Abstract
The use of bacteria in the regression of certain forms of cancer has been recognized for more than a century. Much effort, therefore, has been spent over the years in developing wild-type or modified bacterial strains to treat cancer. However, their use at the dose required for therapeutic efficacy has always been associated with toxicity problems and other deleterious effects. Recently, the old idea of using bacteria in the treatment of cancer has attracted considerable interest and new genetically engineered attenuated strains as well as microbial compounds that might have specific anticancer activity without side effects are being evaluated for their ability to act as new anticancer agents. This involves the use of attenuated bacterial strains and expressing foreign genes that encode the ability to convert non-toxic prodrugs to cytotoxic drugs. Novel strategies also include the use of bacterial products such as proteins, enzymes, immunotoxins and secondary metabolites, which specifically target cancer cells and cause tumor regression through growth inhibition, cell cycle arrest or apoptosis induction. In this review we describe the current knowledge and discuss the future directions regarding the use of bacteria or their products, in cancer therapy.
Key words: cancer therapy, anticancer agents from microbial sources, drug development, Pseudomonas aeruginosa, azurin, multi-targeting drugs in cancer therapy
Introduction
Each year, tens of millions of people are diagnosed worldwide with cancer, and more than half of these patients eventually die from this disease. In 2002, 10.9 million new cancer cases (excluding skin cancer) were diagnosed, and the number of deaths caused by this disease reached 6.7 million (GLOBOCAN).1 Regarding the industrialized countries of the western world, cancer accounts for about one fifth of all deaths. Likewise, one person out of three will be treated for a severe cancer in their lifetime.2 In Europe, cancer has become a major public health problem with an estimated prevalence of about 3%, increasing to 15% at old age.3 This rising burden is mainly due to a rapidly aging population, and demands a clear and coordinated response from oncologists, public health professionals, policy-makers and researchers.
Conventional cancer treatments, such as surgery, chemotherapy and radiotherapy, often fail to achieve a complete cancer remission. Moreover, it has been widely recognized that radiotherapy and/or chemotherapy are likely to cause significant side effects. This has prompted the development of many new approaches for the treatment of cancer. One such example involves the use of live, attenuated bacteria or their purified products.
Live, Attenuated and Engineered Bacterial Strains in the Treatment of Cancer
In 1890, William B. Coley, a surgeon in the Memorial Hospital in New York, described for the first time bacteria as anticancer agents.4 He observed that several patients showed tumor regression after being infected with pathogenic bacteria. Later, in 1935, Connell used sterile filtrates from Clostridium histolyticum to treat advanced cancers and observed tumor regression, which he pointed to be the result of enzymes' production.5 Many scientists since then, and even today, know that certain live, attenuated and engineered microorganisms such as Clostridium, Bifidobacterium, Salmonella, Mycobacterium, Bacillus and Listeria have the ability to selectively target cancer cells and act as anticancer agents. They grow in the hypoxic core region of solid tumors, with very little oxygen and where most of the times radiation or chemotherapy are unsuccessful.6 Because of their selectivity for tumor micro-environment, these bacteria are also promising vectors for delivering therapeutic genes for anticancer therapies.
Clostridia are a group of anaerobes and, therefore, constitute an ideal tool to target solid tumors since in most cases these tumors show increased levels of hypoxia.7 In 1947, it was shown that the injection of spores of Clostridium histolyticus caused oncolysis, then referred to as “liquefaction,” of a transplanted mouse sarcoma.8 Clostridium tetani spores were also used and tumor-bearing mice died 48 hours after the treatment in contrast to the healthy controls.9 This experience proved that a specific microenvironment is required to bacterial survival and development but also that toxicity remained a problem with live bacteria. Several approaches were used in an attempt to overcome this problem and increase the tolerance of this therapy. Using Clostridium oncolyticum M-55, Carey et al. showed the benign activity of this strain by injecting themselves without any dangerous effect.10 It was tested against several transplanted tumors in different animal models and the results indicated the need of several conditions for the success: (1) a threshold in the tumor size (3 cm3 or 2 g of tumor weight); (2) a spore dose of 106–9 and (3) intratumoral or intravenous injection modes.5 A clinical trial using C. oncolyticum M55 spores to treat glioblastomas resulted in oncolysis with almost all gliobastomas converted into brain abscesses one week after injection. However, in the 49 patients treated none exhibited an extension in their life span and the recurrence rate remained unaltered.11,12 In an effort to improve the efficiency of this therapy, combined chemotherapy agents plus the spores were tested. One of them consisted in the administration of 5-fluorodeoxyuridine, an antineoplastic antimetabolite that is metabolized to fluorouracil (5-FU) and alkylating agents of the ethyleneimino type, which induced sarcoma regression in mice.13 Raising the temperature inside the tumors up to 42–44°C (hyperthermia) in combination with radiotherapy was another approach developed for C. oncolyticum M55, creating a more favorable environment for the development of the microorganisms. Mice bearing three different types of neck tumors (Ehrlich adenocarcinoma, Harding-Passey-melanoma and fibrosarcoma) were treated with this strategy, and in all cases tumor lysis was increased due to the bacteria development.14 A third approach developed was by the modification on the level of oxygen given, reducing its content in the air inhaled by the animals to 11–12%. Oncolysis was observed and 30% of the animals were cured with this approach.5 Nevertheless, these approaches were not very successful in their clinical outcomes since there was tumor recurrence in the patients from the viable outer rim of the tumor.7
In the middle of the last decade, the use of engineered Clostridial strains to increase anti-tumor effects gathered more attention. Saccharolytic, non-pathogenic strains were reevaluated and it was seen that spores of C. beijerinckii NCIMB 8052 germinated in EMT6 tumor-bearing mice and spores of C. beijerinckii ATCC 17778, C. limosum DSM1400, C. acetobutylicum ATCC 824 and NI-4082 germinated in WAG/Rij rats with syngeneic rhabdomyosarcomas. In all cases, vegetative bacteria were only present in tumor regions but not in healthy tissues.15,16 C. beijerinckii NCIMB 8052 was also used to clone Escherichia coli cytosine deaminase (CD) and nitroreductase (NTR) genes, and in the presence of the correct prodrug, these enzymes were present in sufficient amount to induce EMT6 cells death. However, the studies showed that only intratumoral administration of the spores, but not intravenous, was able to produce a therapeutic effect.15,17 Genetically saccharolytic C. acetobutylicum strains containing cloned murine tumor necrosis factor α (mTNFα) or rat interleukin 2 (rIL2) were also constructed. The engineered strains gained the ability to produce significant amounts of these antitumor factors, but upon injection of the spores in rhabdomyosarcoma-bearing rats, tumor regression was not successful, even in combination with radiotherapy. This is likely to be due to the less ability of saccharolytic clostridia to colonize tumors when compared to proteolytic bacteria.18
Though proteolytic clostridia presented higher ability to colonize tumors, they were more difficult to manipulate. After a systemic administration of spores from both Clostridia types, tumor colonization from proteolytic Clostridia was about 1,000-fold higher than that of saccharolytic Clostridia.19 The proteolytic strain C. oncolyticum M-55 was transformed with a plasmid containing the CD gene and injected intravenously together with the prodrug 5-fluorocytosine (5-FC). The results demonstrated higher antitumor activity than with other drugs tested, but failed to maintain the effect for more than one week, even though the bacteria remained in the tumor. More recently, this strain has been genetically engineered to overexpress the enzyme nitroreductase from Haemophilus influenza, producing better antitumor results.20
Volgstein et al. assessed the ability of several anaerobes in tumor therapy, using xenografted colorectal carcinoma, as model system. They compared 26 species belonging to the Clostridium, Lactobacillus and Bifidobacterium genera and found two with higher tumor colonization capacity, namely Clostridium novyi and Clostridium sordelli. In spite of presenting this higher capacity, more than one third of the tumor-bearing mice injected with these spores died within 16–18 hours due to a lethal toxin (α) produced by the strains. In C. novyi, the α-toxin was removed through heating (it was located in a phage) and the strain became non-toxic and was renamed as C. novyi-NT. This strain is very sensitive to oxygen and, thus, grows only in the core hypoxic region of the tumors. For this reason when it reached the well oxygenated rim area of the tumors, bacteria didn't survive thus tumor recurrence remained a concern.21,22 Resembling the other strains, alternative therapeutics were tested, combining the C. novyi-NT treatment with conventional chemotherapeutics (a strategy named COBALT—Combined bacteriological therapy). As example, the simultaneous use of the C. novyi-NT spores with a DNA-damaging drug and an anti-vascular agent, mitomycin C and dolastin-10, respectively, resulted in very satisfactory tumor shrinking, however it was always accompanied by severe toxicity.23
Other drugs were or are being tested in combination with these spores: taxanes like docetaxel and MAC-321 which are microtubules stabilizers and have demonstrated decreased toxicity, although with slow tumor regression. In fact, with this combined strategy none of the animals died after treatment.
Also, combinations of C. novyi-NT with radiotherapy were evaluated which showed positive effects in several tumor models. Interestingly, this combination acts in distinct cell components. In solid tumors, radiation hits the rapidly proliferating and highly oxygen-exposed cells, and C. novyi-NT hits those cells resistant to radiation.5 The key advantage of this therapy is that the doses of radiotherapy can be much smaller than the ones used now, not damaging healthy tissues and with obvious advantages for patients' welfare.
Another genus with promising results in anticancer treatment is Bifidobacterium. These are anaerobic bacteria, non-motile, and, unlike the Clostridium genus, non-sporulating. This is the most abundant genus inhabiting human colon and it has been used as a probiotic for many years.5 In the 1980s, it was shown that Bifidobacteria selectively grow in the hypoxic areas of solid mouse tumors upon intravenous injection of 5 × 106 cfu (colony forming units), and 96 hours after injection, virtually no bacteria were found in other tissues.24 Bifidobacterium not only inhibit cancer progression in mice but have also shown the ability to induce a powerful immune response, enhancing the killing activity of NK cells and recruiting the activity of IL-2, INFγ and INFα.25
Several genes have been cloned into natural Bifidobacterium plasmids. pBLES100 was used to clone spectinomycin adenyltransferase, transformed into B. longum 105-A or 108-A and introduced to tumor-bearing mice where they were found to colonize specifically tumoral tissues.26 The same thing happened when this strain was inoculated in rats with chemically induced tumors.26 Cytosine deaminase (CD) of E. coli was also cloned in pBLES100 under the control of a strong promoter (HU gene) and the B. longum strain carrying the recombinant plasmid was able to convert the prodrug 5-FC into 5-FU, suggesting the strain as an effective enzyme/prodrug therapeutic agent.27,28 The CD gene, cloned into the pGEX-1λT plasmid, was also transformed in B. infantis and the positive results obtained in a mice melanoma model also supported the hypothesis of using this genus in cancer gene therapy.29
Endostatin gene was cloned into the pBV220 plasmid and transformed into B. adolescentis and B. longum and both demonstrated the potential to induce tumor growth inhibition. B. adolescentis presented around 70% inhibition when compared to control mice after a systemic administration.30 B. longum (orally administrated) selectivelly recognizes xenographfted hepatocarcinoma cells.31 In addition, no biological effect or plasmid stability's lost occurred when selective antibiotic-resistance genes were removed, improving the ability of this system to act as a safe anticancer deliver gene therapy.32
A number of other genes were also successfully expressed in Bifidobacterium. Very recently, an apoptosis-inducer gene, TRAIL (Tumor-necrosis factor-related apoptosis-inducing ligand) was introduced in the pBV22210 plasmid and transformed to B. longum (B. longum-TRAIL). Tumor-bearing mice were intravenously injected with this strain and sacrificed at several time points to analyze the tumor regression. Results indicated that almost all bacilli were specifically found in tumoral tissue region and only a few in apparently healthy tissues.33 Comparisons in tumor weight and volume were made between B. longum-TRAIL and B. longum-Endostatin, alone or in combination, with the best results being obtained with the combination. Tumor was suppressed in weight by 79.6 and 73.6%, and in volume by 82.6 and 76.7% when compared to a dextrose-saline solution control and B. longum wild-type, respectively. B. longum-Endostatin was also combined with Adriamycin (5 mg/kg) and its antitumor effect was enhanced.33
Despite the great interest of using these plasmids in anticancer therapies since they are all commercially available, they have a high molecular weight, making it somehow hard to engineer. Also, the oral administration of the strains can subject them to acid digestion in the stomach. However, once such obstacles are overcome, their activity can be of significant clinical impact. Bifidobacterium can inhibit cancer growth while it also enhances the immune system response, and growing in the host intestine, they can easily carry anticancer genes and become the reservoir of anticancer medicine.
Salmonella species are facultative anaerobes known to selectively colonize tumoral cells of solid tumors showing a replication ratio between tumor and healthy tissues of 1,000:1.34 However, most strains are pathogenic, causing significant immunostimulation due to the presence of lipopolysaccharide (LPS) and other virulence factors.5 To solve this problem, Salmonella thyphimurium was genetically modified in order to attenuate its virulence, disrupting the msbB gene, responsible for the terminal myristalization of lipid A, and purI, which introduced the need of an external adenine source. After showing the lower toxicity in several animal models, Pawelek and colleagues injected it into tumor-bearing mice and showed its specificity to tumors.35 Additionally, Salmonella strains were engineered to produce several proteins and toxins with anticancer activity: CD, TNFα, mitomycin C, herpes simplex virus thymidine kinase (HSV-tk) and colicin E3. More recently, reports have appeared of attenuated strains with the ability to delay tumor growth, including metastasis, in several models.5 A vaccine strain of Salmonella choleraesuis was developed and used both as a single anticancer agent and in combination with low-dose cisplatin (a platinum-based chemotherapy drug) in lung tumor and hepatocarcinoma in mice models. This strain was capable of delaying tumor growth in subcutaneous tumors and metastasis models. In combination with cisplatin, this strain increased the rate of infiltrating neutrophils, CD8+ T cells, enhancing an antitumor immune response and inducing a higher level of tumor apoptosis, causing an extended life span of the mice.36
Another developed strain has appeared based on S. typhimurium, termed A1. This strain is auxotrophic for leucine and arginine, but receives sufficient amounts of these amino acids from the tumor environment.37 Inoculation of wild type S. typhimurium caused death of the mice after only 2 days, but those inoculated with the A1 strain survived as long as the control mice. The ability of this strain to induce tumor regression was proved on a xenografted human prostate cancer.38 Although the results obtained with S. typhimurium A1 showed significant clinical potential, a new strain aiming at improving of the A1 tumor virulence was isolated. In order to isolate this variant, S. typhimurium A1 strain expressing GFP were injected in nude mice grafted with a colorectal derived cell line (HT-29). GFP expressing bacterial cells could then be isolated from the tumor tissue and cultured. Since it was a re-isolated A1 bacteria strain, the authors named it A1-R and both strains were compared in their adherence to tumor cells. Results showed that this re-isolated strain was capable of adhering to cancer cells, using HT-29 as model system, about 6 times more than the parental A1 strain. Using the prostate cancer cell line PC3 as model, it was also seen that A1-R strain killed cancer cells in about 2 hours, whereas the strain A1 showed the same result only after 24 hours.39 The results obtained with A1-R were validated in other tumoral models. The same was verified in pancreatic tumors, where treatment with A1-R showed a clear reduction in the tumor size and in other primary tumoral models and metastases.40 As an example, A1-R strain has demonstrated increased tumor regression, decreased metastasis number and enhanced animal survival in primary osteosarcoma and respective lung metastasis,41 spinal cord glioma,42 experimental lymph node metastasis, and in lung metastasis.43
In the specific area of increased immune responses, Listeria monocytogenes is another example of bacteria that can be used to treat cancer. It is an intracellular bacterium, capable of infecting phagocytic and non-phagocytic cells.44,45 Upon infection with Listeria, a significant immune response is elicited to clear the organism.46 A variety of live attenuated L. monocytogenes strains expressing viral and tumor antigens as fusion proteins have been produced during the last several years: HPV-16 E7,47 Her-2/neu,48,49 HMW-MAA,50 influenza NP51 and PSA.52,53 These recombinant strains cause specific CD4+ and CD8+ T cell responses in mice. In this bacterium, Listeriolysin O (LLO), a pore forming hemolysin, is the major virulence factor. Fusion of the antigens to a non-hemolytic truncated form of LLO resulted in enhanced immunogenicity and anti-tumor efficacy.47,49 It had the ability to induce inflammatory cytokines and an active strong immune response. Preclinical studies in several tumor models have proved the efficacy of this therapy. It also had the advantage of having already been found inside tumors and persisted in there for 10 days, while in the spleen and liver they only persisted for 3 days.54 Clinical trials using this bacterium have already been performed. In 2002, an indication of safety was obtained through oral administration of an attenuated strain carrying no antigen to 20 healthy volunteers, without detection of any serious adverse effects or long-term sequels.55 More recently, 15 patients with progressive cell carcinoma of the cervix, which previously failed to respond to chemotherapy, radiotherapy and surgery, were enrolled in a study with a live-attenuated vaccine strain of Listeria monocytogenes, carrying a vector expressing an LLO-HOV-16 E7 fusion protein.56 Three different groups of five patients each were treated with escalating intravenous doses of bacteria, followed by ampicillin 5 days after the injection. The main toxicity problems associated with the treatment were flu-like symptoms, resembling those observed with interleukin-2, probably related to a strong immune response triggered by the bacteria. Though it was not the purpose of the study, patients' survival was analyzed and the results were very promising. Usually, patients diagnosed with this type of cancer present only a median survival time of 6–7 months, but the overall median survival time for those subjected to this clinical study was 2x fold higher (347 days), and three patients were still alive, over two years, at the time of the report (2009).56 L. monocytogenes is not only a trigger of a potential immune response and an efficient antigen delivery system but mostly a new promising anticancer agent.
By far, the most successful case of live bacteria in the treatment of cancer is Mycobacterium bovis Bacillus Calmete-Guerin (BCG). Its major application is in bladder cancer, where a complete response is given in approximately 80% of the patients.57 BCG was originally developed as a vaccine against tuberculosis, but in 1950s BCG was studied as an anticancer agent. Despite the encouraging effects in some tumor models, further clinical trials showed delayed hypersensitivity type reactions when tumor implantations were attempted in the locals of infection.6 In 1976, Alvaro Morales established a therapeutic protocol for bladder cancer treatment which took in consideration the time intervals needed for the hypersensitivity reactions to occur and considered, for the first time, intradermal BCG vaccination. The good results obtained with his first seven patients led to two larger clinical trials which confirmed the efficacy of this therapy. It was the beginning of the successful story of BCG in bladder cancer treatment. Today, the therapeutics protocols involve the intra-bladder administration of BCG bacteria using a urethral catheter, which is then removed leaving the bacteria inside.58 The main action induced by bacteria is a massive immune response, with many different cytokines and immune cells involved in the process. BCG binds to FAP (fibronectin attachment protein) and is internalized by urothelial and inflammatory cells.59 It is interesting to emphasize that BCG treatment is dependent on a functional immune system. CD4 and CD8 T cells,60 Natural Killer cells61 and neutrophils62 have been shown to be necessary to achieve a full response, since their abrogation in murine models eliminated the BCG response. Monocytes and macrophages have also been linked to BCG response.63 Several cytokines have been reported to be increased in patients treated with BCG: Interleukin-2, 6, 8, 10, 12, TNF and INFα,γ.64 TRAIL is capable of inducing apoptosis in neoplastic cells but not in normal cells65 and it appears to be of pivotal importance in BCG-response. Neutrophils are its primary producers and its levels are increased in the urine of BCG-responders compared to BCG non-responders.66 Since high levels of cytokines appeared following BCG treatments, several attempts have been made to overcome the possible side effects on live bacteria injections. However, all the clinical trials produced no effective results, suggesting that a complicated cascade of immune responses is activated only when bacteria are present and that it is virtually impossible to replicate it only with cytokines treatment.67 However, combination of BCG with cytokines were more effective, particularly when INFa was administered in addition to BCG.68
Bacteria-Derived Anticancer Agents
Not only live bacteria have applications in cancer therapies, but also bacterially-derived products have been tested and some of them successfully used for this purpose. The mode of action of some strains is through the production of cytotoxic factors, enzymes, antibiotics and others secondary metabolites that can be used or adapted in a proper manner to specifically target cancer cells.
A major aspect in cancer, related to bad prognosis of the cancer patients, is its ability to metastasize. Cancer metastasis occurs during tumor progression and cause 90% of human cancer deaths. The homing of metastases is not a random process, is organ specific, and depends on the histotype of the primary tumor. In this process, tumor cells use chemokine-mediated mechanisms. Chemokines are molecules produced in order to direct the migration of cells toward their gradient, through binding to specific cell membrane-attached molecules: G-protein-coupled receptors, as example. This migratory behavior of cancer cells also occurs in embryonic development and immunity.69 Interestingly, bacteria naturally produce several chemokine/adhesion receptor inhibitors, so this appears as a new appealing approach as an anticancer therapy.
Staphylococcal superantigens-like (SSL) are a class of bacterial proteins produced by Staphylococcus aureus capable of binding several eukaryotic receptors overexpressed in cancer cells. SSL10 binds CXCR4, a GPCR expressed on human T-ALL lymphoma and cervical carcinoma cells. The natural ligand for CXCR4 is CXCL12, but SSL10's action clearly inhibited the chemotactic response of HeLa (cervical carcinoma) cells towards the natural ligand.70
The class of bacterial protein SSL5 has been demonstrated to bind to the receptor for P-selectin glycoprotein-1 (PSGL-1), inhibiting rolling of neutrophils on a surface by acting as a decoy and thus hampering the interaction with its natural ligand, the P-selectin. The inhibitory action of SSL5, without any observed toxicity, was demonstrated in HL-60 leukemia cells since these cells express the receptor P-selectin glycoprotein-1 (PSGL-1). Besides the novel therapeutic action towards hematological malignancies, the bacterial protein SSL5 also binds epithelial cells that circulate in the blood stream during metastasis and thus can be a very interesting therapeutic tool for metastatic carcinoma.71
Some bacterial enzymes are also candidate therapeutic agents for cancer treatment. This is the case of the amino acid-degrading enzyme arginine deiminase of Mycoplasma arginini (Ma-ADI), a tumor growth inhibitor and potentially a therapeutic agent for the treatment of in vitro and in vivo tumors, such as hepatocellular carcinoma, melanoma, leukemia, renal cell carcinoma and prostate cancer.72 Such inhibition of cancer cell growth by ADI is believed to be due to depletion of arginine.73 Interestingly, Ma-ADI has not only anticancer activity, but also has been implicated in inhibiting the growth of viruses such as HIV-1 and hepatitis C.74 These pleiotropic effects appear to suggest that the enzymatic activity of ADI, leading to arginine depletion, may not be the only contributing factor in the anticancer/antiviral activity of MA-ADI. Das et al. reported the crystal structure of ADI from Mycoplasma arginini where the N-terminal part of this enzyme harbored a putative CARD-like domain (caspase activation domain) with no significant amino acid sequence homology to any mammalian CARD proteins but with a low but discernable structural similarity to the CARD motif.75 Likewise, P. aeruginosa also produces ADI (Pa-ADI) and its 5′-end of the sequence containing the CARD-like domain was cloned and expressed as a 17 kDa polypeptide (Pa-CARD). Unlike the full size (46 kDa) protein, the truncated form had no ADI enzymatic activity, but presented anticancer activity in fibrosarcoma, breast cancer and leukemia cells. Thus, it appears that ADI's anticancer activity via enzymatic depletion of arginine is not the only way but a second potent route exists, which is through the N-terminal putative CARD-like domain without ADI activity.76
Two enzymes are required for the synthesis of arginine from citrulline: first, argininosuccinate synthetase (ASS) convertes citrulline to argininosuccinate and then, this is converted to arginine by argininosuccinate lyase (ASL). Conversely, ADI converts arginine to citrulline and ammonia. While arginine is a non-essential amino acid in humans, certain cancers such as hepatocellular carcinoma, melanoma or renal cell carcinomas do not express ASS in vivo, making them sensitive to arginine deprivation due to ADI action.
ADI causes an immune response when injected in humans and therefore multiple injections are necessary to consume all arginine due to the enzyme's short half-life. To reduce immunogenicity, ADI was covalently bound to PEG 20,000 and renamed as ADI-PEG20 (Polaris, Inc.), maintaining approximately 50% of its specific activity and increasing half-life in the animal models.77 It was shown that ADI-PEG20 inhibited in vivo and in vitro growth of human melanoma and hepatocellular carcinoma, which were found as auxotrophic for arginine due to a deficiency in ASS.78,79 Phase I and II clinical trials have been performed in patients with unresectable hepatocellular carcinoma and melanoma.80–86 The results of the hepatocellular carcinoma showed only a moderate antitumor activity with tolerable toxicity, and in some cases, some patients couldn't complete the therapy as a result of allergic diseases. In the malignant melanoma clinical trials a higher anti-tumor activity was observed.86 Recent studies appear to indicate that a major advantage of ADI seems to be as a part of combinatorial therapies with interleukin 2, reducing the hypotension cause by nitric oxide, a common feature when high doses of IL-2 are given to patients, and its ability to cause nutritional stresses in cancer cells may be positive for the action of chemotherapeutic agents.
Recombinant immunotoxins based on Pseudomonas aeruginosa exotoxin A (PE) are also promising anticancer agents. These chimeric proteins contain the Exotoxin A (a toxic domain for cytotoxicity) fused either to monoclonal antibodies or antibody fragments or physiologically important ligands like cytokines and growth factors. Exotoxin A from P. aeruginosa is a potent virulence factor that catalyzes the ADP-ribosylation of the eukaryotic elongation factor 2 (eEF-2) in host cells, affecting protein synthesis and cell viability.87 It shares an A-B structure with other bacterial toxins. The B-domain is responsible for the interaction with eukaryotic host cell receptors, after which the A-domain translocates to the cytoplasma and exerts its action.88,89 Recombinant Exotoxin A-immunotoxins kill cancer cells by binding specifically to overexpressed cell-surface receptors, which carries them into the cell interior, where they arrest protein synthesis and induce apoptosis.87
Clinical trials with different Exotoxin A-immunotoxins have already been performed with positive results in leukemia and bladder cancer.88–93 However, it was frequently observed that these immunotoxins missed the ability to exert a strong cytotoxic activity against solid tumors, with better results obtained with hematologic tumors.94 These cells are more accessible for immunotoxins than solid tumors because these proteins need to overcome several natural barriers, which leads to the loss of their efficacy. Protein engineering has recently been developed to increase affinity towards cancer cells, increasing specificity, cytotoxicity and maximum tolerated doses in the treated mice and also PEGylated forms of immunotoxins were tested.95 Previous studies showed cytotoxicities were similar to the native form, but half-lives were prolonged and animal toxicity was considerably reduced.96
Microbial secondary metabolites, such as antibiotics and pigments, are also a source of new drugs for cancer treatment. Pharmaceutical industries have invested in the identification of their biological activities, but in the beginning of the 1990s the appearance of new techniques favoring chemical synthetic compounds downsized the research funding of new natural compounds. Nowadays, research in this area is re-gaining strength and new natural or natural-derived products are emerging.97 Farnesyltransferases inhibitors, prodiginines and epothilones are three examples of these products.
Activating K-ras mutations represents the most common type of abnormality of a dominant oncogene in human cancer, with specificity and type of mutation varying in relation to tumour type.98 Among the critical transforming alterations occurring in cancer it is widely accepted that mutations in K-ras are very frequent genetic somatic events, occurring in about 30% of all tumour types. Further, Ras activation is crucial to activate signaling pathways involved in cancer progression, namely proliferation, differentiation, cell survival and migration. Ras is a GTP-binding protein and in normal cells it switches between an active form, GTP-bound, and an inactive form, GDP-bound. Ras mutations cause a constitutively active form resulting in the constant activation of its downstream signaling pathways, MAP-kinase as example.99
In order to be functional proteins, Ras needs to be anchored in the inner side of plasma membrane which requires farnesylation. Farnesyltransferase inhibitors (FTI) are metabolites capable of blocking this step and thus impair Ras activation, even in the presence of a mutant Ras. Several FTIs of microbial origin have been discovered in the past few years, namely Manumycin A and Gliotoxin. Manumycin A is an antibiotic produced by Streptomyces parvulus and is one of the most studied, which has shown both in vitro cytotoxic activity against several cell lines (human pancreatic tumor, thyroid carcinoma, leukemias, myeloma and hepatocellular carcinoma) and in vivo against human cancer xenografted models (pancreatic cancer and thyroid cancer).100–107 A combined treatment with Manumycin and Paclitaxel (a mitotic inhibitor used in cancer chemotherapy), had a synergistic cytotoxic effect on anaplastic thyroid carcinoma cells.107 Angiogenesis and Vascular Endothelial Growth Factor are also inhibited by this combination, showing higher cytotoxic effects than when used singly.
TAN-1813, a farnesyltransferase inhibitor isolated from a fungus strain, Phoma sp. FL-4151, was shown to arrest a mouse embryonic fibroblast cell line NIH3T3 at G1 and the G2/M phases impairing cell proliferation. In this cancer model, xenografted tumors in nude mice have also been inhibited by this metabolite.108
Overall, several farnesyltransferase inhibitors of microbial origin can induce apoptosis in cancer cells through the disruption of farnesylation or the farnesylated proteins, but more insights in the mechanisms are needed to optimize their use alone or combined with other chemotherapeutic agents.
Prodiginines are bacterial secondary metabolites with immunosuppressive and anticancer properties, characterized by a pyrrolydipyrromethene skeleton. The anticancer activity has been shown in several cancer derived cell lines (liver, spleen, blood, colon, gastric, lung, breast and chronic myeloid leukemia) and also in vivo in mice with a xenografted liver cancer. Moreover, these metalolites show little or no activity at all in normal cells.109 Prodiginines are capable of inducing apoptosis and act through different modes targeting multiple cellular pathways: (1) inducing or correcting DNA damage, (2) by cell cycle arrest; (3) changes of intracellular pH and (4) showing resistance to multidrug pumps that are often responsible for inducing resistance to other anticancer agents. Prodigiosin, a red pigment produced by many strains of the bacterium Serratia marcescens, is a prodiginine already tested against dozens of cancer cell lines exhibiting an average inhibitory concentration of 2.1 µM.110
Obatoclax (GX15-070) is a synthetic derivative of natural prodiginines developed at Gemin X Pharmaceuticals.111 This compound was designed to antagonize the effects of Bcl-2 anti-apoptotic proteins family, through binding and sequestering Bcl-2 proteins, releasing the pro-apoptotic Bax and Bak proteins. Obatoclax is the leading prodiginine candidate to be applied in clinics. This new drug is now undergoing multiple phase I and phase II clinical trials against several forms of cancer both as a single agent and in combined therapies.109,111–113
Epothilones were discovered as cytotoxic metabolites in the myxobacterium Sorangium cellulosum. They show anti-tumor activity in cancer cell lines, many of those multidrug-resistant or paclitaxel-resistant (Taxol®, Bristol-Myers Squibb).114 Epitholones bind to the α, β-tubulin dimer of microtubules, inducing its polymerization and stabilization which causes cell cycle arrest at the G2/M transition and apoptosis. The first studies on the mechanism of binding seemed to indicate that these natural drugs shared pharmacophores with taxanes; however, recent crystallography results with epothilones indicated a unique tubulin-binding pocket that is not common to taxanes.115
Natural epothilones B and D and five derivatives (ixabepilone, BMS-310705, ZK-EPO, ABJ879, KOS-1584) are under investigation in several pre-clinical, and phase I and II clinical trials (reviewed in ref. 116). The modifications in the derivatives are made in order to improve specific properties that hamper the biological activity like water and plasma solubility, toxicity and binding ability to the microtubules.114 Unlike paclitaxel, ZK-EPO, one of epitholone B derivatives, is able to cross the blood-brain barrier and, therefore, is a potential agent for penetration into the central nervous system. Pre-clinical studies have demonstrated in vitro activity in breast, lung and colon cancer cell lines, amongst others. These natural drugs have shown an average activity one order of magnitude more potent than taxanes in cancer cell lines with an inhibitory concentration (IC50) in the sub or low nanomolar range.117,118 In murine xenograft tumor models with both intravenous and oral administration of ixabepilone, a second generation epothilone B,118 successful tests have been conducted, particularly for multidrug resistant models, such as MCF7/ADR and 16C/ADR breast, Pat-7 ovarian and HCT116/VM46 human colon carcinoma.119 Breast cancer models of metastasis have striking tumor growth inhibition when treated with ZK-EPO.120 Different schedules for drug administrations have been reported for different cancer models in order to determine the maximum tolerated doses and the dose-limiting toxicities (DLT). The most common DLT encountered was diarrhea for epothilone B and its derivatives and sensory neuropathy for epothilone D.114 More ongoing clinical trials will bring better insights regarding the precise effects of epothilones whilst more research exploring new derivatives or new natural epothilones will allow extensive studies on the clinical and pharmacological use of this new class of drugs.
Bacterial Azurin as a Novel and Promising Anticancer Agent
It has now been demonstrated that Pseudomonas aeruginosa produces at least two cytotoxic proteins against cancer cells. One protein, interestingly, is not known as a virulence factor or an enzyme but a water soluble low molecular weight copper-containing redox protein named azurin (128 aa–14 kDa) involved in the electron transport chain. Zaborina et al. showed that azurin has cytotoxic activity against murine macrophage cell line J774 when its release was observed in the presence of eukaryotic proteins in the growth media.121 Besides azurin, cytochrome c551, believed to be a partner of azurin in electron transfer, also demonstrated somewhat reduced cytotoxic activity towards the macrophage cells. Since J774 is a tumor cell line, it was of interest to see if azurin would demonstrate similar cytotoxicity towards human cancer cells. Melanoma (UISO-Mel-2)122 and breast cancer (MCF-7)123,124 were two models tested, where the efficacy of azurin to induce apoptosis and allow significant regression of these two cancers was demonstrated. In addition, intraperitoneal administration of azurin in nude mice xenografted with the two human cancer cells led to statistically significant tumor regression in vivo, with no apparent toxic effects to the animals.122,124
A variant of azurin termed Laz has also been characterized from gonococci and meningococci such as Neisseria meningitidis, which can cause meningitis, an inflammation of the brain meninges. Contrasting with other bacterial azurins, this Neisserial azurin has an extra epitope (39 amino acids) in the N-terminal called H.8. This epitope is responsible for entry in gliobastoma cells, an ability that azurin does not possess. Brain is a highly protected organ exhibiting blood-brain barrier (BBB) which restricts the uptake of various compounds including drugs into the brain. This epitope was cloned in the N-terminal part of P. aeruginosa azurin, allowing its entry and cytotoxic activity in gliobastoma cells. This H.8 epitope may as well be useful to allow entry of other drugs in the brain.125
Recently, azurin and Laz have been demonstrated to exhibit cytotoxic activity against liquid cancers, in addition to the solid tumours already described. Using HL60, an acute myeloid cell line, and K562, a chronic myeloid cell line, Kwan et al. demonstrated the high cytotoxicity of Laz towards both HL60 and K562 cells at modest concentrations (10 µM induced a loss in cell viability of about 90%).126
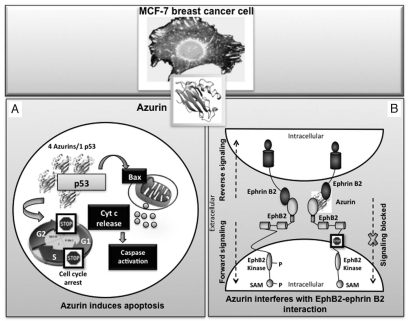
So far, three different modes of azurin's action towards cancer cells have been proposed. Azurin has been shown to bind avidly to the intracellular tumour suppressor p53, stabilizing it and, thus, leading to increased expression of pro-apoptotic protein Bax and Bax-dependent apoptosis in cancer cells;122,123,127 it also binds to several Eph receptor tyrosine kinases, a family of extracellular receptor proteins known to be upregulated in many tumors,128 and this binding with EphB2 interferes in its phosphorylation at the tyrosine residue, resulting in inhibition of cell signalling and cancer growth (Fig. 1).6
Figure 1.
The mode of action of azurin in the induction of apoptosis and growth inhibition of a MCF-7 breast cancer cell is shown schematically. Azurin can enter the cancer cell to form a complex with tumor suppressor protein p53 (4 azurin molecules per 1 p53 molecule), stabilizing it and enhancing its intracellular level, which leads to apoptosis via caspase-mediated mitochondrial cytochrome c release pathways (A). Azurin can also bind avidly to the surface—exposed receptor tyrosine kinase EphB2, interfering in its binding with the ligand ephrinB2, and thereby preventing cell signaling that promotes cancer cell growth (B).
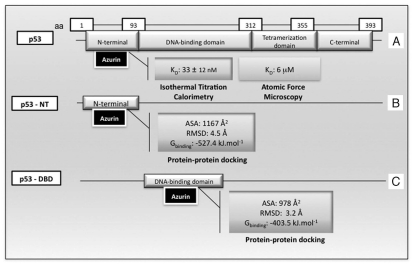
With regard to azurin-p53 interaction and induction of apoptosis,129–131 several recent studies have been conducted to understand the nature of such interactions. p53 is a four-chain, 393 residue protein, organized into four functional domains: an N-terminal domain (NTD), a sequence-specific DNA-binding domain (DBD), a tetramerization domain (TD) and a C-terminal regulatory domain (CTD) (Fig. 2). The DBD is the largest domain and its structure has been solved by NMR. This p53 domain is relatively unstable and often suffers mutations capable of altering its stability. The TD has also been characterized by NMR. In contrast, the NTD and CTD are predominantly unstructured under normal conditions.132–135 The first evidence of complex formation between p53 and azurin came from glycerol gradient and column binding, co-elution experiments.130 Subsequent experiments with isothermal calorimetry demonstrated that four azurin molecules bind per 1 monomer of p53 with a dissociation constant of 33 ± 12 nM, presumably in the p53 NTD, according to the observed quenching of tryptophan residues (Fig. 2).127 Regarding azurin, a specific region has been implicated in the complex formation by site-directed mutagenesis: amino acids Met-44 and Met-64, located in a hydrophobic patch of the protein, have been shown to be important for the interactions with p53 and their substitutions resulted in altered complex formation with p53.123,129,131 Further, more insight in the interaction between these two molecules at the single molecule level has been obtained by atomic force microscopy (AFM).136 The p53 protein was immobilized in a gold substrate and azurin was tethered in the AFM tip. The results confirmed the interaction between both proteins with an estimated dissociation constant of 6 µM (Fig. 2), lower than that estimated before in bulk, and establishing a strong affinity in the range of others like the transient complex azurin-cytochrome c551 (1–100 µM)137 and close to that of an antigen-antibody pair (0.1–10−5 µM).136 Through protein docking, the nature of the complex formed between azurin and DBD and NTD of p53 was investigated. p53 DBD acquires a β-sandwich fold (PDB entry 1TUP_B), formed by two antiparallel β-sheets, SI and SII, with 4 and 5 strands, respectively (s1, s2, s3, s8 and s4, s6, s7, s9 and s10). This structure is a scaffold for two large loops, L2 and L3, and a loop-sheet-helix (LI-SIII-H2).138,139 The Zdock docking program, was used to model the complex structure (p53 DBD domain_Azurin) generating more than 2,000 predicted structures.140 Only the models where Met-44 and Met-64 were within a reasonable distance to be involved in the binding process were accepted, reducing the number to 194 models. Moreover, the models were grouped in clusters according to their similarities and the 3D structures were compared to eliminate geometric similar structures, obtaining a final number of ten candidates.140 Physical properties of each model was calculated like the ASA, the accessible surface area, and all of them presented values between 690–800 Å2 before molecular dynamic (MD) simulations, comparable to the values for transient complexes (400–1,000 Å2).141 MD simulations and estimation of the free energy of binding were performed to refine the models. The best model with the lowest binging energy (−403.5 kJ mol−1), was found to involve the L1 loop and strands s7 and s8 in the p53 DBD binding interface and the residues in the hydrophobic patch of azurin (Fig. 2). The authors proposed that the loops in p53 belong to the most flexible region of the domain, thus capable of a strong structural adaptation, observed in the MD simulations, which lead to a tighter packing of both proteins and resulted in constructive Van der Waals contacts. Also, since the p53 DBD regions involved in the binding are in a peripheral position, this flexibility restriction can be one of the reasons for the azurin-induced stability of p53.140 The NT domain of p53 has also been implicated in the binding to azurin and as for the DBD, Zdock, MD and free energy calculations have been applied to determine a good model for the complex.138 The NTD structure is predicted to be organized in an α-helix, HI, and two turns assuming another α-helix structure, HII and HIII, linked by a fragment of 30–32 residues. As for the DBD, 2,000 models were generated and clustered by analysis of the 3D structures into five groups. The calculated ASA values were again in the range of the transient complexes. After the MD simulations, the most probable complex conformation is characterized by a free energy of −527.4 kJ mol−1 (Fig. 2). The regions involved in the binding are the helices HII and HIII of p53, and the simulation showed a strong adaptation of this region to the azurin shape, increasing packing between both proteins, through numerous and favourable Van der Waals interactions. Apiyo and Wittung-Stafsede127 had already suggested this region as the one involved in the binding due to the quenching of the tryptophan emission which was corroborated by this model because Trp-23 is located at the protein interface and exposed to be quenched by azurin binding.138 When one compares the two models for azurin and p53 binding in the two different p53 domains, the best result is obtained by the latter, with a lower free energy. Since it was also shown by the same authors that 4 azurin monomers bind to each p53 monomer, one cannot exclude the fact that more than one configuration is possible, as highlighted by the authors of this study.138
Figure 2.
Studies of complex formation between the bacterial protein azurin and the transcription factor p53. (A) Binding studies using Isothermal Titration Calorimetry126 and Atomic Force Microscopy.135 (B and C) Docking studies and free energy simulation for the interaction between azurin and p53 N-terminal (p53-NT)138 and azurin and p53 DNA binding domain (p53-DBD).139
In order to interact with p53, azurin must come out of the periplasmic space of P. aeruginosa and enter mammalian cells. Indeed, azurin has been shown to be secreted to the outside medium when P. aeruginosa cells are exposed to human cancer cells, but less so when exposed to normal cells.142 Azurin was proficient in entering the human cancer cells but was deficient in entering the normal cells.143 The entry of azurin in cancer cells has been studied by Yamada et al.143 through a series of GST fusions of truncated forms of azurin at the central, N- and C-terminal regions. Azurin was internalized but GST alone was not and several fragments of azurin were tested to uncover the entry domain of this bacterial protein. The preferential entry of azurin in cancer cells has been identified to be a domain between amino acids 50 and 77 of azurin, termed p28, which can be used as a vehicle to transport cargo proteins.143 p28 forms an extended amphipathic helix with both a hydrophobic (amino acids 50 to 66) and a hydrophilic (amino acids 67 to 77) domain with antiangiogenic activity.144
A chemically-synthesized version of the p28 peptide has not only entry specificity in cancer cells, but similar to azurin, has been shown to interfere with angiogenesis, inhibiting the formation of capillary tube formation of the human umbilical vein endothelial cells (HUVEC) in a dose dependent mode.6,145,146 Using a scratch wound migration assay, it was shown that p28 inhibited the migration of these cells in Matrigel, proving that this peptide has broad range effects in the morphological characteristic of angiogenesis. Interestingly, in addition to its ability to kill cancer cells, azurin and p28 can also interfere in oncogenic transformation to prevent precancerous lesion formation in mouse alveolar and ductal mammary glands exposed to a carcinogen Dimethyl-benz-anthracene (DMBA).147
Taylor et al.146 provided new information regarding the entry of the chemically-synthesized peptide p28 in human cancer cells. They refined the protein transduction domain (PTD) of 28 amino acid (p28) to only 18 (p18) in the N-terminal region, from amino acids 50 to 67. They showed that the entry of p28 and p18 occurs mainly via a nonendocytic rather than an endocytic membrane receptor mediated process. The results also suggested that the entry occurs without loss of membrane integrity. Recently, a start-up company CDG Therapeutics has initiated phase I human clinical trials of p28 for its anticancer activity (http://clinicaltrials.gov; clinical trials.gov identifier: NCT00914914).
Another important feature of azurin is its ability to recognize and bind to certain cellular receptors exposed at the cells' surface. Eph receptor tyrosine kinases are a family of 14 extracellular receptor proteins which bind to their ligands, the ephrins. These receptors are generally divided in two classes (A and B): A1 to A8 are cell-membrane linked proteins while B1 to B6 have highly conserved intracellular domains as well as a transmembrane domain.6 After the receptor-ligand interaction, a series of cellular signalling processes are initiated resulting in the proliferation, migration, invasion and angiogenesis of many types of human tumors. Different Eph receptors and ephrin ligands are present in distinct locations and some have been shown to be upregulated in some tumors, as it was for the EphB2 which is upregulated in gliobastoma, hepatocellular carcinoma, gastrointestinal and renal carcinomas, and prostate, lung and ovarian cancers.128 Chaudari et al.128 tested three different cupredoxin proteins which exhibited structural similarities with the ephrinB2 ectodomain and found high values of interaction between azurin and EphB2 and also EphA6, using Surface Plasmon Resonance (SPR). However, since both EphB2 and azurin have been involved in cancer cell signalling or cancer cell death, the authors proposed that this interaction could be a way of blocking the signalling process, antagonizing the Eph-ephrin mediated tumor progression (Fig. 1). In particular, a fragment of azurin, Azu 88-113, coincident with the G-H loop of ephrinB2-Fc, had a high affinity for binding with EphB2 (12 nM). Moreover, pre-incubation of EphB2-Fc with azurin diminished protein binding to an immobilized surface with ephrinB2 by up to 99% at stoichiometric or excess concentrations of the bacterial protein. Azurin and a fragment of it comprising amino acids 88 to 113 (Azu 88-113) showed the highest inhibition abilities. Altogether, the binding and the inhibition data indicate an affinity of azurin to interfere with the EphB2-ephrinB2 binding and the signalling process that can lead to tumor progression.128
Unlike the rationally designed drugs that target a specific step in the signaling pathway involved in cancer, azurin intervenes in multiple pathways of cancer growth, thus exhibiting promiscuity. This is probably due to the fact that azurin has some interesting 3D-structural features. Azurin has structural similarity with variable domains of various immunoglobulins, thereby clearly demonstrating its single antibody-like structure as represented by ribbon drawing of eight antiparallel strands stabilized by a disulphide bridge-giving rise to a β-sandwich core, an immunoglobulin fold.147,148 It has a 28 amino acid (p28) extended a-helix PTD in the middle part that allows its entry specificity in cancer cells. It also has four exposed loop regions that are believed to be involved in its bindings with other proteins. By far the most interesting characteristic of azurin is its ability to bind various unrelated mammalian proteins relevant in cancer, conferring on it the property of a natural scaffold protein.148 The scaffold proteins are non-antibody recognition proteins belonging to different and divergent families but that demonstrate immunoglobulin-like binding characteristics.149 The ability of the azurin peptide p28 to act as a vehicle to carry cargo proteins inside cancer cells and azurin's ability to bind many different proteins because of its unique structural features makes azurin a potentially important natural scaffold protein for therapeutic purposes. A further development in the concept of “scaffold” implies the generation of azurin variants displaying multiple affinities to pre-defined targets relevant in cancers. This strategy can be reached by randomized substitution of amino acids or substitutions of only a few selected residues within preexisting binding sites, specifically the exposed a-helix (p28) and the four surface exposed loops.
Future Perspectives
In the field of cancer drug discovery, recent developments are often related to rationally designed drugs, targeting a specific molecule or a signaling pathway. The use of high-throughput assays and combinatorial chemistry has supported the development of new synthetic chemical compounds with specific properties and structures directed to a specific receptor or protein that blocks a reaction or signaling cascade promoting cancer cell growth, survival and/or invasion. Different targets are used in anticancer drugs development, including those in growth signaling cascades and in DNA replication, cell division, angiogenesis and apoptosis. Overall different therapeutic modalities are now commercially available and in clinical practice, including monoclonal antibodies, drugs and prodrugs combinations and small natural or synthetic molecules.1,6 However, cancer cells, as with other disease agents, have the ability to mutate rapidly, causing a significant loss of therapeutic efficacy. Combinations of different drugs are often used to overcome this problem but this can cause a considerable increase in the costs and poses a problem to acquired resistance to multiple drugs. This has prompted the development of novel and alternative approaches for the treatment of cancer.
Microbial-based therapy of cancer is one of the emerging cancer treatment modalities. Over the past few years, important advances have been made to study and develop live bacteria, with or without additional cloned genes that encode toxins targeted specifically to cancer cells, or bacterial products with cancer killing ability. Various types of obligate anaerobic and facultative anaerobic bacteria can preferentially target cancer cells for growth in the hypoxic regions of the solid tumour. Attention has also recently been directed not only to the use of live bacteria, but bacterial proteins that can enter preferentially to cancer cells and disrupt their growth or kill them by multiple mechanisms.
During the past decade, cancer drug discovery or development is based on the single-target, single-compound paradigm. This concept contrasts with a more recent drug design approach, where a single drug can target with high efficacy multiple steps in cancer progression. Such second-generation drugs are often much more efficacious with little side effects. Of particular interest in this respect is azurin, a protein produced by the pathogenic bacteria P. aeruginosa. Azurin induces apoptosis through complex formation with tumor suppressor protein p53 or inhibits growth of cancer cells by interfering in receptor tyrosine kinase-mediated cell signaling or preventing angiogenesis.150 In vivo cancer regression by azurin has also been demonstrated. The ability of a single bacterial protein, azurin, to interfere in the growth of cancer, is an interesting example of a potential drug candidate that can target multiple unrelated targets, interfering in multiple steps in the disease progression.
Footnotes
Previously published online: www.landesbioscience.com/journals/biobugs/article/10903
References
- 1.Hayat MA, editor. Methods of Cancer Diagnosis, Therapy and Prognosis: Gastrointestinal Cancer. 1st Edition. Springer; 2008. [Google Scholar]
- 2.Schulz WA. Molecular Biology of Human Cancers. 1st Edition. Springer; 2005. [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarty AM. Microorganisms and Cancer: Quest for a therapy. J Bacteriol. 2003;185:2683–2686. doi: 10.1128/JB.185.9.2683-2686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei MQ, Mengesha A, Good D, Anné J. Bacterial targeted tumour therapy-dawn of a new era. Cancer Letters. 2008;259:16–27. doi: 10.1016/j.canlet.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 6.Fialho AM, das Gupta TK, Chakrabarty AM. Promiscuous drugs from pathogenic bacteria in the post-antibiotics era. In: Sleator R, Hill C, editors. Patho-Biotechnology. Landes Bioscience; 2008. pp. 145–162. [Google Scholar]
- 7.Minton NP. Clostridia in cancer therapy. Nat Rev Microbiol. 2003;1:237–242. doi: 10.1038/nrmicro777. [DOI] [PubMed] [Google Scholar]
- 8.Parker RC, Plummber HC, Siebenmann CO, Chapman MG. Effect of hystolyticus infection and toxin on transplantable mouse tumors. Proc Soc Exp Biol Med. 1947;66:461–465. doi: 10.3181/00379727-66-16124. [DOI] [PubMed] [Google Scholar]
- 9.Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15:473–478. [PubMed] [Google Scholar]
- 10.Carey RW, Holland FW, Whang HY, Neter E, Bryant B. Clostridial oncolysis in man. Eur J Cancer. 1967;3:37–46. [Google Scholar]
- 11.Heppner F, Mose JR. The liquefaction (oncolysis) of malignant gliomas by a non pathogenic Clostridium. Acta Neurochir (Wien) 1978;42:123–125. doi: 10.1007/BF01406639. [DOI] [PubMed] [Google Scholar]
- 12.Heppner F, Trtthart H, Holzer P. Surgical therapy of traumatic intracerebral hematomas. Wien Med Wochenschr. 1978;128:635–636. [PubMed] [Google Scholar]
- 13.Thiele EH, Arison RN, Boxer GE. Oncolysis by Clostridia III. Effects of clostridia and chemotherapeutic agents on rodent tumors. Cancer Res. 1964;24:222–223. [PubMed] [Google Scholar]
- 14.Morse SS. Evolving views of viral evolution: towards an evolutionary biology of viruses. Hist Philos Life Sci. 1992;14:215–248. [PubMed] [Google Scholar]
- 15.Lemmon MJ, van Zijl P, Fox ME, Mauchline ML, Giaccia AJ, Minton NP, et al. Anaerobic bacteria as a gene deliver system that is controlled by the tumor microenvironment. Gene Ther. 1997;4:791–796. doi: 10.1038/sj.gt.3300468. [DOI] [PubMed] [Google Scholar]
- 16.Lambin P, Theys J, Landuyt W, Rijken P, van der Kogel A, van der Schueren E, et al. Colonisation of Clostridium in the body is restricted to the hypoxic areas of tumors. Anaerobe. 1998;4:183–188. doi: 10.1006/anae.1998.0161. [DOI] [PubMed] [Google Scholar]
- 17.Fox ME, Lemmon MJ, Mauchline ML, Davis TO, Giaccia AJ, Minton NP, et al. Anaerobic bacteria as a delivery system for cancer gene therapy: in vitro activation of 5-fluorocytosine by genetically engineered clostridia. Gene Ther. 1996;3:173–178. [PubMed] [Google Scholar]
- 18.Mellaert LV, Wei MQ, Anné J. Live Clostridia: A powerful tool in tumor biotherapy. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 19.Lambin P, Nuyts S, Landuyt W, Theys J, de Bruijn E, Anné J, et al. The potential therapeutic gain of radiation-associated gene therapy with the suicide gene cytosine deaminase. Int J Radiat Biol. 2000;76:285–293. doi: 10.1080/095530000138628. [DOI] [PubMed] [Google Scholar]
- 20.Theys J, Pennington O, Dubois L, Vaughan T, Mengesha A, Landuyt W, et al. Repeated cycles of Clostridium-directed enzyme prodrug therapy result in sustained antitumor effects in vivo. Br J Cancer. 2006;95:1212–1219. doi: 10.1038/sj.bjc.6603367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal N, Bettegowda C, Cheong I, Geschwind JF, Drake CG, Hipkiss EL, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci USA. 2004;101:15172–15177. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettegowda C, Dang LH, Abrams R, Huso DL, Dillehay L, Cheong I, et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc Natl Acad Sci USA. 2003;100:15083–15088. doi: 10.1073/pnas.2036598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Nat Acad Sci USA. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura NT, Taniguchi S, Aoki K. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res. 1980;40:2061–2068. [PubMed] [Google Scholar]
- 25.Fu GF, Yin Y, Hu B, Xu GX. Bifidobacterium as a delivery system of functional genes for cancer gene therapy. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 26.Yazawa K, Fujimori M, Nakamura T, Sasaki T, Amano J, Kano Y, et al. Bificobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumours. Breast Cancer Res Treat. 2001;66:165–170. doi: 10.1023/a:1010644217648. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Sasaki T, Fujimori M, Yazawa K, Kano Y, Amano J, et al. Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci Biotechnol Biochem. 2002;66:2362–2366. doi: 10.1271/bbb.66.2362. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T, Fujimori M, Hamaji Y, Amano J, Taniguchi S. Genetically engineered Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy of autochthonous mammary tumors in rats. Cancer Sci. 2006;97:649–657. doi: 10.1111/j.1349-7006.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi C, Huang Y, Guo ZY, Wang SR. Antitumor effect of cytosine deaminase/5-fluorocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma. Acta Pharmacol Sin. 2005;26:629–634. doi: 10.1111/j.1745-7254.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Fu GF, Fan YR, Liu WH, Liu XJ, Wang JJ, et al. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003;10:105–111. doi: 10.1038/sj.cgt.7700530. [DOI] [PubMed] [Google Scholar]
- 31.Fu GF, Li X, Hou YY, Fan YR, Liu WH, Xu GX. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer. Cancer Gene Ther. 2005;12:133–140. doi: 10.1038/sj.cgt.7700758. [DOI] [PubMed] [Google Scholar]
- 32.Xu YF, Zhu LP, Hu B, Fu GF, Zhang HY, Wang JJ, et al. A new expressing plasmid in Bifidobacterium Longum as a delivery system of endostatin for cancer gene therapy. Cancer Gene Ther. 2007;14:151–157. doi: 10.1038/sj.cgt.7701003. [DOI] [PubMed] [Google Scholar]
- 33.Hu B, Kou L, Li C, Zhu LP, Fan YR, Wu ZW, et al. Bifidobacterium longum as a delivery system of TRAIL and endostatin cooperates with chemotherapeutic drugs to inhibit hypoxic tumor growth. Cancer Gene Ther. 2009;16:655–663. doi: 10.1038/cgt.2009.7. [DOI] [PubMed] [Google Scholar]
- 34.Ryan RM, Green J, Lewis CE. Use of bacteria in anti-cancer therapies. Bioessays. 2005;28:84–94. doi: 10.1002/bies.20336. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman RM. Salmonella thyphimurium mutants selected to grow only in tumors and eradicate them in nude mouse models. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 36.Lee CH, Wu CL, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther. 2005;12:175–184. doi: 10.1038/sj.cgt.7700777. [DOI] [PubMed] [Google Scholar]
- 37.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao M, Yang M, Ma H, Li X, Tan X, Li S, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Nat Acad Sci USA. 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, et al. Efficacy of a genetically-modified Salmonella typhimurium against metastatic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–1878. [PubMed] [Google Scholar]
- 41.Mengesha A, Dubois L, Lambin P, Landuyt W, Chiu RK, Wouters BG, et al. Development of a flexible and potent hypoxia-inducible promoter for tumor-targeted gene expression in attenuated Salmonella. Cancer Biol Ther. 2006;5:1120–1128. doi: 10.4161/cbt.5.9.2951. [DOI] [PubMed] [Google Scholar]
- 42.Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, et al. Targeted therapy of spinal cord glioma with a genetically-modified Salmonella typhimurium. Cell Proliferation. 2010;43:41–48. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhymurium. J Cell Biochem. 2009;106:992–998. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camilli A, Tilney LG, Portnoy DA. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman J, Wallecha A, Maciag PC, Riviera S, Sahabi V, Paterson Y. The use of Listeria monocytogenes as an active immunotherapy for the treatment of cancer. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 46.Paterson Y, Maciag PC. Listeria-based vaccines for cancer treatment. Curr Opin Mol Ther. 2005;7:454–460. [PubMed] [Google Scholar]
- 47.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 48.Seavey MM, Pan ZK, Maciag PC, Wallecha A, Rivera S, Paterson Y, et al. A Novel Human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8+ T cell responses and impact the growth and spread of Her-2/neu+ Breast Tumors. Clin Cancer Res. 2009;15:924–932. doi: 10.1158/1078-0432.CCR-08-2283. [DOI] [PubMed] [Google Scholar]
- 49.Singh R, Dominiecki ME, Jaffee EM, Paterson Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J Immunol. 2005;175:3663–3673. doi: 10.4049/jimmunol.175.6.3663. [DOI] [PubMed] [Google Scholar]
- 50.Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y. Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res. 2008;68:8066–8075. doi: 10.1158/0008-5472.CAN-08-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikonomidis G, Paterson Y, Kos FJ, Portnoy DA. Delivery of a viral antigen to the class I processing and presentation pathway by Listeria monocytogenes. J Exp Med. 1994;180:2209–2218. doi: 10.1084/jem.180.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shahabi V, Reyes-Reyes M, Wallecha A, Rivera S, Paterson Y, Maciag P. Development of a Listeria monocytogenes based vaccine against prostate cancer. Cancer Immunol Immunother. 2008;57:1301–1310. doi: 10.1007/s00262-008-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallecha A, Maciag PC, Rivera S, Paterson Y, Shahabi V. Construction and characterization of an attenuated Listeria monocytogenes cancer immunotherapeutic for clinical use. Clin Vaccine Immunol. 2008;16:96–103. doi: 10.1128/CVI.00274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang B, Zhao J, Shen S, Li H, He KL, Shen GX, et al. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. 2007;67:4346–4352. doi: 10.1158/0008-5472.CAN-06-4067. [DOI] [PubMed] [Google Scholar]
- 55.Angelakopoulos H, Loock K, Sisul DM, Jensen ER, Miller JF, Hohmann EL. Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: a dose escalation study of oral inoculation. Infect Immun. 2002;70:3592–3601. doi: 10.1128/IAI.70.7.3592-3601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 57.Cheadle EF, Jackson AM. Bugs as drugs for cancer. Immunology. 2002;107:10–19. doi: 10.1046/j.1365-2567.2002.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–1694. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 59.Kavoussi LR, Brown EJ, Ritchey JK, Ratliff TL. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990;85:62–67. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. 1993;150:1018–1023. doi: 10.1016/s0022-5347(17)35678-1. [DOI] [PubMed] [Google Scholar]
- 61.Brandau S, Riemensberger J, Jacobsen M, Kemp D, Zhao W, Zhao X, et al. NK cells are essential for effective BCG immunotherapy. Int J Cancer. 2001;92:697–702. doi: 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 62.Suttmann H, Riemensberger J, Bentien G, Schmaltz D, Stöckle M, Jocham D, et al. Neutrophil granulocytes are required for effective Bacillus Calmette-Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res. 2006;66:8250–8257. doi: 10.1158/0008-5472.CAN-06-1416. [DOI] [PubMed] [Google Scholar]
- 63.Suttmann H, Jacobsen M, Reiss K, Jocham D, Bohle A, Brandau S. Mechanisms of bacillus Calmette-Guerin mediated natural killer cell activation. J Urol. 2004;172:1490–1495. doi: 10.1097/01.ju.0000131944.52354.63. [DOI] [PubMed] [Google Scholar]
- 64.Shintani Y, Sawada Y, Inagaki T, Kohjimoto Y, Uekado Y, Shinka T. Intravesical instillation therapy with bacillus Calmette-Guerin for superficial bladder cancer: study of the mechanism of bacillus Calmette-Guerin immunotherapy. Int J Urol. 2007;14:140–146. doi: 10.1111/j.1442-2042.2007.01696.x. [DOI] [PubMed] [Google Scholar]
- 65.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 66.Ludwig AT, Moore JM, Luo Y, Chen X, Saltsgaver NA, O'Donnell MA, et al. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette-Guerin-induced antitumor activity. Cancer Res. 2004;64:3386–3390. doi: 10.1158/0008-5472.CAN-04-0374. [DOI] [PubMed] [Google Scholar]
- 67.Belldegrun AS, Franklin JR, O'Donnell MA, Gomella LG, Klein E, Neri R, et al. Superficial bladder cancer: the role of interferon-alpha. J Urol. 1998;159:1793–1801. doi: 10.1016/S0022-5347(01)63160-4. [DOI] [PubMed] [Google Scholar]
- 68.Kresowik TP, Griffith TS. Bacillus Calmete-Guerin (BCG) for urothelial carcinoma of the bladder. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 69.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–2029. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 70.Walenkamp AME, Boer IG, Bestebroer J, Rozeveld D, Timmer-Bosscha H, Hemrika W, et al. Staphylococcal superantigen-like 10 inhibits CXCL12-induced human tumor cell migration. Neoplasia. 2009;11:333–344. doi: 10.1593/neo.81508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walenkamp AME. Bacterial proteins against metastasis. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 72.Lind DS. Arginine and cancer. J Nutr. 2004;134:2837–2841. doi: 10.1093/jn/134.10.2837S. [DOI] [PubMed] [Google Scholar]
- 73.Barile MF, Leventhal BG. Possible mechanism for Mycoplasma inhibition of lymphocyte transformation induced by phytohaemagglutinin. Nature. 1968;219:750–752. doi: 10.1038/219751a0. [DOI] [PubMed] [Google Scholar]
- 74.Feun L, Kuo MT, You M, Wangpaichitr M, Savaraj N. Arginine deiminase and cancer therapy. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 75.Das K, Butler GH, Kwiatkowski V, Clark AD, Jr, Yadav P, Arnold E. Crystal structures of arginine deaminase with covalent reaction intermediates: implications for catalytic mechanism. Structure. 2004;12:657–667. doi: 10.1016/j.str.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 76.Kundu M, Thomas J, Fialho AM, Kwan JM, Moreira L, Mahfouz M, et al. The anticancer activity of the N-terminal CARD-like domain of arginine deiminase (ADI) from Pseudomonas aeruginosa. Lett Drug Des Dicov. 2009:6. [Google Scholar]
- 77.Holtsberg FW, Ensor CM, Steiner MR, Bomalaski JS, Clark MA. Poly(ethylene glycol) (PEG) conjugated arginine deiminase: effects of PEG formulations on its pharmacological properties. J Control Release. 2002;80:259–271. doi: 10.1016/s0168-3659(02)00042-1. [DOI] [PubMed] [Google Scholar]
- 78.Takaku H, Misawa S, Hayashi H, Miyazaki K. Chemical modification by polyethylene glycol of the anti-tumor enzyme arginine deiminase from Mycoplasma arginini. Jpn J Cancer Res. 1993;84:1195–1200. doi: 10.1111/j.1349-7006.1993.tb02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, et al. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100:826–833. doi: 10.1002/cncr.20057. [DOI] [PubMed] [Google Scholar]
- 80.Curley SA, Bomalaski JS, Ensor CM, Holtsberg FW, Clark MA. Regression of hepatocellular cancer in a patient treated with arginine deiminase. Hepatogastroenterology. 2003;50:1214–1216. [PubMed] [Google Scholar]
- 81.Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De Rosa V, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004;22:1815–1822. doi: 10.1200/JCO.2004.11.120. [DOI] [PubMed] [Google Scholar]
- 82.Delman K, Brown T, Thomas M, Ensor CM, Holtsberg FW, Bomalaski JS, et al. PhaseI/II trial of pegylated arginine deiminae (ADI-PEG20) in unresectable hepatocellular carcinoma. Proc Am Soc Clin Oncol. 2005;23:342. [Google Scholar]
- 83.Feun L, Savaraj N, Wu C, You M, Wangpaichitr M, Marini A, et al. Clinical and pharmacologic sudy of ADI-PEG in hepatocellular carcinoma. Proceedings of the American Society of Clinical Oncology. 2008:15584. [Google Scholar]
- 84.Shen LJ, Lin WC, Beloussow K, Shen WC. Resistance to the anti-proliferative activity of recombinant arginine deiminase in cell culture correlates with the endogenous enzyme, argininosuccinate synthetase. Cancer Lett. 2003;191:165–170. doi: 10.1016/s030-43835(02)00693-6. [DOI] [PubMed] [Google Scholar]
- 85.Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23:7660–7668. doi: 10.1200/JCO.2005.02.0933. [DOI] [PubMed] [Google Scholar]
- 86.Feun L, Savaraj N, Marini A, Wu C, Robles C, Herrera C, et al. Phase 2 study of pegylated arginine deiminase (ADI-PEG20), a novel targeted therapy for melanoma. Proc Am Soc Clin Oncol. 2005;23:236. [Google Scholar]
- 87.Wolf P, Elsasser-Beile U. Pseudomonas Exotoxin A based immunotoxins for targeted cancer therapy. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 88.Frankel AE, Kreitman RJ, Sausville EA. Targeted toxins. Clin Cancer Res. 2000;6:326–334. [PubMed] [Google Scholar]
- 89.Michl P, Gress TM. Bacteria and bacterial toxins as therapeutic agents for solid tumors. Curr Cancer Drug Targets. 2004;4:689–702. doi: 10.2174/1568009043332727. [DOI] [PubMed] [Google Scholar]
- 90.Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 91.Kreitman RJ, Squires DR, Stetler-Stevenson M, Noel P, FitzGerald DJ, Wilson WH, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–6729. doi: 10.1200/JCO.2005.11.437. [DOI] [PubMed] [Google Scholar]
- 92.Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Giardina S, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 93.Kreitman RJ, Wilson WH, Robbins D, Margulies I, Stetler-Stevenson M, Waldmann TA, et al. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood. 1999;94:3340–3348. [PubMed] [Google Scholar]
- 93.Biggers K, Scheinfeld N. VB4-845, a conjugated recombinant antibody and immunotoxin for head and neck cancer and bladder cancer. Curr Opin Mol Ther. 2008;10:176–186. [PubMed] [Google Scholar]
- 94.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 95.Weldon JE, Xiang L, Chertov O, Margulies I, Kreitman RJ, FitzGerald DJ, et al. A protease-resistant immunotoxin against CD22 with greatly increased activity against CLL and diminished animal toxicity. Blood. 2009;113:3792–3800. doi: 10.1182/blood-2008-08-173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsutsumi Y, Onda M, Nagata S, Lee B, Kreitman RJ, Pastan I. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc Natl Acad Sci USA. 2000;97:8548–8553. doi: 10.1073/pnas.140210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lam KS. New aspects of natural products in drug discovery. Trends Microbiol. 2007;15:279–289. doi: 10.1016/j.tim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Jiang Y, Kimchi ET, Staveley-O'Carroll KF, Cheng H, Ajani JA. Assessment of K-ras mutation: a step toward personalized medicine for patients with colorectal cancer. Cancer. 2009;115:3609–3617. doi: 10.1002/cncr.24434. [DOI] [PubMed] [Google Scholar]
- 99.Pan J, Yeung S-CJ. Farnesyltransferase inhibitors of microbial origins in cancer. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 100.Ito T, Kawata S, Tamura S, Igura T, Nagase T, Miyagawa JI, et al. Suppression of human pancreatic cancer growth in BALB/c nude mice by manumycin, a farnesyl:protein transferase inhibitor. Jpn J Cancer Res. 1996;87:113–116. doi: 10.1111/j.1349-7006.1996.tb03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kainuma O, Asano T, Hasegawa M, Kenmochi T, Nakagohri T, Tokoro Y, et al. Inhibition of growth and invasive activity of human pancreatic cancer cells by a farnesyltransferase inhibitor, manumycin. Pancreas. 1997;15:379–383. doi: 10.1097/00006676-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 102.Yeung SC, Xu G, Pan J, Christgen M, Bamiagis A. Manumycin enhances the cytotoxic effect of paclitaxel on anaplastic thyroid carcinoma cells. Cancer Res. 2000;60:650–656. [PubMed] [Google Scholar]
- 103.She M, Pan I, Sun L, Yeung SC. Enhancement of manumycin A-induced apoptosis by methoxyamine in myeloid leukemia cells. Leukemia. 2005;19:595–602. doi: 10.1038/sj.leu.2403691. [DOI] [PubMed] [Google Scholar]
- 104.Frassanito MA, Cusmai A, Piccoli C, Dammacco F. Manumycin inhibits farnesyltransferase and induces apoptosis of drug-resistant interleukin 6-producing myeloma cells. Br J Haematol. 2002;118:157–165. doi: 10.1046/j.1365-2141.2002.03559.x. [DOI] [PubMed] [Google Scholar]
- 105.Zhou JM, Zhu XF, Pan QC, Liao DF, Li ZM, Liu ZC. Manumycin inhibits cell proliferation and the Ras signal transduction pathway in human hepatocellular carcinoma cells. Int J Mol Med. 2003;11:767–771. [PubMed] [Google Scholar]
- 106.Zhou JM, Zhu XF, Pan QC, Liao DF, Li ZM, Liu ZC. Manumycin induces apoptosis in human hepatocellular carcinoma HepG2 cells. Int J Mol Med. 2003;12:955–959. [PubMed] [Google Scholar]
- 107.Pan J, Xu G, Yeung SC. Cytochrome c release is upstream to activation of caspase-9, caspase-8 and caspase-3 in the enhanced apoptosis of anaplastic thyroid cancer cells induced by manumycin and paclitaxel. J Clin Endocrinol Metab. 2001;86:4731–4740. doi: 10.1210/jcem.86.10.7860. [DOI] [PubMed] [Google Scholar]
- 108.Ishii T, Hayashi K, Hida T, Yamamoto Y, Nozaki Y. TAN-1813, a novel Ras-farnesyltransferase inhibitor produced by Phoma sp. taxonomy, fermentation, isolation and biological activities in vitro and in vivo. J Antibiot. 2000;53:765–778. doi: 10.7164/antibiotics.53.765. [DOI] [PubMed] [Google Scholar]
- 109.Williamson NR, Chawrai S, Leeper FJ, Salmond GPC. Prodiginines and their potential utility as proapototic anticancer agents. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]
- 110.Manderville RA. Synthesis, proton-affinity and anti-cancer properties of the prodigiosin-group natural products. Curr Med Chem Anti-Canc Agents. 2001;1:195–218. doi: 10.2174/1568011013354688. [DOI] [PubMed] [Google Scholar]
- 111.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 113.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 114.Fumoleau P, Coudert B, Isambert N, Ferrant E. Novel tubulin-targeting agents: anticancer activity and pharmacological profile of epothilones and related analogues. Annals of Oncolology. 2007;18:9–15. doi: 10.1093/annonc/mdm173. [DOI] [PubMed] [Google Scholar]
- 115.Nettles JH, Li H, Cornett B, Krahn JM, Snyder JP, Downing KH. The binding mode of epothilone A on alpha, beta-tubulin by electron crystallography. Science. 2004;305:866–869. doi: 10.1126/science.1099190. [DOI] [PubMed] [Google Scholar]
- 116.Cheng KL, Bradley T, Daniel RB. Novel microtubule-targeting agents—the epothilones. Biologics: Targets & Therapy. 2008;2:789–811. doi: 10.2147/btt.s3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, et al. Epothilones, a new class of microtubule-stabilizing agents with a Taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 118.Lee FY, Borzilleri R, Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 119.Lee FY, Camuso A, Castenada S, Fager K, Flefleh C, Inigo I, et al. Preclinical studies of ixabepilone (BMS-2475550) demonstrate optimal antitumor activity against both chemotherapy-sensitive and -resistant tumor types. Proc Amer Assoc Cancer Res. 2006:47. [Google Scholar]
- 120.Hoffman J, Buchmann B, Schwedde W, Skuballa W, Lichtner RB, Pietsch H, et al. New synthetic epothilone derivative ZK-EPO inhibits breast cancer metastasis. Proc Amer Assoc Cancer Res. 2005:46. [Google Scholar]
- 121.Zaborina O, Dhiman N, Chen ML, Kostal J, Holder IA, Chakrabarty AM. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiology. 2000;146:2521–2530. doi: 10.1099/00221287-146-10-2521. [DOI] [PubMed] [Google Scholar]
- 122.Yamada T, Goto M, Punj V, Zaborina O, Chen ML, Kimabara K, et al. Bacterial redox protein azurin, tumor suppressor protein p53 and regression of cancer. Proc Natl Acad Sci USA. 2002;99:14098–14103. doi: 10.1073/pnas.222539699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yamada T, Hiraoka Y, Ikehata M, Kimbara K, Avner BS, Das Gupta TK, et al. Apoptosis or growth arrest: modulation of tumor suppressor p53's specificity by bacterial redox protein azurin. Proc Natl Acad Sci USA. 2004;101:4770–4775. doi: 10.1073/pnas.0400899101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Punj V, Bhattacharyya S, Saint-Dic D, Vasu C, Cunningham EA, Graves J, et al. Bacterial Cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene. 2004;23:2367–2378. doi: 10.1038/sj.onc.1207376. [DOI] [PubMed] [Google Scholar]
- 125.Hong CS, Yamada T, Hashimoto W, Fialho AM, Das Gupta TK, Chakrabarty AM. Disrupting the entry barrier and attacking brain tumors: the role of the Neisseria H.8 epitope and the Laz protein. Cell Cycle. 2006;5:1633–1641. doi: 10.4161/cc.5.15.2991. [DOI] [PubMed] [Google Scholar]
- 126.Kwan JM, Fialho AM, Kundu M, Thomas J, Hong CS, Das Gupta TK, et al. Bacterial proteins as potential drugs in the treatment of leukemia. Leuk Res. 2009;33:1392–1399. doi: 10.1016/j.leukres.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 127.Apiyo D, Wittung-Stafshede P. Unique complex between bacterial azurin and tumor-suppressor protein p53. Biochem Biophys Res Commun. 2005;332:965–968. doi: 10.1016/j.bbrc.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 128.Chaudhari A, Mahfouz M, Fialho AM, Yamada T, Granja AT, Zhu Y, et al. Cupredoxin-cancer interrelationship: azurin binding with EphB2, interference in EphB2 tyrosine phosphorylation, and inhibition of cancer growth. Biochemistry. 2007;46:1799–1810. doi: 10.1021/bi061661x. [DOI] [PubMed] [Google Scholar]
- 129.Yamada T, Goto M, Punj V, Zaborina O, Kimbara K, Das Gupta TK, et al. The bacterial redox protein azurin induces apoptosis in J774 macrophages through complex formation and stabilization of the tumor suppressor protein p53. Infect Immun. 2002;70:7054–7062. doi: 10.1128/IAI.70.12.7054-7062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Punj V, Das Gupta TK, Chakrabarty AM. Bacterial cupredoxin azurin and its interactions with the tumor suppressor protein p53. Biochem Biophys Res Commun. 2003;312:109–114. doi: 10.1016/j.bbrc.2003.09.217. [DOI] [PubMed] [Google Scholar]
- 131.Yamada T, Hiraoka Y, Das Gupta TK, Chakrabarty AM. Regulation of mammalian cell growth and death by bacterial redox proteins. Relevance to ecology and cancer therapy. Cell Cycle. 2004;3:752–755. [PubMed] [Google Scholar]
- 132.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 133.Clore GM, Omichinski JG, Sakaguchi K, Zambrano N, Sakamoto H, Appella E, Gronenborn AM. High-resolution structure of the oligomerization domain of p53 by multidimensional NMR. Science. 1995;265:386–391. doi: 10.1126/science.8023159. [DOI] [PubMed] [Google Scholar]
- 134.Jeffrey PD, Gorina S, Pavletich NP. Crystal structure of the tetramerization domain of the p53 tumor suppressor at 1.7 angstroms. Science. 1995;267:1498–1502. doi: 10.1126/science.7878469. [DOI] [PubMed] [Google Scholar]
- 135.Klein C, Planker E, Diercks T, Kessler H, Kunkele KP, Lang K, et al. NMR spectroscopy reveals the solution dimerization interface of p53 core domains bound to their consensus DNA. J Biol Chem. 2001;276:49020–49027. doi: 10.1074/jbc.M107516200. [DOI] [PubMed] [Google Scholar]
- 136.Taranta M, Bizzarri AR, Cannistraro S. Probing the interaction between p53 and the bacterial protein azurin by single molecule force spectroscopy. J Mol Recognit. 2008;21:63–70. doi: 10.1002/jmr.869. [DOI] [PubMed] [Google Scholar]
- 137.Wilson MT, Greenwood C, Brunori M, Antonini E. Electron transfer between axurin and cytochrome c-551. Biochem J. 1975;145:449–457. doi: 10.1042/bj1450449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taranta M, Bizzarri AR, Cannistraro S. Modeling the interaction between the N-terminal domain of the tumor-supressor p53 and azurin. J Mol Recognit. 2009;22:215–222. doi: 10.1002/jmr.934. [DOI] [PubMed] [Google Scholar]
- 139.Chen R, Weng Z. Docking unbound proteins using shape complementary, desolvation and electrostatics. Proteins: Struct Funct Genet. 2002;47:281–294. doi: 10.1002/prot.10092. [DOI] [PubMed] [Google Scholar]
- 140.De Grandis V, Bizzarri AR, Cannistraro S. Docking study and free energy simulation of the complex between p53 DNA-binding domain and azurin. J Mol Recognit. 2007;20:215–226. doi: 10.1002/jmr.840. [DOI] [PubMed] [Google Scholar]
- 141.Nooren IMA, Thorton JM. Structural characterization and functional significance of transient protein-protein interactions. J Mol Biol. 2003;325:991–1018. doi: 10.1016/s0022-2836(02)01281-0. [DOI] [PubMed] [Google Scholar]
- 142.Mahfouz M, Hashimoto W, Das Gupta TK, Chakrabarty AM. Bacterial proteins and CpG-rich extrachromosomal DNA in potential cancer therapy. Plasmid. 2007;57:4–17. doi: 10.1016/j.plasmid.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 143.Yamada T, Fialho AM, Punj V, Bratescu L, Das Gupta TK, Chakrabarty AM. Internalization of bacterial redox protein azurin in mammalian cells: entry domain and specificity. Cell Microbiol. 2005;7:1418–1431. doi: 10.1111/j.1462-5822.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 144.Punj V, Chakrabarty AM. Redox proteins in mammalian cell death: an evolutionary conserved function in mitochondria and prokaryotes. Cell Microbiol. 2003;5:225–231. doi: 10.1046/j.1462-5822.2003.00269.x. [DOI] [PubMed] [Google Scholar]
- 145.Metha RR, Taylor BN, Yamada T, Beattie CW, Das Gupta TK, Chakrabarty AM. US Patent Application Serial No. 11/488, 693: PCT/US06/27940. Compositions and methods to control angiogenesis with cupredoxins. 2006
- 146.Taylor BN, Metha RR, Yamada T, Lekmine F, Christov K, Chakrabarty AM, et al. Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res. 2009;69:537–546. doi: 10.1158/0008-5472.CAN-08-2932. [DOI] [PubMed] [Google Scholar]
- 147.Das Gupta TK, Chakrabarty AM. U.S. Patent US2008/013947. Compositions and methods to prevent cancer with cupredoxins. 2008
- 148.Fialho AM, Stevens FJ, Das Gupta TK, Chakrabarty AM. Beyond host-pathogen interactions: microbial defense strategy in the host environment. Curr Opin Biotechnol. 2007;18:279–286. doi: 10.1016/j.copbio.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 149.Hey T, Fielder E, Rudolph R, Fiedler M. Artificial, nonantibody binding proteins for pharmaceutical and industrial applications. Trens Biotechnol. 2005;23:514–522. doi: 10.1016/j.tibtech.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 150.Fialho AM, Chakrabarty AM. Promiscuous drugs from pathogenic bacteria: rational versus intelligent drug design. In: Fialho AM, Chakrabarty AM, editors. Emerging Cancer Therapy: Microbial approaches and Biotechnological Tools. Nutley N.J.: John Wiley; 2009. In press. [Google Scholar]