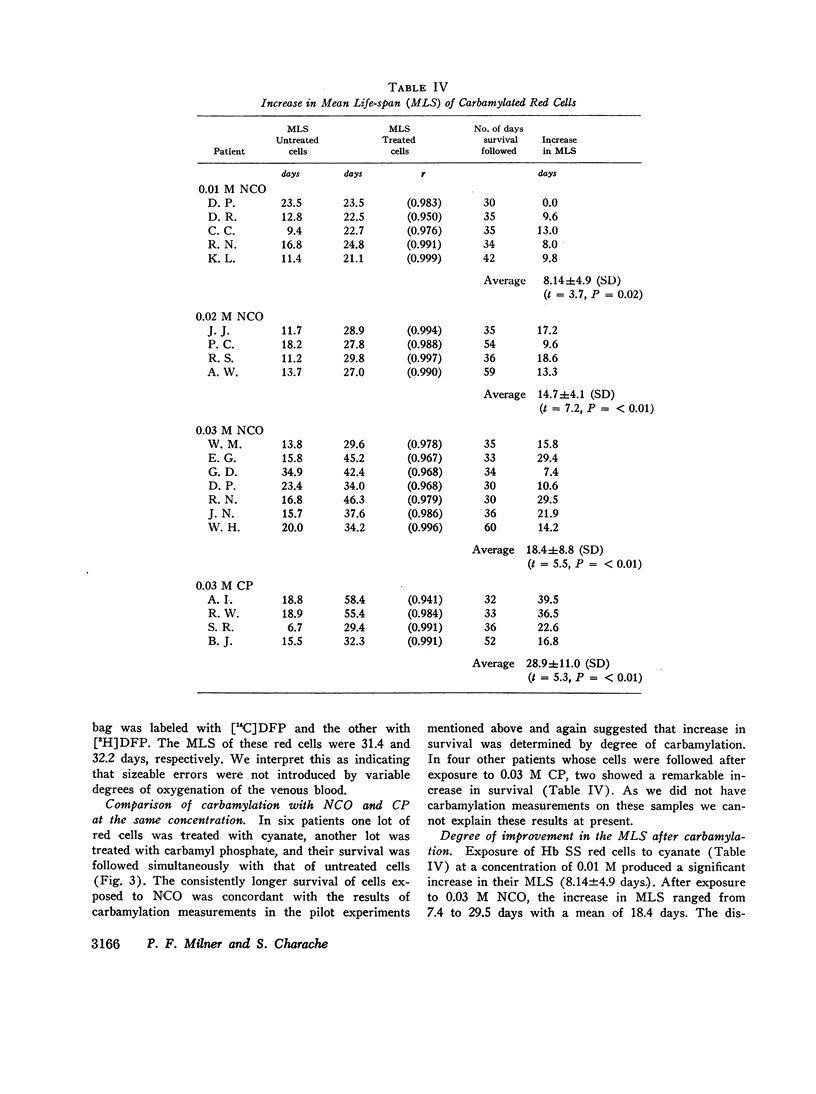
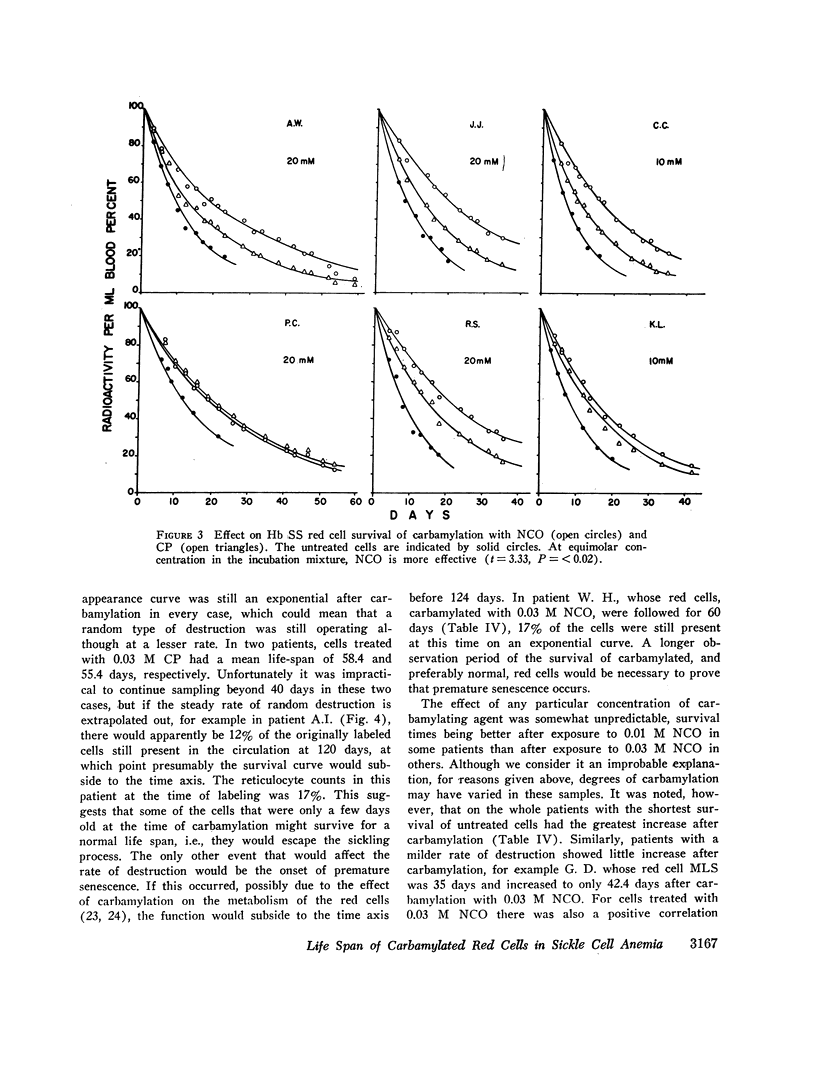
The MLS of red cells in the patients studied averaged 15.2±6.3 (SD) days. After carbamylation the increase in red cell life span was linearly proportional to the concentration of cyanate used, so that at 0.01. 0.02, and 0.3 M NCO (approximately 1, 1.6, and 2 mol NCO/mol Hb) the average increase in MLS was 8.14±4.9 days, 14.7±4.1 days, and 18.4±8.8 days, respectively.
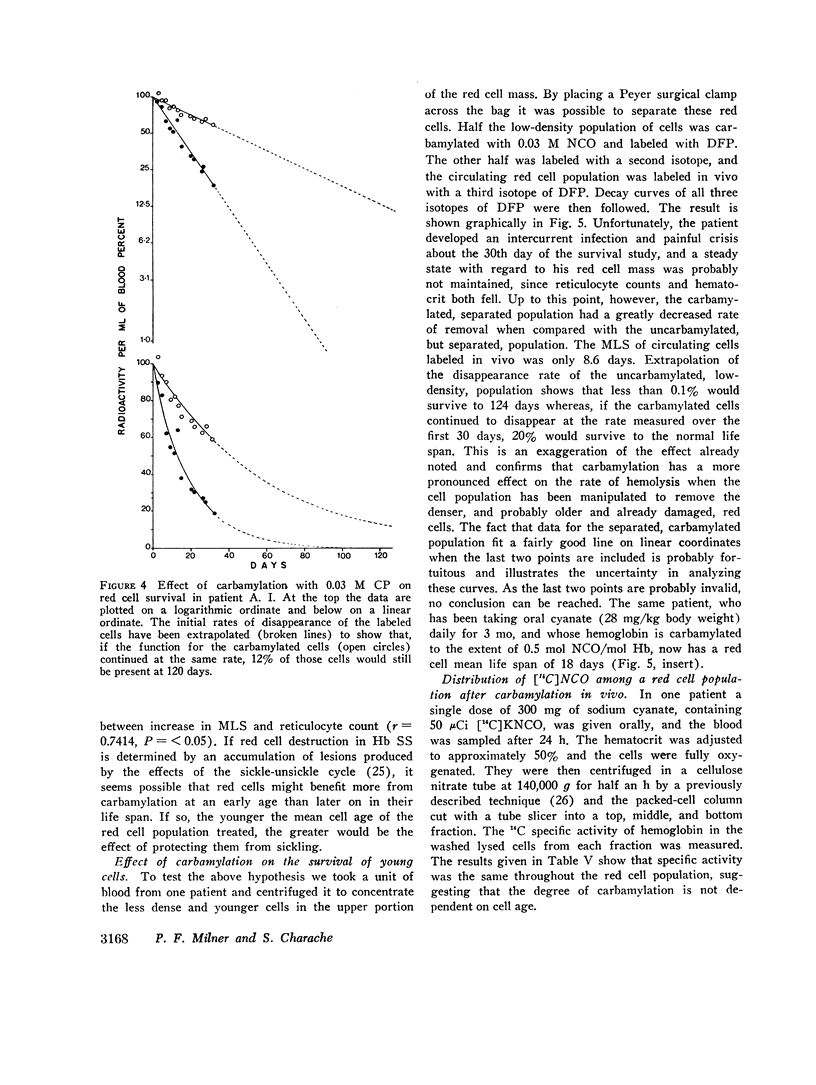
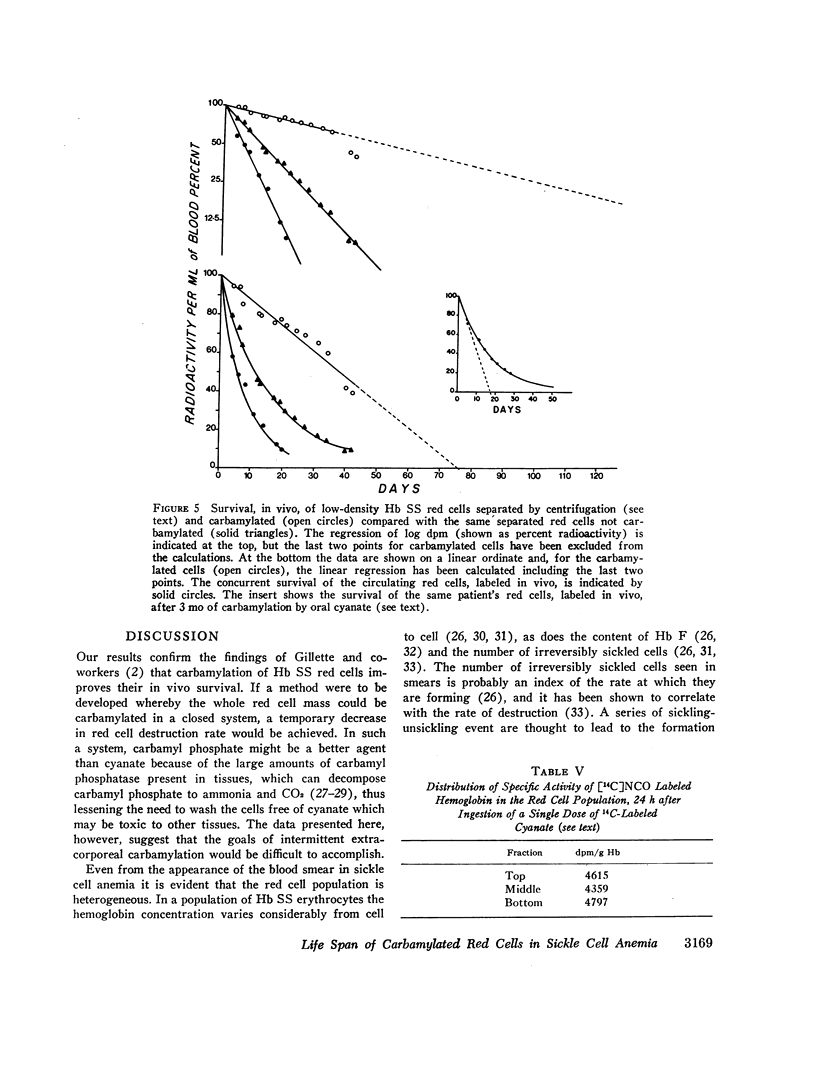
Analysis of survival curves and the results of an experiment using a population of Hb SS cells separated by centrifugation indicated that carbamylation had a disproportionate effect on the survival of the youngest cells in the population. Improvement in MLS correlated with the reticulocyte count of the cells carbamylated. This finding is explained on the hypothesis that the life span of irreversibly sickled and other damaged cells is not improved by carbamylation but that carbamylation greatly improves the life span of the young, and as yet undamaged, cells. For this reason extracorporeal carbamylation is not favored as a form of therapy. At the level of carbamylation attainable by oral therapy, however, it would appear likely that only a modest increase in red cell life span will be achieved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Kan Y. W., Nathan D. G. Reticulocyte survival in sickle cell anemia: effect of cyanate. Blood. 1972 Nov;40(5):733–739. [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLINE M. J., BERLIN N. I. AN EVALUATION OF DFP AND CR AS METHODS OF MEASURING RED CELL LIFE SPAN IN MAN. Blood. 1963 Oct;22:459–465. [PubMed] [Google Scholar]
- CLINE M. J., BERLIN N. I. Simultaneous measurement of the survival of two populations of erythrocytes with the use of labeled diisopropylfluorophosphate. J Lab Clin Med. 1963 Feb;61:249–257. [PubMed] [Google Scholar]
- CLINE M. J., BERLIN N. I. The red cell chromium elution rate in patients with some hematologic diseases. Blood. 1963 Jan;21:63–69. [PubMed] [Google Scholar]
- Carreras-Barnes J., Diederich D. A., Grisolia S. Some comparative aspects of carbamylation of human blood and of hemoglobin with cyanate and carbamyl phosphate. Eur J Biochem. 1972 May;27(1):103–108. doi: 10.1111/j.1432-1033.1972.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Cerami A., Manning J. M. Potassium cyanate as an inhibitor of the sickling of erythrocytes in vitro. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1180–1183. doi: 10.1073/pnas.68.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S., Usami S., Bertles J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):623–634. doi: 10.1172/JCI106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORNHORST A. C. The interpretation of red cell survival curves. Blood. 1951 Dec;6(12):1284–1292. [PubMed] [Google Scholar]
- De Furia F. G., Miller D. R., Cerami A., Manning J. M. The effects of cyanate in vitro on red blood cell metabolism and function in sickle cell anemia. J Clin Invest. 1972 Mar;51(3):566–574. doi: 10.1172/JCI106845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich D., Grisolia S. Purification and properties of acyl phosphatase from heart muscle. Comparative properties of acyl phosphatase from several sources. Biochim Biophys Acta. 1971 Jan 13;227(1):192–198. doi: 10.1016/0005-2744(71)90179-3. [DOI] [PubMed] [Google Scholar]
- Diederich D. Relationship between the oxygen affinity and in vitro sickling propensity of carbamylated sickle erythrocytes. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1255–1261. doi: 10.1016/s0006-291x(72)80110-4. [DOI] [PubMed] [Google Scholar]
- EADIE G. S., BROWN I. W., Jr Red blood cell survival studies. Blood. 1953 Dec;8(12):1110–1136. [PubMed] [Google Scholar]
- EERNISSE J. G., van ROOD J. Erythrocyte survival-time determinations with the aid of DF32P. Br J Haematol. 1961 Jul;7:382–404. doi: 10.1111/j.1365-2141.1961.tb00348.x. [DOI] [PubMed] [Google Scholar]
- GARBY L. Analysis of red-cell survival curves in clinical practice and the use of di-iso-propylfluorophosphonate (DF32P) as a label for red cells in man. Br J Haematol. 1962 Jan;8:15–27. doi: 10.1111/j.1365-2141.1962.tb06490.x. [DOI] [PubMed] [Google Scholar]
- Gillette P. N., Manning J. M., Cerami A. Increased survival of sickle-cell erythrocytes after treatment in vitro with sodium cyanate. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2791–2793. doi: 10.1073/pnas.68.11.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glader B. E., Conrad M. E. Cyanate inhibition of erythrocyte glucose-6-phosphate dehydrogenase. Nature. 1972 Jun 9;237(5354):336–338. doi: 10.1038/237336a0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. The binding of carbon dioxide by horse haemoglobin. Biochem J. 1971 Aug;124(1):31–45. doi: 10.1042/bj1240031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus L. M., Kraus A. P. Carbamyl phosphate mediated inhibition of the sickling of erythrocytes in vitro. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1381–1387. [PubMed] [Google Scholar]
- Landaw S. A. The use of 14 C-cyanate for red blood cell survival studies. Proc Soc Exp Biol Med. 1973 Feb;142(2):712–715. doi: 10.3181/00379727-142-37099. [DOI] [PubMed] [Google Scholar]
- Manning J. M., Lee C. K., Cerami A., Gillette P. N. Gas chromatographic determination of the carbamylation of hemoglobin S by cyanate. J Lab Clin Med. 1973 Jun;81(6):941–945. [PubMed] [Google Scholar]
- May A., Bellingham A. J., Huehns E. R., Beaven G. H. Effect of cyanate on sickling. Lancet. 1972 Mar 25;1(7752):658–661. doi: 10.1016/s0140-6736(72)90462-x. [DOI] [PubMed] [Google Scholar]
- McCurdy P. R. 32-DFP and 51-Cr for measurement of red cell life span in abnormal hemoglobin syndromes. Blood. 1969 Feb;33(2):214–224. [PubMed] [Google Scholar]
- Padilla F., Bromberg P. A., Jensen W. N. The sickle-unsickle cycle: a cause of cell fragmentation leading to permanently deformed cells. Blood. 1973 May;41(5):653–660. [PubMed] [Google Scholar]
- SHEPARD M. K., WEATHERALL D. J., CONLEY C. L. Semi-quantitative estimation of the distribution of fetal hemoglobin in red cell populations. Bull Johns Hopkins Hosp. 1962 Jun;110:293–310. [PubMed] [Google Scholar]
- Seakins M., Gibbs W. N., Milner P. F., Bertles J. F. Erythrocyte Hb-S concentration. An important factor in the low oxygen affinity of blood in sickle cell anemia. J Clin Invest. 1973 Feb;52(2):422–432. doi: 10.1172/JCI107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R., Serjeant B. E., Milner P. F. The irreversibly sickled cell; a determinant of haemolysis in sickle cell anaemia. Br J Haematol. 1969 Dec;17(6):527–533. doi: 10.1111/j.1365-2141.1969.tb01403.x. [DOI] [PubMed] [Google Scholar]


