Abstract
Retrieval is often subdivided into recollection and familiarity. Memory-strength and reaction time (RT) differ for each, complicating fMRI studies of these processes. Recollection leads to greater activity in the hippocampus and default network (DN). Increased DN activity with recollection is thought to reflect self-referential processes, but prior studies have not accounted for varying RT, which modulates DN activity and is consistently faster for recollection than familiarity. This study examined the influence of RT and memory-strength on recollection and familiarity activity. The results show the hippocampus functionally dissociated from DN during retrieval. DN was generally influenced by RT and signal was suppressed when subjects were task-engaged in recollection or familiarity; suppression was greater for slower trials of either type. The hippocampus showed a positive deflection of fMRI activity only for recollection trials; activation was greater for slower recollection trials, but RT did not influence hippocampal activity during familiarity trials.
Keywords: memory recall, medial temporal lobe, precuneus, parietal, fMRI
Introduction
In memory studies, retrieved episodic memories are often classified as ‘remembered’ or ‘known’ (Mandler, 1980; Rajaram, 1996). ‘Remember’ is a judgment of recollection, defined as not only recalling an event, but also recalling specific details about the encoding event. ‘Know’ is a judgment that an item is familiar, but without the retrieval of specific details about the encoding event. Many studies of recollection and familiarity have focused on the medial temporal lobe (MTL), specifically the hippocampus, since its involvement in memory encoding, consolidation, and retrieval is well known (Bayley & Squire, 2005; Cohen & Squire, 1980; Scoville & Milner, 1957).
In functional brain imaging studies of memory, hippocampal activity has been associated with memory encoding, consolidation, and retrieval, but during rest, activity in the hippocampus is generally correlated with a set of regions commonly identified as the default network (DN). The DN is a set of brain regions less active during task performance than during rest or fixation (Raichle, et al., 2001). It includes a distributed network of midline and lateral brain regions (for review see Buckner, Andrews-Hanna, & Schacter, 2008). Elevated DN activity during fixation suggests that, in the presence of a task, DN would show a negative deflection from baseline with greater deflection for longer time spent ‘on-task.’ Indeed, prior studies have shown an association between reaction time (RT) and DN suppression (McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003; Park, Polk, 2010, Hebrank, & Jenkins; Weissman, Roberts, Visscher, & Woldorff, 2006).
Brain regions with greater activity during confident memory retrieval have been termed the ‘retrieval-network.’ In a meta-analysis of memory tasks and the parietal lobe, Wagner, Shannon, Kahn, & Buckner (2005) report greater blood oxygen level dependent (BOLD) signal in parietal-lobe subregions across multiple memory experiments with comparisons of recollection/familiarity, correct rejections, false alarms, and misses (Dobbins, Rice, Wagner, & Schacter, 2003; Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000; Henson, Rugg, Shallice, Josephs, & Dolan, 1999; Wheeler & Buckner, 2004). Whether the comparison is old versus new judgments, hits versus correct rejections, hits versus misses, source hits versus source misses, or any other “stronger” vs. “weaker” memory comparison, a relative “positive activation” is seen in precuneus, lateral parietal cortex, posterior cingulate, retrosplenial cortex, and intraparietal sulcus. These regions, most active for stronger memories, overlap with the posterior portion of the DN (Cavanna, 2007). While not all retrieval-network regions are part of the DN, a subset appears to be modulated by the simple performance of any of a wide variety of tasks.
Since the retrieval-network shows increased activity during confident memory retrieval, it is likely that some activity attributed to retrieval might, in fact, represent a broader modulation of DN activity; hippocampal activity, consistent with findings from hippocampal lesion literature, might be more specific to memory tasks. Imaging studies show increased hippocampal activity during episodic recollection (Henson, 2005; Schacter, Alpert, Savage, Rauch, & Albert, 1996) and for recollection relative to familiarity (Cabeza, Rao, Wagner, Mayer, & Schacter, 2001; Cansino, Maquet, Dolan, & Rugg, 2002; Eldridge, et al., 2000; Schacter, et al., 1996). Recollection and familiarity differentially modulate both the hippocampus and posterior DN (Rugg & Henson, 2002), but only recently has evidence existed for task-based dissociations amongst cortical DN subcomponents (Kim, 2010) or for general dissociations between cortical DN subcomponents and the hippocampus (Israel, Seibert, Black, & Brewer, 2010). A similar dissociation between DN and hippocampus for recollection and familiarity judgments might be expected, given RT differences (modulating DN activity) and memory differences (modulating hippocampal activity) for these judgments.
Many studies have noted that confident memory retrieval is performed faster than judgments of familiarity or retrieval with lower confidence (Dewhurst, Holmes, Brandt, & Dean, 2006; Rotello & Zeng, 2008; Wixted, 2009; Wixted & Stretch, 2004). Increased RT for familiarity relative to recollection could be due to amount of search needed to bring up a memory, post-retrieval processing required for memory judgment, or other components of retrieval. RT is a confounding component uncontrolled in most studies that use the ‘remember/know’ paradigm. In fact, it is plausible that increased activity commonly observed in the retrieval-network could simply reflect decreased suppression of DN for tasks performed more quickly and easily. Thus, an analysis of ‘remember/know’ judgments constrained by RT may help disentangle DN from retrieval-related activation.
This study examined how RT influences BOLD activity in the hippocampus and DN during correct ‘remember/know’ responses. Since the hippocampus is functionally connected with other DN regions at rest, the goals of this study were to determine if hippocampus modulates with DN during tasks of memory retrieval and if RT may account for the common DN and hippocampal activations identified in memory studies that use the ‘remember/know’ paradigm. We expect that if DN and hippocampus are part of the same network during memory retrieval, RT should similarly influence their activity. Conversely, if DN and hippocampus comprise separate networks in the brain that are dissociable during memory retrieval, slower RT would yield differential and possibly divergent hippocampal activity from DN activity during recollection, and possibly familiarity.
Method
Twelve healthy right-handed subjects were recruited from the University of California, San Diego (UCSD) community and surrounding area (mean age = 23 ± 3 years, 2 male). Subjects received $40 for their participation and gave informed consent approved by the Institutional Review Board of UCSD. Stimuli were 384 color images of common, namable objects (Bakker, Kirwan, Miller, & Stark, 2008), separated into 256 shown in a pre-scan test and an additional 128 novel images used during the scan.
Prior to scanning, subjects visually studied 256 nameable objects, each for 3 seconds, and made a living/non-living judgment. During scanning, subjects saw all 256 studied objects and 128 novel objects and were asked to judge each with ‘remember,’ ‘know,’ or ‘novel.’ Subjects were instructed to respond “remember” if they saw the image during the study task and could recall specific details about its presentation, “know” if the image was familiar but they did not recall specific details about seeing it before, or “new” if the image was not presented during the study session (Yonelinas, 2001). Subjects responded by pressing one of three buttons on a button box held in their right hand. Each image was presented for 3 seconds. Trials were jittered with 0, 1.5, 3, or 4.5 seconds of fixation-cross baseline to optimize the study design (Dale, 1999). Each subject underwent a single session of four 530-second runs. Only correctly identified ‘remember’ and ‘know’ trials (hits) were used in further analysis. ‘Novel’ and ‘miss’ trials were analyzed, but not further explored with attention to reaction time.
Imaging was done in a 3T GE scanner at the Keck Center for Functional MRI at the University of California, San Diego. Functional images were acquired using a gradient echo echo-planar, T2*-weighted pulse sequence (repetition time = 1.5 s, one shot per repetition, echo time = 30, flip angle = 90°, bandwidth = 31.25 MHz). Twenty-two slices covering the brain were acquired perpendicular to the long axis of the hippocampus with 3.4 × 3.4 × 7 mm voxels, allowing greater summation of activity along the hippocampal axial plane (Brewer & Moghekar, 2002). A T1-weighted high resolution (1 × 1 × 1 mm), three-dimensional fast spoiled gradient-recalled anatomical dataset was collected.
Data from each run were field-map corrected to account for inhomogeneities in the magnetic field. Using AFNI (Cox, 1996), slices were reconstructed to a 3-dimensional volume, temporally aligned, co-registered, and motion corrected. A general linear model (GLM) was constructed using multiple regression analysis and included six motion regressors and regressors for ‘remember’ and ‘know,’ hit and miss responses and ‘novel’ correct rejections and false alarms. A second GLM was constructed to examine ‘remember’ and ‘know’ hit trials longer and shorter than 1500 msec. A third GLM was constructed with one column for all task types to examine task versus no task activity (used to mask the ‘remember minus know’ activation map in Figure 2). Areas with negative deflection during task engagement, as revealed by the task versus no task GLM, were defined functionally as the default network (Raichle, et al., 2001). Standard landmarks were defined manually, and the anatomical and functional scans were transformed into Talairach space (Talairach & Tournoux, 1998). For each condition, a hemodynamic-response function was estimated for 15 seconds following the onset of the stimulus using signal deconvolution. Amplitude-modulated regression (http://afni.nimh.nih.gov/pub/dist/doc/misc/Decon/AMregression.pdf) was used to identify active voxels whose activation level also depended on RT for the ‘remember’ and ‘know’ hits. Two regressors were constructed to find the mean BOLD response and areas where BOLD response correlated with RT. Again, a hemodynamic-response function was estimated for 15 seconds following the onset of the stimulus using signal deconvolution. Cluster maps were displayed using SUMA (Saad, Reynolds, Argall, Japee, & Cox, 2004) on the pial surface of the Talairach and Tournoux N27 average brain from Freesurfer (http://surfer.nmr.mgh.harvard.edu). In order to improve alignment of MTL structures, the region of interest large deformation diffeomorphic metric mapping alignment technique (Miller, Beg, Ceritoglu, & Stark, 2005) was used.
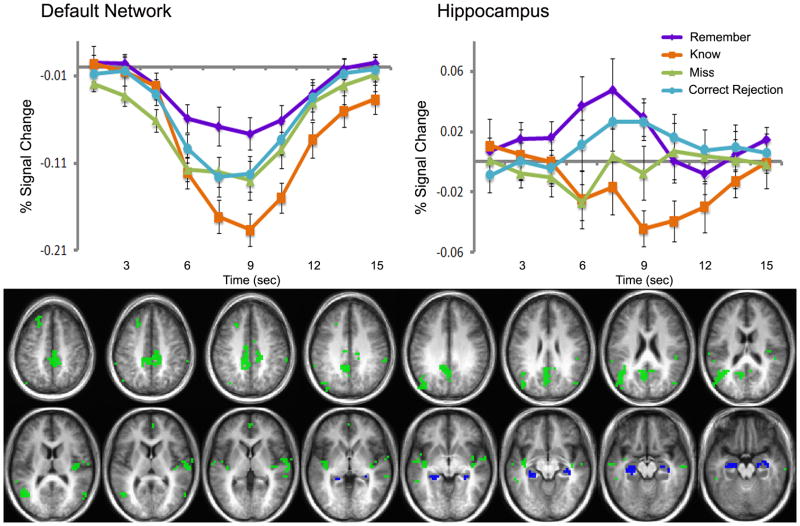
Figure 2.
Default network and hippocampus activity in the ‘remember minus know’ contrast (p<.01). Cluster map (below) shows regions of overlap between the default network (defined as regions of deactivation in task minus no task contrast, green) and hippocampus (defined anatomically, blue) and the ‘remember minus know’ contrast (p<.01). Clusters overlaid on an average anatomical brain of all 12 study participants. Impulse response curves in the default network (top left) and hippocampus (top right) for ‘remember’ (purple), ‘know’ (orange), ‘misses’ (green), and ‘correct rejection’ (light blue) judgment trials. Y-axis is percent signal change; X-axis is time (seconds) after the onset of the stimulus.
After individual deconvolution analysis, single-subject parameter estimates were entered into group level analyses. Voxel-wise t-tests (two-tailed) were performed to compare average area under the curve between conditions and between the presence and absence of a task. Clusters were defined with a connectivity of 4mm between voxel-centers and included at least 5 voxels for a whole brain significance of p<.05 and a voxel-wise significance of p<.001 when corrected for multiple comparisons (using alpha probability simulations calculated with the AFNI plugin, AlphaSim). Clusters were extracted at p<.01 (two-tailed, corrected for multiple comparisons) and were displayed as a statistical map overlaid onto an average structural image. The hemodynamic response function, estimated in the signal deconvolution analysis, was extracted for each subject in each cluster of interest and then averaged. Reaction time histograms were constructed using the sum of all hit/correct trials for all subjects in each 250 msec bin for remember, know, and novel responses.
Results
In this study of memory retrieval, more BOLD activation was observed in DN and hippocampus during ‘remember’ hits than during ‘know’ hits. However, activity in these regions was dissociated; hippocampus showed a response-curve with a positive deflection from baseline for ‘remember,’ while DN showed negatively-deflecting response-curves that dipped more negatively for ‘know’ than for ‘remember.’
Regions more active for ‘Remember’ than ‘Know’
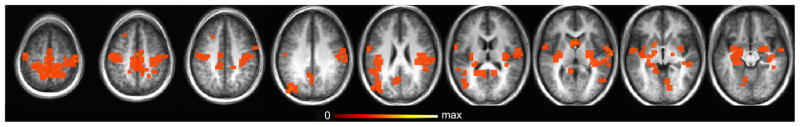
Old items were judged as ‘remember’ 60.1 ± 6.2% of the time and as ‘know’ 17.8 ± 3.2% of the time. Novel items were judged as ‘new’ (correct rejection) 84.2 ± 2.4% of the time. Brain regions showing greater BOLD activity during ‘remember’ trials than during ‘know’ trials (p<0.01, corrected for multiple comparisons) are identified in Figure 1 (Figure 1 about here). There were no voxels showing the opposite relationship. Active voxels in this contrast include typical DN regions (bilateral postcentral gyrus, medial parietal lobe, precuneus, insula, and hippocampus). In order to more closely examine the BOLD dynamics in DN and hippocampus separately, all regions of differential activity in the ‘remember minus know’ contrast were masked by the anatomically defined hippocampus and functionally defined DN for use in further analyses. Clusters of activation in hippocampus extended beyond the anatomical boundaries of the structure, so an anatomical mask of hippocampus was used to constrain the voxels included in the analysis. Activity that was modulated by the remember-know contrast and that fell within the DN (Figure 2, green) or hippocampus (Figure 2, blue) was explored for differences between ‘remember’ and ‘know’ hits and for relationships with RT.
Figure 1.
Cluster map of ‘remember minus know’ (p<.01, corrected for multiple comparisons). Clusters overlaid on an average anatomical brain of the 12 study participants.
Inspection of impulse-response functions for hits in DN and hippocampus confirm greater activity during ‘remember’ than during ‘know’ trials (Figure 2); however, the impulse-responses in these regions were in opposite directions and were differentially influenced by ‘remember’ and ‘know’ judgments. The DN showed a negative deflection of activity that was of greater amplitude during ‘know’ trials than during ‘remember’ trials (Figure 2, top left). Impulse-response curves for ‘remember’ (purple), ‘know’ (orange), ‘misses’ (green), and ‘correct rejection’ (light blue) responses were all decreased from baseline (Remember: t(11)=−2.869, p<.05; Know: t(10)=−3.676, p<.01; Misses: t(11)=−4.358, p<.01; Correct Rejection: t(11)=−3.215, p<.05). Hippocampus showed a positive deflection of activity during ‘remember’ hits and ‘novel’ correct rejections (Remember: t(11)=2.996, p<.05; Correct Rejection: t(11)=2.429, p<.05), no change in activity during ‘misses’ (t(11)=−1.161, p=.275), and a decrease in activity during ‘know’ hits (t(10)=−2.547, p<.05), (Figure 2, top right), all compared to a fixation-cross baseline. (Figure 2 about here)
RT Differences Across Trial Types
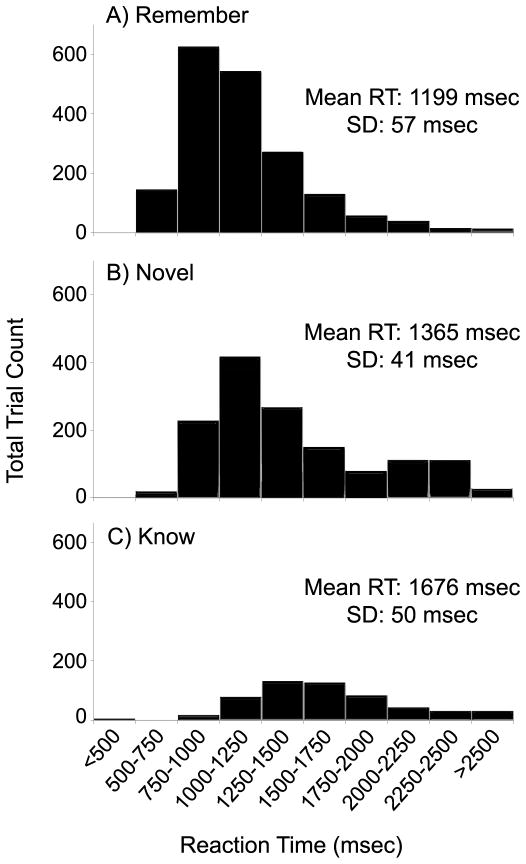
‘Remember’ and ‘know’ hits and ‘novel’ correct rejections had significantly different RTs [F(2,33) = 23.762, p<.001; R-K: t(11)= −4.981, p<.001; R-N: t(11)= −2.220, p<.05; K-N: t(11)=6.199, p<.001]. ‘Remember’ hits responses were fastest (1199±57 msec), followed by ‘novel’ correct rejections (1365±41 msec), then ‘know’ hits (1676±50 msec) (distributions displayed in Figures 3A-C). Examining these reaction time distributions together with the impulse-response curves in Figure 2, it is noted that average activity decreased with longer RT in the hippocampus and DN, suggesting a potential association between neural activity and RT in these regions. (Figure 3 about here)
Figure 3.
Reaction time distribution varies for ‘remember,’ ‘novel’ and ‘know’ trials. Reaction time distribution and mean RT for A) ‘remember,’ B) ‘novel,’ and C) ‘know’ trials. X-axis is reaction time (msec); Y-axis is number of trials in each bin, summed across all 12 subjects. Only correct ‘remember,’ ‘novel,’ and ‘know’ responses are included.
DN and Hippocampal Activity
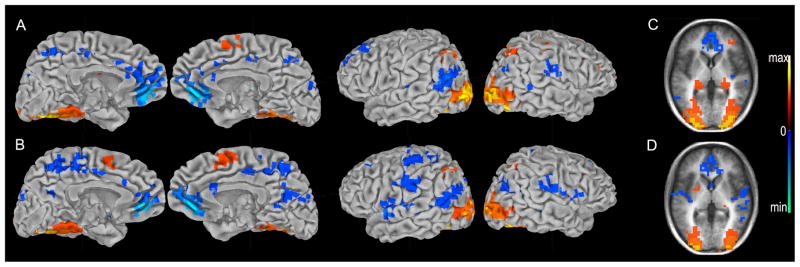
Amplitude regression analysis identified brain regions whose activity correlated with RT for ‘remember’ and ‘know’ hits (Figure 4). There were inverse correlations of both ‘remember’ and ‘know’ trials with RT in DN (p<.01, Figure 4A,B). Hippocampus, however, showed a positive correlation for ‘remember’ (p<.01, Figure 4C) and no correlation for ‘know’ (p>.01, Figure 4D). (Figure 4 about here)
Figure 4.
Regions correlated with reaction time. Map of regions positively and negatively correlated with reaction time for ‘remember’ trials (A, C) and ‘know’ trials (B, D). Cluster maps for correct ‘remember’ and ‘know’ trials are presented on the pial surface of the Talaraich and Tournoux N27 average brain (A, B), and on an average anatomical brain of the 12 study participants (C, D). (p<.01, warm colors = positive correlation, cool colors = negative correlation)
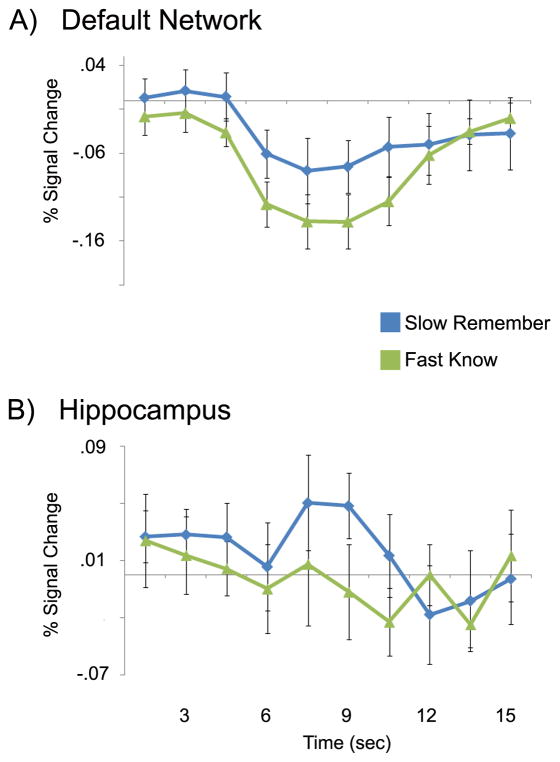
To examine the effects of RT and memory-strength, ‘remember’ and ‘know’ hits were split into those with RT slower and faster than 1500 msec. Fast ‘know’ trials were compared with slow ‘remember’ trials, allowing a near-optimal balance in trial number for the two conditions while equating memory-strength and reversing the confounding directionality of RT present in unconstrained analyses. Typically, RT and false-alarm rates are higher for ‘know’ than for ‘remember.’ In this analysis, RT was longer for slow ‘remember’ hits (trials: 259, Average RT: 1852 msec, FA rate: 1%) than for long ‘know’ hits (trials: 233, Average RT: 1224 msec, FA rate: 0%) and false-alarm rates were equated; this provides a test of whether hippocampal and DN activity remains higher for ‘remember’ than for ‘know’ despite equal memory-strength and slower RT. Despite this, activity was still greater in DN (Figure 5A) and hippocampus (Figure 5B) for slow ‘remember’ trials than fast ‘know’ trials, and hippocampus still showed a significant increase during ‘remember’ trials compared with baseline (Slow remember: t(11)=2.387, p<.05; Fast know: t(10)=−.043, p=1) while DN showed a decrease for both ‘remember’ and ‘know’ trials (Slow remember: t(11)=−2.718, p<.05; Fast know: t(10)=−5.772, p<.001). (Figure 5 about here)
Figure 5.
Reaction time does not account for all task-related differences in ‘remember’ and ‘know’ trial activity in the default-mode network and hippocampus. Impulse response curves for the A) default-mode network and B) hippocampus for ‘remember’ trials with reaction time slower than 1500 msec (‘slow remember,’ blue) and ‘know’ trials with reaction time faster than 1500 msec (‘fast know,’ green). Masks used to extract impulse response curves are the same as those in Figure 2. Y-axis is percent signal change; X-axis is time (seconds) after the onset of the stimulus. All impulse response curves are for correct trials only.
General Discussion
During episodic retrieval, more activity was elicited for ‘remember’ than for ‘know’ hits in areas commonly identified as the DN. In a meta-analysis of DN activity by Buckner et al. (2008), activation from the ‘remember-know’ contrast and hippocampal-correlated DN activation overlapped in the hippocampus and posterior DN regions (Cavanna, 2007; Vincent, et al., 2006; Wagner, et al., 2005). By examining the timecourse of this activity, the present study demonstrates that the hippocampus dissociates from DN during ‘remember’ and ‘know’ judgments and also demonstrates why these regions are co-identified using subtraction analyses. The hippocampus responds to ‘remember’ trials with a positive deflection from baseline that is of greater amplitude with slower RT, while the DN shows a negative deflection from baseline that is of greater amplitude with slower RT. The DN responds to ‘know’ trials with an even deeper negative deflection from baseline that is also of greater amplitude with slower RT, while the hippocampus shows a negative deflection from baseline during these trials that is not sensitive to RT. Though the hippocampus is considered to be part of and is typically functionally correlated with the DN, during episodic retrieval, hippocampal activity dissociates from DN activity.
The present study demonstrates that even though RT is correlated with hippocampal activity during recollection, selective hippocampal involvement during ‘remember’ trials remains even after controlling RT. This activation, unseen during ‘know’ hits, lends support to the dual process model of memory recall, where hippocampus supports recollection but not familiarity. Even in ‘remember’ trials with a slower RT and equated confidence, the hippocampus still showed a positive deflection from baseline only for ‘remember’ and no deflection for ‘know.’
Prior studies noted increased hippocampal activity for recollection-based but not for familiarity-based responses (Eldridge, et al., 2000); however, it was unknown whether these effects were due to the brain bases of the recollection/familiarity dissociation, due to differences in memory-strength, or primarily driven by differences in DN, since MTL regions are functionally linked to the DN during rest. If ‘remember’ judgments yield elevated hippocampal activity even when ‘remember’ and ‘know’ judgments are equated for memory-strength and RT, it would support selective hippocampal involvement in recollection. The present study did not additionally obtain subjects’ subjective ratings of memory-strength, given potential influences of such metamemory judgments, themselves, on hippocampal and DN activity and a desire to maintain consistency with prior imaging studies of recollection/familiarity. Nevertheless, such ratings of retrieval confidence might allow greater flexibility in examining the confound of memory-strength present in prior studies.
Hippocampal activity diverges from other DN activity during memory-related tasks and is differentially influenced by RT during recollection- and familiarity-based memory retrieval. Task-related activity differences in these regions survive correction for differences in RT and memory-strength. ‘Remember’ and ‘know’ judgments might require different amounts of search or might rely on different metamemory processes to make the judgments themselves. Despite the wealth of imaging studies on memory, these additional components of memory retrieval are poorly understood, calling for further research to determine how regional brain activity might be affected by the interactive components of memory-strength and RT.
Acknowledgments
This work was supported by NINDS 1K23 NS050305 and the General Electric Medical Foundation.
References
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319(5870):1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Squire LR. Failure to acquire new semantic knowledge in patients with large medial temporal lobe lesions. Hippocampus. 2005;15(2):273–280. doi: 10.1002/hipo.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Moghekar A. Imaging the medial temporal lobe: exploring new dimensions. Trends Cogn Sci. 2002;6(5):217–223. doi: 10.1016/s1364-6613(02)01881-8. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci U S A. 2001;98(8):4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12(10):1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cavanna AE. The precuneuos and consciousness. CNS Spectr. 2007;12(7):545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210(4466):207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst SA, Holmes SJ, Brandt KR, Dean GM. Measuring the speed of the conscious components of recognition memory: remembering is faster than knowing. Conscious Cogn. 2006;15(1):147–162. doi: 10.1016/j.concog.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41(3):318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3(11):1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Henson R. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. Q J Exp Psychol B. 2005;58(3–4):340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel SL, Seibert TM, Black ML, Brewer JB. Going their separate ways: dissociation of hippocampal and dorsolateral prefrontal activation during episodic retrieval and post-retrieval processing. J Cogn Neurosci. 2010;22(3):513–525. doi: 10.1162/jocn.2009.21198. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage. 2010;50(4):1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognising: the judgment of previous occurrence. Psuchological Review. 1980;87:252–271. [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc Natl Acad Sci U S A. 2005;102(27):9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram S. Perceptual effects on remembering: recollective processes in picture recognition memory. J Exp Psychol Learn Mem Cogn. 1996;22(2):365–377. doi: 10.1037//0278-7393.22.2.365. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Zeng M. Analysis of RT distributions in the remember-know paradigm. Psychon Bull Rev. 2008;15(4):825–832. doi: 10.3758/pbr.15.4.825. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: Parker AE, Wilding EL, Bussey T, editors. The cognitive neuroscience of memory encoding and retrieval. Psychology Press; 2002. [Google Scholar]
- Saad ZS, Reynolds RC, Argall B, Japee S, Cox RW. SUMA: an interface for sufrace-based intra- and inter-subject analysis with AFNI. Arlington, VA: 2004. [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS. Conscious recollection and the human hippocampal formation: evidence from positron emission tomography. Proc Natl Acad Sci U S A. 1996;93(1):321–325. doi: 10.1073/pnas.93.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A Co-Planar stereotaxic Atlas of the Human Brain. Thieme; Stuttgard: 1998. [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. NeuroImage. 2004;21(4):1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Remember/Know judgments in cognitive neuroscience: An illustration of the underrepresented point of view. Learn Mem. 2009;16(7):406–412. doi: 10.1101/lm.1312809. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychon Bull Rev. 2004;11(4):616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philos Trans R Soc Lond B Biol Sci. 2001;356(1413):1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]