Abstract
Natural killer T (NK T) cells play a central role as intermediates between innate and adaptive immune responses important to induce anti-tumour reactivity in cancer patients. In two of 14 renal cell carcinoma (RCC) patients, treated with interferon (IFN)-α, we detected significantly enhanced numbers of circulating NK T cells which were typed phenotypically and analysed for anti-tumour reactivity. These NK T cells were T cell receptor (TCR) Vα24/Vβ11+, 6B11+ and bound CD1d tetramers. No correlation was observed between NK T frequencies and regulatory T cells (Tregs), which were also enhanced. NK T cells expressed CD56, CD161, CD45RO and CD69 and were predominantly CD8+, in contrast to the circulating T cell pool that contained both CD4+ and CD8+ T cells, as is found in healthy individuals. It is unlikely that IFN-α triggered the high NK T frequency, as all other patients expressed low to normal NK T numbers. A parallel was observed in IFN-α-related increase in activation of NK T cells with that in conventional T and non-T cells. Normal interleukin (IL)-7, IL-12 and IL-15 plasma levels were found. In one of the patients sporadic NK T cells were detected at the tumour site. α-Galactosylceramide (αGalCer) stimulation of peripheral blood mononuclear cells or isolated NK T cell lines from both patients induced IFN-γ, but no IL-4 and no response towards autologous tumour cells or lysates. The clinical course of disease in both patients was not exceptional with regard to histological subtype and extent of metastatic disease. Therefore, despite a constitutive high peripheral frequency and in vitroαGalCer responsiveness, the NK T cells in the two RCC patients did not show anti-tumour responsiveness.
Keywords: IFN-α therapy, immunotherapy, NK T cells, renal cell carcinoma
Introduction
Invariant NK T cells are a distinct set of T cells characterized by expression of an invariant T cell receptor (TCR) Vα14-Jα18 chain, coupled preferentially to Vβ8·2,7 or -2 in mice or TCR Vα24-Jα18 and Vβ11 in humans [1]. NK T cells recognize glycolipids, rather than peptide antigens, presented by the major histocompatibility complex class I-like molecule CD1d. This results in rapid release of large amounts of T helper type 1 (Th1) [interferon (IFN)-γ] or Th2 [interleukin (IL)-4] cytokines, which in turn can activate dendritic cells, NK cells and B cells as well as conventional CD4+ and CD8+ T cells [2,3]. Thereby, NK T cells play a pivotal role as intermediates between the innate and the adaptive immune system and have the capacity to enhance host immunity to microbial infections and cancer as well as prevent autoimmunity [4–6].
In healthy individuals, the frequency of NK T cells in the peripheral blood is relatively low and ranges between 0·01% to 0·2% of total lymphocytes [7–9].
In cancer patients, NK T cell counts are reduced further compared to age- and gender-matched healthy controls [7,8] and usually defective in IFN-γ production upon stimulation [10,11]. Low circulating NK T cell numbers were found to predict poor clinical outcome in patients with head and neck cancer [12]. Attempts have been made to stimulate NK T cell expansion with the glycolipid α-galactosylceramide (αGalCer) in order to stimulate anti-tumour responses in cancer patients [13–18]. In 10 of 17 non-small cell lung cancer patients this resulted in prolonged median survival time [19].
In an IFN-α trial of patients with metastatic renal cell carcinoma (RCC), a disease that has not been associated with high NK T cell numbers previously, we detected unusually high levels of circulating NK T cells in two of 14 patients. This prompted us to characterize these cells further to elucidate whether they were related to the therapy and had anti-tumour effectivity.
Materials and methods
Patients
All patients had primary metastatic RCC, patient B2 had clear cell RCC with sarcomatoid component and patient B7 had papillary RCC. All patients gave informed consent to participate in a randomized Phase II trial of nephrectomy followed by IFN-α (arm A) versus IFN-α followed by deferred nephrectomy (arm B). The trial was approved by the local ethical committee and closed prematurely after the clinical implementation of tyrosine kinase inhibitors. IFN-α therapy consisted of subcutaneously applied escalating doses of a 2-month induction regimen of IFN-α2b (Roferon®, Hoffman-LaRoche, Nutley, NJ, USA): 2 weeks 5 × 3 × 106; 2 weeks 5 × 6 × 106; 2 weeks 5 × 9 × 106; and 2 weeks 3 × 9 × 106 IU/week). Tumour and lymph node tissues were obtained at nephrectomy. Peripheral blood mononuclear cells (PBMC) were harvested at regular time-points pre-, during and post-therapy by Ficoll-Hypaque, washed and resuspended in phosphate-buffered saline (PBS) complemented with 0·5% bovine serum albumin (BSA; Sigma Aldrich, Zwijndrecht, the Netherlands) and cryopreserved in liquid nitrogen for later analysis.
Cell lines
RCC tumour cell lines were established from fresh tumour (patient B2) or tumour-involved lymph node (patient B7) after digestion with collagenase type 4 (1 mg/ml; Sigma-Aldrich Chemie B.V., Zwijndrecht, the Netherlands) and expressed the epidermal growth factor receptor (EGFR) and clear cell RCC-associated G250 antigen. Established Epstein–Barr virus (EBV)-transformed B cell lines used were JY, C1R and C1R-huCD1d, the latter transduced with human CD1d (C1R and C1R-huCD1d [20], kindly provided by Dr V. Cerundolo, Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, UK). All cell lines were cultured in RPMI-1640 (Invitrogen Life Sciences/Gibco, Invitrogen Corporation Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS) (inactivated; Greiner Bio-one GmbH, Frickenhausen, Germany), penicillin (100 U/ml) and streptomycin (100 µg/ml) (Roche Diagnostics, Mannheim, Germany) and were refreshed twice a week.
Establishment and culture of NK T cell lines
NK T cell lines from patients B2 and B7 were established by fluorescence activated cell sorting (FACS) of cells labelled with anti-TCR Vα24 plus Vβ11 antibodies (Beckman Coulter, Woerden, the Netherlands), cultured for 1–3 weeks in serum-free Iscove's modified Dulbecco's medium (IMDM; Invitrogen Life Sciences/Gibco) supplemented with 2% normal human serum (Invitrogen, Brown Deer, WI, USA), penicillin/streptomycin and IJssel's supplements [21] in the presence of IL-2 (100 U/ml; Eurocetus, Amsterdam, the Netherlands) and IL-15 (5 ng/ml, Peprotech, London, UK) and were refreshed twice a week.
Preparation of RCC tumour cell lysates
Tumour cell lysates were prepared from tumour cell lines or tumour-involved lymph node tissues which were suspended in 250 µl PBS, followed by snap-freezing three times and sonification on ice.
Enzyme-linked immunospot (ELISPOT) assay
IFN-γ and IL-4 ELISPOT assays were carried out according to the manufacturer's instructions (U-cytech Biosciences, Utrecht, the Netherlands), as described previously [22]. Briefly, flat-bottomed 96-well plates (Costar 3799) were incubated with coating antibody (U-cytech) overnight at 37°C, washed with PBS and incubated with coating buffer for 2 h. The patient PBMC (obtained from samples during IFN-α therapy) were thawed, washed and incubated for 1 h in IMDM supplemented with 5% FCS (Greiner Bio-one) at 2 × 106 cells/ml. The coating buffer was removed from the plates, optimal concentrations of 2 × 105 responder cells in IMDM with 5% FCS were put into each well and 5 × 104 tumour target cells or lysates (equivalent of 2 × 104 cells), optimal concentrations of αGalCer (100 ng/ml, obtained from Dr H. Ovaa, the Netherlands Cancer Institute, Amsterdam, the Netherlands) or phorbol myristate acetate (PMA) (50 ng/ml) plus ionomycin (1 µg/ml) were added and the plates were incubated overnight at 37°C. In experiments with NK T cell lines, optimal concentrations of responders were used at 5 × 102/well, targets at 2 × 103 cells/well and external antigen-presenting cells C1R-huCD1d or C1R at 2 × 103 cells/well. After removal of the cell suspension, the plates were washed with PBS, developed according to the manufacturer's instructions and read using the Bioreader 4000 pro-X ELISPOT reader (Bio-sys, Karben, Germany).
Enzyme-linked immunosorbent assay (ELISA) for IL-7, IL-12 and IL-15
Plasma samples were obtained at various time-points during IFN-α therapy, preserved at −70°C and tested using ELISA according to the manufacturer's instructions (human IL-7 Quantikine ELISA kit HS750, human IL-12 Quantikine ELISA kit D1200 and human IL-15 Quantikine ELISA kit D1500; R&D Systems, Abington, UK).
Flow cytometry
PBMC subset analysis was performed as described previously [23]. Briefly, cells or cell lines were stained for 20 min at room temperature followed by washing steps in PBS containing 0·5% BSA with the following conjugated antibodies directed at: CD3-fluorescein isothiocyanate [fluoroscein isothiocyanate (FITC)/CD(16+56]-phycoerythrin (PE) (B&D Biosciences), CD8-FITC, CD56-PE cyanine5 (PC5), CD19-PC5, CD69-PE Texas Red [electrochemical detection (ECD)], CD8-PC5, CD3-PE cyanine7 (PC7), CD45-FITC/CD14-PE, CD45RO-ECD and CD4-ECD (all from Beckman Coulter, Woerden, the Netherlands). For detection of NK T cells, staining with anti-TCR Vα24-FITC and Vβ11-PE in combination with anti-CD3-PC7 was used; in some experiments, NK T cells were measured using anti-TCR Vβ11-PE in combination with 6B11-FITC [24] (BD Biosciences Pharmingen, San Diego, CA, USA). Further NK T subset typing was performed using antibodies to CD4-ECD, CD8-PC5, CD56-PC5, CD69-ECD, CD45RO-ECD (all Beckman Coulter) and CD161-biotin (Ancell, Bayport, MN, USA). For enumeration of regulatory T cells (Treg), antibodies were used directed at: CD4-FITC, CD8-PE, CD45-ECD, CD25-PC5, CD3-PC7 (Beckman Coulter) and forkhead box P3 (FoxP3) (eBioscience kit; eBioscience, Inc. San Diego, CA, USA). In all experiments gates were set on viable [propidium iodide (PI)-negative] cells and fluorochrome-labelled isotype control antibodies were included in each assay to determine background staining. FACS analysis was performed with a Beckman Coulter flow cytometer FC500 and computer software Beckman Coulter program CXP.
CD1d tetramer assay
The capacity of NK T cells to bind CD1d-presented ligand was tested using PE-conjugated CD1d tetramer, complexed to PBS57, an αGalCer analogue, obtained through the NIH Tetramer Facility (NIH Tetramer Facility, Germantown, MD, USA). HIV tetramer (Sanquin, Amsterdam, the Netherlands) served as negative control (< 0·05% positive). We measured CD1d tetramer binding to T cells that were negative for a mixture of FITC-conjugated anti-CD13 (Beckman Coulter), anti-CD14, anti-CD16 and anti-CD19 (B&D Biosciences, San Jose, CA USA) instead of positive for CD3 antibody to avoid blocking or hindering of tetramer binding.
In situ immunofluorescence multi-colour staining
NK T cells in tissues were examined by triple immunofluorescence staining by anti-CD3 antibody combined with anti-TCR Vα24 and Vβ11 antibodies and analysis by confocal laser scanning microscopy, as described previously [25,26]. In brief, 4-µm cryostat sections from primary tumour and lymph nodes from patients B2 and B7 were air-dried overnight, fixed in acetone for 10 min at room temperature, preincubated in 5% (vol/vol) normal goat serum (Sanquin) and incubated successively with mouse anti-CD3 antibody (Dako A/S, Glostrup, Denmark), biotinylated goat anti-mouse antibody (Dako), normal mouse serum (Sanquin), mouse anti-human TCR Vα24-FITC, mouse anti-human TCR Vβ11-PE (Beckman Coulter) and rabbit anti-PE antibody (Biogenesis, Poole, UK), followed by Cy3-conjugated goat anti-rabbit antibody and Cy5-conjugated streptavidin (Jackson Immunoresearch Laboratories, Inc., Palo Alto, CA, USA). Between incubations, sections were rinsed extensively in PBS. For each fluorochrome label, isotype-matched control antibodies were included and found negative. For counting of NK T cells, 2000 CD3+ T cells in two separate tissue sections were examined.
Confocal laser scanning microscopy analysis
Confocal fluorescence images were obtained on a Leica TCS SP (Leica Microsystems, Heidelberg, Germany) confocal system, equipped with an Argon/Krypton/HeliumNeon laser combination. Images were taken using a 40× 1·25 NA objective. Possible spectral leak-through between FITC, Cy3 and Alexa 647, which could give rise to false-positive co-localization of different signals, was avoided by careful selection of the imaging conditions. Colour photomicrographs were taken from electronic overlays.
Statistical analysis
Statistical significance was determined using the Student's t-test.
Results
Identification of peripheral NK T cells
Immunomonitoring of RCC patients in the IFN-α trial revealed an exceptionally high percentage of circulating CD3+CD56+ T cells in patient B2 (Table 1). Further analysis indicated that this patient and patient B7 showed significantly elevated levels of NK T cells expressing TCR Vα24/Vβ11 in their peripheral blood compared to a panel of healthy donors (Table 1). There were no large differences between NK T cell numbers pre-, during and post-treatment in each patient, as is reflected in the relatively low standard deviation (s.d.) values for the mean (Table 1). The increase in NK T cell number in patients B2 and B7 is unlikely to be due to the IFN-α treatment, as the number of circulating NK T cells in all other RCC patients, regardless of time of treatment, was in the low to normal range (Table 1). Moreover, patient B7 had already presented with high NK T frequency before the start of the IFN-α therapy (see Fig. 3b; no pre-therapy sample available from patient B2). PBMC subset analysis of the RCC patients in the two treatment arms of the IFN-α trial showed normal absolute numbers of CD3, CD4 or CD8 T cells, NK cells or monocytes (Fig. 1).
Table 1.
Peripheral CD3+CD56+ T cells and natural killer (NK) T cells in renal cell carcinoma patients of interferon (IFN)-α trial arms A and B
| Patient | % CD3+CD56+ | n† | % Vα24/Vβ11+ | n |
|---|---|---|---|---|
| A1‡ | 8·0 ± 4·1§ | 10 | 0·02 ± 0·02 | 12 |
| A2 | 0·7 ± 0·7 | 5 | 0·02 ± 0·01 | 5 |
| A3 | 3·1 ± 1·8 | 3 | 0·01 ± 0·00 | 4 |
| A4 | 6·9 ± 2·0 | 6 | 0·02 ± 0·03 | 6 |
| A5 | n.t. | 0·01 ± 0·01 | 2 | |
| A6 | 8·6 ± 4·9 | 2 | 0·32 ± 0·11 | 3 |
| B1 | 0·9 ± 0·1 | 2 | 0·04 ± 0·01 | 5 |
| B2 | 36·8 ± 9·3 | 4 | 1·65 ± 0·54¶ | 5 |
| B3 | 8·3 ± 2·2 | 4 | 0·05 ± 0·02 | 6 |
| B4 | 8·5 ± 3·3 | 4 | 0·01 ± 0·01 | 4 |
| B5 | 7·0 ± 4·1 | 3 | 0·14 ± 0·04 | 3 |
| B6 | 7·0 ± 2·6 | 2 | 0·00 ± 0·00 | 4 |
| B7 | 9·1 ± 5·8 | 3 | 7·64 ± 1·54¶ | 9 |
| B8 | 4·9 ± 2·6 | 2 | 0·13 ± 0·10 | 2 |
| Total without B2 and B7 | 0·06 ± 0·09 | 12 | ||
| Healthy donors | 5·1 ± 5·1 | 11 | 0·13 ± 0·15 | 14 |
Number of tests.
Patients receiving IFN-α treatment after (A) or before and after (B) nephrectomy.
Mean percentage ± standard deviation of CD3+ T cells in peripheral blood mononuclear cells, calculated from all samples taken pre-, during and post-IFN-α therapy.
Significantly different from healthy donor controls (P < 0·001)
n.t.: not tested.
Fig. 3.
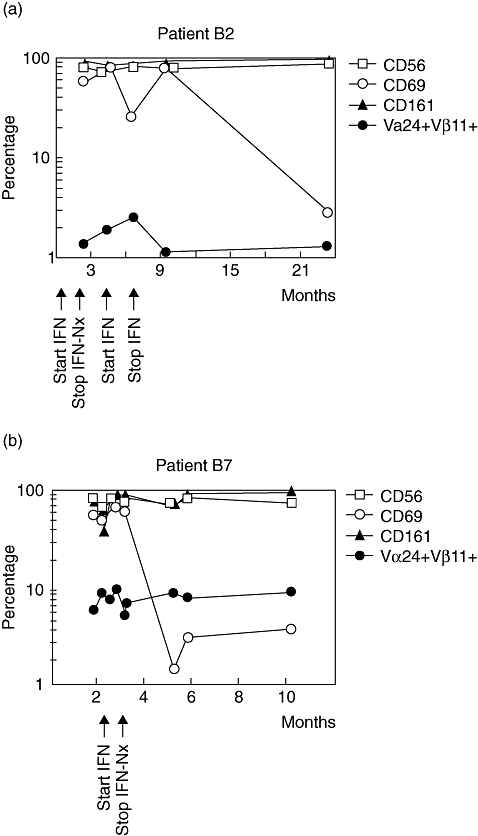
Kinetics of natural killer (NK) T cells and their subsets during interferon (IFN)-α treatment in patients B2 (a) and B7 (b). NK T cells are detected as T cell receptor Vα24/Vβ11-expressing T cells and shown as percentage of total CD3+ T cells. CD56, CD161, CD45RO and CD69 NK T subsets are shown as percentage of total NK T cells. Nx, nephrectomy.
Fig. 1.
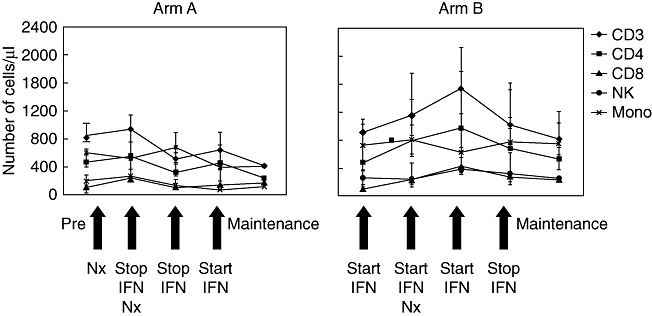
Analysis of peripheral blood mononuclear cell lineages during the course of interferon (IFN)-α treatment in renal cell carcinoma patients of trial arms A and B. Absolute cell counts for CD3+CD56− T cells, CD3+CD4+ T cells, CD3+CD8+ T cells, CD3−CD16+56+ natural killer (NK) cells and CD14+ monocytes were determined at various time-points pre-, during and post-IFN-α therapy. Patient values are within the normal range (for CD3+ T cells 700–1900, CD4+ T cells 400–1300, CD8+ T cells 200–700, NK cells 100–400 and for monocytes 100–1000 cells/µl). Nx, nephrectomy.
In addition, Tregs, measured as the percentage of FoxP3+ cells within the CD4+ T cell population, were increased in RCC patients at nephrectomy and during therapy, significantly in B2 compared to 10 healthy donors (8·0 ± 3·9% versus 3·0 ± 2·4%, mean ± s.d.; P < 0·05) (Table 2). No significant differences were found between RCC patients in arm A and arm B (Table 2).
Table 2.
Percentages of regulatory T cells in renal cell carcinoma patients in relation to natural killer (NK) T cells
| % Vα24/Vβ11+ | % FoxP3 | % Vα24/Vβ11+ | n† | % FoxP3 | n† | |
|---|---|---|---|---|---|---|
| Patient | At Nx‡ | During treatment period§ | ||||
| A1¶ | 0·02 | 6·5 | 0·02 ± 0·02 | 12 | 10·6 ± 8·0 | 11 |
| A2 | 0·03 | 12·0 | 0·02 ± 0·01 | 5 | 12·8 ± 1·3 | 2 |
| A3 | 0·01 | 4·9 | 0·01 ± 0·00 | 4 | 5·1 ± 1·2 | 3 |
| A4 | 0·06 | 6·1 | 0·02 ± 0·03 | 6 | 8·0 ± 2·0 | 3 |
| A6 | 0·27 | 3·7 | 0·32 ± 0·11 | 3 | 4·3 ± 0·9 | 2 |
| 8·2 ± 4·0†† | ||||||
| B1 | 0·03 | 5·6 | 0·04 ± 0·01 | 5 | 6·1 ± 0·4 | 4 |
| B2 | 1·41 | 9·2 | 1·65 ± 0·54 | 5 | 8·0 ± 3·9‡‡ | 5 |
| B3 | 0·05 | 2·3 | 0·05 ± 0·02 | 6 | 5·0 ± 6·3 | 4 |
| B4 | 0·01 | 10·0 | 0·01 ± 0·01 | 4 | 10·0 | 1 |
| B7 | 7·00 | 12·2 | 7·64 ± 1·54 | 9 | 7·9 ± 6·7‡‡ | 5 |
| B8 | 0·15 | 8·0 | 0·13 ± 0·10 | 2 | 10·2 ± 3·1 | 2 |
| 7·9 ± 2·1†† | ||||||
| Healthy donors | 0·13 ± 0·15 | 14 | 3·0 ± 2·4 | 10 | ||
Number of tests.
% forkhead box P3 (FoxP3) of CD3+CD4+ T cells tested at time of nephrectomy (Nx).
Mean % FoxP3 ± standard deviation of CD3+CD4+ T cells tested in multiple peripheral blood mononuclear cell samples during treatment period.
Patients receiving interferon-α treatment after (A) or before and after (B) nephrectomy.
% FoxP3 not significantly different between arm A and arm B (P > 0·5).
% FoxP3 in B2 significantly (P < 0·05) and in B7 not significantly (P > 0·05) different from healthy donors.
Peripheral NK T cells and subsets
As shown in Fig. 2a, NK T cells were detected similarly by staining with antibodies to TCR Vα24/Vβ11 as by staining with CD1d tetramer, indicating that the NK T cells could bind CD1d-presented ligand. In addition, NK T cells were also positive for the NK T marker 6B11 (Fig. 2b). Comparable low percentages within the CD3 population were found for NK T cell frequencies (range < 0·01–0·09%), either tested by Vα24/Vβ11 or Vβ11/6B11 monoclonal antibody (mAb) combinations in RCC patients A1, A2, A3, A4, A7, B1 or B3 (data not shown).
Fig. 2.
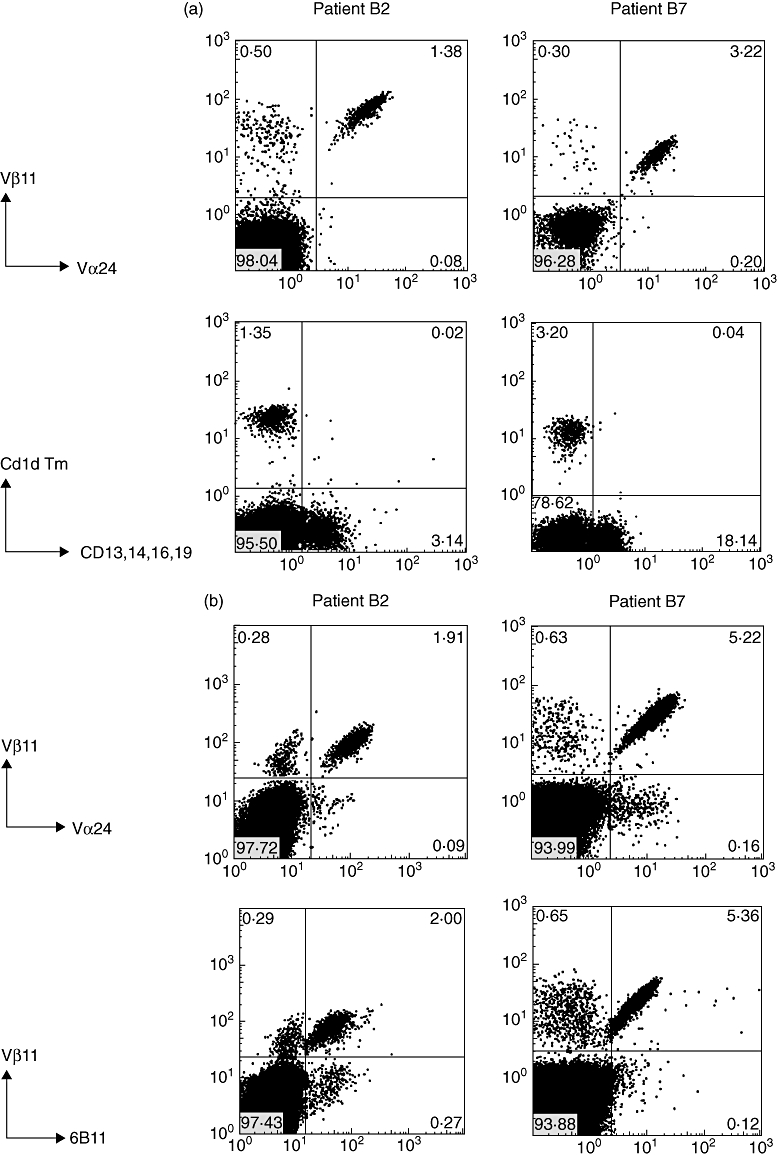
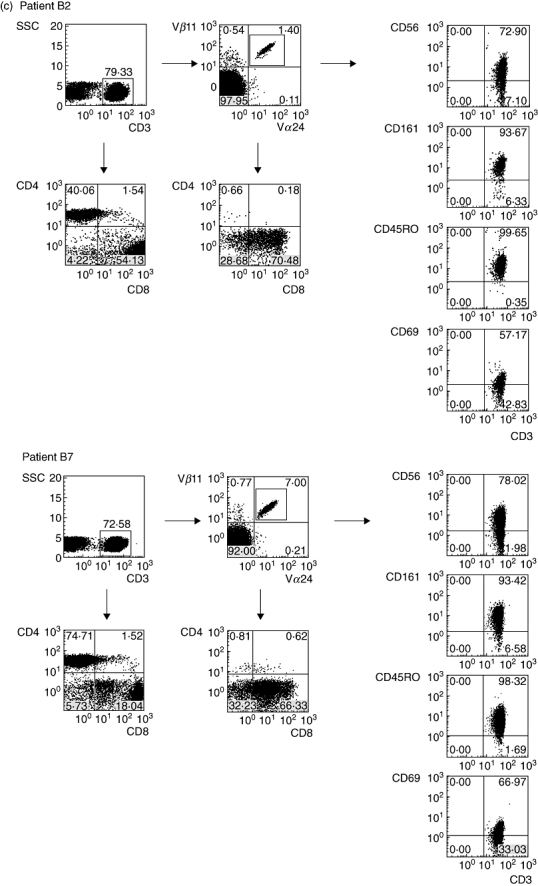
T cell receptor (TCR) Vα24/Vβ11 and 6B11 expression, CD1d tetramer staining and natural killer (NK) T subset analysis of peripheral blood mononuclear cells of patients B2 and B7. (a) Cells double-stained with anti-TCR Vα24-fluorescein isothiocyanate (FITC) and Vβ11-phycoerythrin (PE) are detected within lymphocyte and CD3 gate; for CD1d tetramer staining, cells were stained with PE-conjugated PBS57 (αGalCer homologue) loaded CD1d tetramer combined with negative selection antibodies (a mixture of FITC-conjugated anti-CD13, 14, 16, 19). Numbers indicate the percentage of positive cells in each quadrant; (b) cells double-stained with anti-6B11-FITC and Vβ11-PE are detected within lymphocyte and CD3 gate; (c) expression of CD4, CD8, CD56, CD161, CD45RO and CD69 on peripheral blood NK T cell subsets of patients B2 and B7 analysed within the indicated TCR Vα24/Vβ11 gate compared to CD4- and CD8-positive T cell subsets analysed within the indicated CD3 gate. Note absence of CD3+CD4+ cells within the NK T cell population and presence within the total peripheral blood T cell population of patients B2 and B7.
The main phenotype of the NK T cells in both patients was CD3+CD4−CD8+, with a minor fraction being CD3+CD4−CD8− and virtually no cells being CD3+CD4+CD8−, in contrast to the total peripheral blood T cell pool that contained both CD4−CD8+ and CD4+CD8− T cells (Fig. 2c, Table 3). In RCC patients and healthy individuals with NK T cell numbers in the normal range, both CD4−CD8+ and CD4+CD8− NK T subsets were detectable. No association was found between NK T frequency and patient age. NK T cells in patients B2 and B7 expressed NK T-associated antigens CD45RO, CD161, CD56 and were CD69+ (Fig. 2c). During IFN-α treatment, this phenotype remained stable except that CD69 expression was lost upon withdrawal of therapy (Fig. 3). Expression of CD69 in patients B2, B7, A6 and in healthy donors was relatively high on NK T cells compared to conventional T and non-T cells. IFN-α treatment of our patients does not appear to be a trigger for high NK T frequency, but was found to enhance the activation state in a co-stimulatory manner. As shown in Table 4, it increased CD69 expression of NK T cells, sometimes with a short delay. Particularly in patients B2 and B7, changes in activation of conventional T and non-T cells, parallel to NK T cells, were observed, indicating that IFN-α treatment also affected these cell types.
Table 3.
CD3 and natural killer (NK) T cell subsets in relation to NK T cell frequency and age in patient peripheral blood mononuclear cell samples obtained just before nephrectomy and healthy donors
| % of CD3† | % of NK T‡ | ||||||
|---|---|---|---|---|---|---|---|
| Patient | Age | TCR Vα24+Vα11+ | CD4+ | CD8+ | CD4+ | CD8+ | DN |
| B7 | 61 | 7·00§ | 74 | 18 | 1 | 66 | 32 |
| B2 | 46 | 1·41 | 40 | 54 | 1 | 70 | 29 |
| A6 | 60 | 0·27 | 65 | 28 | 31 | 33 | 31 |
| B8 | 55 | 0·15 | 61 | 31 | 22 | 56 | 15 |
| B5 | 27 | 0·15 | 43 | 49 | 15 | 16 | 68 |
| A7 | 60 | 0·08 | 80 | 15 | 30 | 2 | 69 |
| B3 | 56 | 0·05 | 55 | 17 | 87 | 5 | 3 |
| HD | |||||||
| HD1 | 0·45 | 41 | 58 | 4 | 75 | 21 | |
| HD13 | 0·34 | 62 | 25 | 36 | 13 | 50 | |
| HD5 | 0·17 | 55 | 38 | 65 | 8 | 25 | |
| HD6 | 0·14 | 63 | 34 | 50 | 38 | 9 | |
| HD7 | 0·05 | 71 | 27 | 65 | 22 | 10 | |
| HD8 | 0·02 | 74 | 23 | 29 | 13 | 58 | |
| HD9 | 0·02 | 74 | 21 | 18 | 41 | 41 | |
| HD10 | 0·01 | 75 | 20 | 86 | 11 | 2 | |
| HD11 | < 0·01 | 60 | 25 | 70 | 10 | 19 | |
Within CD3 gate.
Within Vα24/Vα11+ gate.
Renal cell carcinoma patient peripheral blood mononuclear cell samples obtained just before nephrectomy.
HD: healthy donor (ages between 20 and 60 years); TCR: T cell receptor.
Table 4.
CD69 expression on natural killer (NK) T, conventional T and non-T cells
| % CD69 | ||||||
|---|---|---|---|---|---|---|
| Time-point | IFN-α treatment† | % Vα24/Vα11+ | Vα24/Vα11+ | Conventional T cells | Non-T cells | |
| Patient | ||||||
| B2 | 1 (Nx) | + | 1·41‡ | 56·0§ | 12·2‡ | 25·3¶ |
| 2 | − | 1·97 | 84·4 | 34·8 | 9·2 | |
| 3 | + | 2·43 | 20·8 | 14·4 | 15·6 | |
| 4 | − | 1·12 | 77·3 | 10·4 | 18·9 | |
| 5 | − | 1·31 | 3·0 | 6·2 | 8·2 | |
| B7 | 1 | − | 5·70 | 56·9 | 3·1 | 6·4 |
| 2 | + | 8·91 | 48·0 | 4·3 | 5·6 | |
| 3 | + | 6·98 | 74·1 | 9·0 | 32·5 | |
| 4 | + | 9·40 | 67·3 | 10·7 | 30·0 | |
| 5 | + | 5·24 | 68·5 | 5·7 | 23·3 | |
| 6 (Nx) | + | 7·00 | 60·3 | 6·8 | 18·6 | |
| 7 | − | 8·98 | 3·4 | 2·5 | 10·4 | |
| 8 | − | 7·63 | 3·1 | 1·6 | 10·0 | |
| 9 | − | 9·03 | 3·8 | 3·1 | 13·6 | |
| A6 | 1 (Nx) | − | 0·45 | 9·7 | 2·3 | 3·0 |
| 2 | + | 0·27 | 22·6 | 2·1 | 7·5 | |
| 3 | + | 0·24 | 39·0 | 2·9 | 8·1 | |
| 4 | − | 0·20 | 40·7 | 3·5 | 8·3 | |
| 5 | − | 0·26 | 60·0 | 6·3 | 8·0 | |
| HD | ||||||
| HD1 | 0·45 | 95·0 | 6·4 | 14·8 | ||
| HD2 | 0·38 | 91·0 | 14·3 | 15·3 | ||
| HD3 | 0·26 | 58·2 | 2·1 | 6·5 | ||
| HD4 | 0·20 | 92·7 | 2·5 | 2·7 | ||
| HD5 | 0·17 | 65·3 | 6·0 | 9·0 | ||
| HD9 | 0·02 | 97·5 | 0·8 | 1·7 | ||
| HD8 | 0·01 | 93·4 | 0·6 | 1·8 | ||
Sample taken at time-point with (+) or without (−) interferon (IFN)-α treatment.
Within CD3 gate.
Within Vα24/Vα11+ gate.
Within lympho- and non-CD3 gate.
HD: healthy donor.
Identification of NK T cells at the tumour site
To examine whether NK T cells could be detected directly in tumour or lymph node tissues, in situ triple-staining analysis of TCR Vα24/Vβ11 combined with CD3 was performed in available tissues, i.e. tumour of both patients and lymph node of patient B7. As presented in Fig. 4, only in the tumour of patient B2 could sporadically triple-positive NK T cells be observed (0·4% triple-positives of 2000 T cells counted). No expression of CD4 or CD8 was found on these NK T cells.
Fig. 4.
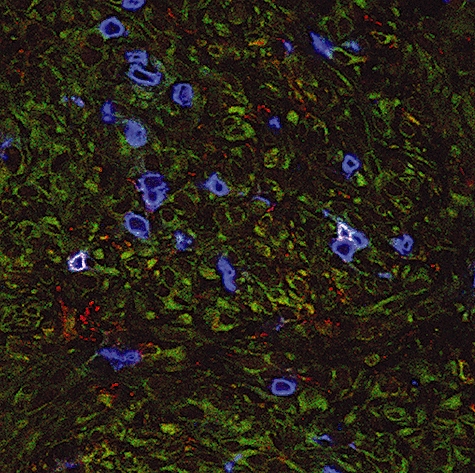
In situ detection of natural killer (NK) T cells in tumour from patient B2. Cryosections of tumour tissue were stained with anti-T cell receptor (TCR) Vα24-fluorescein isothiocyanate (green) in combination with anti-TCR Vβ11-phycoerythrin (red) and anti-CD3 (blue), as described in Materials and methods. Triple-positive NK T cells are detected by a white membrane staining. Renal tumour cells show some aspecific background staining in green. Original magnification × 400.
Functional activity of NK T cells
To investigate whether the NK T cells of patients B2 and B7 responded to their tumours, ELISPOT analysis of PBMC-containing NK T cells was performed. Because no CD1d was found on tumour targets (data not shown), not only tumour cells, but also tumour lysates were tested as targets for which autologous dendritic cells in the PBMC served as antigen-presenting cells. As shown in Table 5, peripheral NK T cells did not react to autologous tumour or lysate and showed IFN-γ, but no IL-4 responses to αGalCer. Several other RCC patients (A1, A2, A3, A4, A6, B1, B3 and B4) and healthy donors did not show any responsiveness to αGalCer (data not shown).
Table 5.
Functional responses of patient B2 and B7 natural killer (NK) T cells in peripheral blood mononuclear cells
| Patient B2 | Patient B7 | |||
|---|---|---|---|---|
| Exp.1 | IFN-α | IL-4 | IFN-α | IL-4 |
| Autologous tumour cells | 3† | 0 | 0 | 0 |
| Autologous tumour lysate | 2 | 0 | 0 | 0 |
| Controls | ||||
| αGalCer‡ | 38 | 0 | 26 | 0 |
| JY§ | > 50 | 8 | 19 | 26 |
| PMA/ionomycin | > 50 | 40 | > 50 | 48 |
| Patient B2 | Patient B7 | |||
|---|---|---|---|---|
| Exp.2 | IFN-α | IL-4 | IFN-α | IL-4 |
| Autologous tumour cells | 3 | 0 | 1 | 0 |
| Autologous tumour lysate | 0 | 0 | 0 | 0 |
| Controls | ||||
| αGalCer | 20 | 0 | 7 | 0 |
| JY | > 50 | 4 | 16 | 8 |
| PMA/ionomycin | > 50 | 29 | > 50 | 40 |
Spots/well of 2 × 105 effector cells co-cultured overnight with 5 × 104 targets in enzyme-linked immunospot assay.
α-Galactosylceramide (αGalCer).
JY cells are used as allogeneic stimulator cells.
IL: interleukin; IFN: interferon; PMA: phorbol myristate acetate.
Because patient PBMC contained enhanced numbers of Treg, NK T cells were isolated from the cells by FACS sorting and in vitro-cultured NK T cell lines were tested as responders, allowing analysis of anti-tumour reactivity in the absence of potential suppressing Treg. As shown in Fig. 5, isolated NK T cell lines cultured for 1–3 weeks could be typed as TCR Vα24/Vβ11-expressing cells that also bound CD1d tetramer.
Fig. 5.
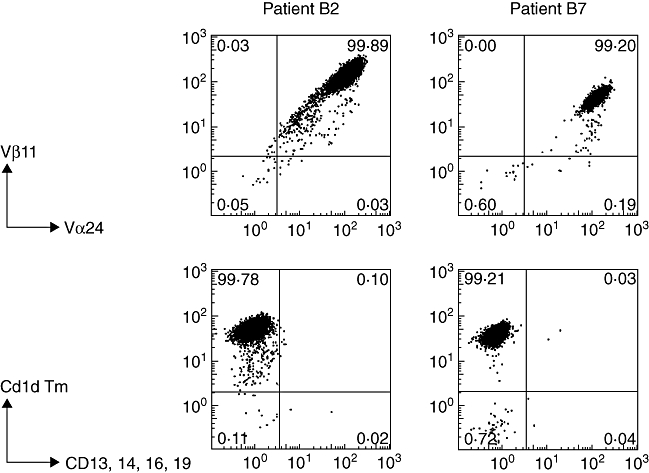
Analysis of T cell receptor (TCR) Vα24/Vβ11 expression and CD1d tetramer staining of natural killer (NK) T cell lines. NK T cell lines were established by fluorescence activated cell sorting of anti-TCR Vα24 plus Vβ11-labelled cells and cultured for 1–3 weeks in medium supplemented with interleukin (IL)-2 and IL-15 (see Materials and methods). Cells double-stained with anti-TCR Vα24- fluorescein isothiocyanate (FITC) and Vβ11-phycoerythrin (PE) are detected within lymphocyte and CD3 gate; for CD1d tetramer staining, cells were stained with PE-conjugated PBS57 (αGalCer homologue) loaded CD1d tetramer combined with negative selection antibodies (a mixture of FITC-conjugated anti-CD13, 14, 16, 19). The percentages of CD1d tetramer binding T cells are shown in the upper left quadrants.
NK T cell lines were tested in the presence of human CD1d-transfected C1R cells as antigen-presenting cells. Unlike conventional T cells, these purified NK T cell lines did not react to the allogeneic cell line C1R (or C1R-huCD1d) (Table 6). As shown in Table 6, the IFN-γ responses of the NK T cell lines were induced by αGalCer (but not in its absence) when presented by C1R-huCD1d cells and not in the presence of the CD1d-negative cell line C1R. B2 autologous tumour did not elicit any response; B7 autologous tumour elicited a variable response that was not consistently positive or negative. Tumour lysates did not induce a response (in the absence of αGalCer), did not enhance the αGalCer response and with the B7 NK T cell line as responder even suppressed this response.
Table 6.
Functional responses of natural killer (NK) T cell lines
| Experiment 1 | Patient B2 | Patient B7 |
|---|---|---|
| Autologous tumour + αGalCer† | 0‡ | 0 |
| Autologous tumour | 1 | 1 |
| Autologous tumour lysate + C1R-huCD1d§ + αGalCer | n.t. | 3 |
| Autologous tumour lysate + C1R-huCD1d | n.t. | 2 |
| Controls | ||
| C1R-huCD1d + αGalCer | 26 | 15 |
| C1R-huCD1d | 2 | 7 |
| C1R + αGalCer | 1 | 0 |
| C1R | 1 | 0 |
| PMA/ionomycin | 11 (61¶) | 14 (54) |
| Medium | 0 | 0 |
| Experiment 2 | Patient B2 | Patient B7 |
|---|---|---|
| Autologous tumour + αGalCer | 0 | 7 |
| Autologous tumour | 0 | 0 |
| Autologous tumour lysate + C1R-huCD1d + αGalCer | 5 | 1 |
| Autologous tumour lysate + C1R-huCD1d | 1 | 0 |
| allogeneic tumour lysate†† + C1R-huCD1d + αGalCer | 5 | 1 |
| allogeneic tumour lysate + C1R-huCD1d | 1 | 0 |
| Controls | ||
| C1R-huCD1d + αGalCer | 7 | 8 |
| C1R-huCD1d | 4 | 0 |
| C1R + αGalCer | 0 | 0 |
| C1R | 0 | 0 |
| PMA/ionomycin | 22 (36) | 22 (23) |
| Medium | 0 | 0 |
α-Galactosylceramide (αGalCer).
Interferon (IFN)-γ spots/well of 5 × 102 effector cells co-cultured overnight with 2 × 103 targets in enzyme-linked immunospot assay.
C1R cell line untransfected (C1R) or transfected with human CD1d (C1R-huCD1d).
Within brackets, responses of allogeneic peripheral blood mononuclear cell effectors.
Lysates of the other NK T patient were used.
PMA: phorbol myristate acetate; n.t.: not tested.
IL-7, IL-12 and IL-15
Enhanced levels of IL-7, IL-12 and IL-15 in the serum of the patients might be an explanation for the high peripheral NK T cell numbers. However, no enhanced levels of these cytokines were found in available plasma samples from patients A1, A2, A4, A5, B1, B3, B5, B6 and B7 (data not shown).
Discussion
In this study, we describe enhanced levels of circulating NK T cells in two of 14 RCC patients treated with IFN-α. The NK T cells expressed TCR Vα24/Vβ11 and the 6B11 NK T cell marker and bound CD1d-presented ligand, confirming their NK T type I character [1]. NK T cells were encountered only sporadically in one of the two patients in the tumour microenvironment.
The clinical course of disease in patients B2 and B7 was not exceptional in comparison to the other patients included in this trial, who had similar histological subtypes and extent of metastatic disease. All patients had advanced metastatic RCC, which was the only clinically detectable disease at evaluation. To the best of our knowledge, no other diseases were present nor were viral, bacterial or other genetic factors involved, which might have served as NK T triggers.
Most of the NK T cells of both patients were CD8+, with minor numbers presenting as double-negative and hardly any as CD4+. This is in contrast to the NK T subsets found usually in the peripheral blood of healthy donors or cancer patients, in which CD4+ NK T cells outnumber double-negative NK T cells and few or virtually no CD8+ NK T cells are found [8,27,28]. Our RCC patient data are in line with the correlation noted in healthy individuals between high peripheral NK T cell frequency and increase in CD4-negative NK T cells [9,28], which has been described to reverse with age [29]. The aberrant CD4-negative (and CD8-positive) NK T phenotype in patients B2 and B7 suggests that progressive differentiation and selected expansion may have occurred [30]. Expression of CD69 and CD161 would suggest that these NK T cells are recently activated and mature [1]. In humans, the number of peripheral CD4+ NK T cells is supported mainly by thymic output and survival and controlled by IL-7 [31], whereas CD4− NK T cells in the periphery are thought to be driven by IL-15-dependent homeostatic proliferation [30,32] Therefore, in the absence of a known antigenic trigger, the high NK T frequency in our patients can most probably be explained by homeostatic expansion, for which the normal levels of IL-15 that are detectable, may be sufficient. Homeostasis would also explain the relatively stable NK T frequency observed in the patients. The strong drop in CD69 expression, but not in NK T cell numbers, after stopping IFN-α treatment (see Table 4), may indicate that IFN-α can influence activation, but has no direct effect on homeostasis.
NK T cells have been described to activate downstream immune effector pathways, and this has prompted combination treatments aimed at activating T cell-mediated anti-tumour responses [3,33,34].
Three factors will determine the outcome of interactions between NK T cells and antigen-presenting cells: (i) frequency, strength and duration of antigenic stimulus; (ii) differentiation state of antigen-presenting cells; and (iii) presence or absence of cytokines that co-stimulate NK T cells, among which is IFN-α[35]. IFN-α treatment of ourpatients does not appear to be a trigger for high NK T frequency, as low to normal NK T cell counts were present in 12 of 14 RCC patients. Furthermore, in patient B7 the high NK T frequency could be shown to be already present before therapy. However, IFN-α was found to enhance the activation state in a co-stimulatory manner. As shown in Table 4, it increased CD69 expression of NK T cells, sometimes with a short delay. Particularly in patients B2 and B7, changes in activation of conventional T and non-T cells, parallel to NK T cells, were observed, indicating that IFN-α treatment also affected these cell types. It can be envisioned that via NK T cell CD40 ligand up-regulation, interactions with CD40-expressing antigen-presenting cells are enabled, which further up-regulate their co-stimulatory and cytokine profile involved in T cell activation [3,33–35]. However, apart from the IFN-α-related effect on CD69 up-regulation, our study does not provide evidence that these activated NK T cells cross-react with and thereby activate antigen-presenting cells, conventional T cells and non-T cells, as we neither detected enhanced T or NK cell numbers, IL-12 expressing DC in situ nor enhanced IL-12, IL-7 or IL-15 plasma levels.
Direct anti-tumour responsiveness by NK T cells in our two patients, as tested by IFN-γ responsiveness to tumours or tumour lysates, however, was not observed either. In vivo, this may be hampered by lack of CD1d expression on the tumours and lack of NK T cell infiltration into the tumour tissues. Alternatively, NK T function may be influenced by Treg cells [36], which are known to be elevated in cancer patients [37] and were found to be enriched, compared to normal individuals, in the peripheral blood of the RCC patients, without any relationship to NK T frequency. To test whether NK T cell-mediated anti-tumour responsiveness might be induced in the absence of Treg cells, NK T cell lines were isolated from the cell populations, cultured in the presence of IL-2 and IL-15 and tested for anti-tumour reactivity. The cell line C1R-huCD1d, expressing human CD1d, was added to serve as antigen-presenting cell in this system. However, despite appropriate CD1d-ligand binding capacity and IFN-γ response to αGalCer by the isolated NK T cell lines, no consistent reactivity to tumours or tumour lysates was observed. Tumour lysates were even found to suppress the αGalCer response of the B7 NK T cell line. These data point to an intrinsic inability of the patient NK T cells to respond to the autologous tumour, even in an activated state and in the absence of Treg cells.
Our observation of highly elevated levels of NK T cells in these RCC patients during an extended period of time bears resemblance to the observations of Chan et al. [38] on a healthy individual at risk for type 1 diabetes, and contrasts with the generally reduced NK T cell numbers in cancer patients [7,8,10,11].
In conclusion, despite the elevated and sustained levels of NK T cells in these patients, any functional role of the NK T cells in these patients thus remains elusive at present and it will be of interest to elucidate whether RCC aetiology is linked with conditions that stimulate NK T cell expansion.
Acknowledgments
We greatly acknowledge Drs S. Horenblas and W. Meinhardt for providing patients, Dr H. Ovaa for providing αGalCer, Dr V. Cerundolo for providing C1R and C1R-huCD1d cell lines, the NIH Tetramer Facility for providing PE-conjugated PBS57 loaded CD1d tetramer, A. Pfauth, F. van Diepen and M. van der Maas for help with flow cytometry and Drs J. Borst and J. Coquet for carefully reading the manuscript.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NK T cells: what's in a name? Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NK T cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowe NY, Coquet JM, Berzins SP, et al. Differential antitumor immunity mediated by NK T cell subsets in vivo. J Exp Med. 2005;202:1279–88. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawano T, Nakayama T, Kamada N, et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NK T cells. Cancer Res. 1999;59:5102–5. [PubMed] [Google Scholar]
- 6.Seino K, Motohashi S, Fujisawa T, Nakayama T, Taniguchi M. Natural killer T cell-mediated antitumor immune responses and their clinical applications. Cancer Sci. 2006;97:807–12. doi: 10.1111/j.1349-7006.2006.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crough T, Purdie DM, Okai M, Maksoud A, Nieda M, Nicol AJ. Modulation of human Valpha24(+)Vbeta11(+) NK T cells by age, malignancy and conventional anticancer therapies. Br J Cancer. 2004;91:1880–6. doi: 10.1038/sj.bjc.6602218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molling JW, Kolgen W, van der Vliet HJ, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NK T cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg JK, Bhardwaj N, Nixon DF. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Valpha24 NK T cell compartment. Eur J Immunol. 2003;33:588–96. doi: 10.1002/eji.200323707. [DOI] [PubMed] [Google Scholar]
- 10.Tahir SM, Cheng O, Shaulov A, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–50. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 11.Yanagisawa K, Seino K, Ishikawa Y, Nozue M, Todoroki T, Fukao K. Impaired proliferative response of V alpha 24 NK T cells from cancer patients against alpha-galactosylceramide. J Immunol. 2002;168:6494–9. doi: 10.4049/jimmunol.168.12.6494. [DOI] [PubMed] [Google Scholar]
- 12.Molling JW, Langius JA, Langendijk JA, et al. Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–8. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 13.Chang DH, Osman K, Connolly J, et al. Sustained expansion of NK T cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–17. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giaccone G, Punt CJ, Ando Y, et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–9. [PubMed] [Google Scholar]
- 15.Ishikawa A, Motohashi S, Ishikawa E, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–17. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 16.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NK T cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 17.Uchida T, Horiguchi S, Tanaka Y, et al. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:337–45. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieda M, Okai M, Tazbirkova A, et al. Therapeutic activation of Valpha24+Vbeta11+ NK T cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–9. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 19.Motohashi S, Nagato K, Kunii N, et al. A phase I–II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 20.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. 1992;360:593–7. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 21.Yssel H, De Vries JE, Koken M, Van Blitterswijk W, Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–27. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 22.Bins A, Mallo H, Sein J, et al. Phase I clinical study with multiple peptide vaccines in combination with tetanus toxoid and GM-CSF in advanced-stage HLA-A*0201-positive melanoma patients. J Immunother. 2007;30:234–9. doi: 10.1097/01.cji.0000211333.06762.47. [DOI] [PubMed] [Google Scholar]
- 23.de Gast GC, Klumpen HJ, Vyth-Dreese FA, et al. Phase I trial of combined immunotherapy with subcutaneous granulocyte macrophage colony-stimulating factor, low-dose interleukin 2, and interferon alpha in progressive metastatic melanoma and renal cell carcinoma. Clin Cancer Res. 2000;6:1267–72. [PubMed] [Google Scholar]
- 24.Exley MA, Hou R, Shaulov A, et al. Selective activation, expansion, and monitoring of human iNK T cells with a monoclonal antibody specific for the TCR alpha-chain CDR3 loop. Eur J Immunol. 2008;38:1756–66. doi: 10.1002/eji.200737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verra N, de Jong D, Bex A, et al. Infiltration of activated dendritic cells and T cells in renal cell carcinoma following combined cytokine immunotherapy. Eur Urol. 2005;48:527–33. doi: 10.1016/j.eururo.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Vyth-Dreese FA, Kim YH, Dellemijn TA, et al. In situ visualization of antigen-specific T cells in cryopreserved human tissues. J Immunol Methods. 2006;310:78–85. doi: 10.1016/j.jim.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Gadola SD, Dulphy N, Salio M, Cerundolo V. Valpha24-JalphaQ-independent, CD1d-restricted recognition of alpha-galactosylceramide by human CD4(+) and CD8alphabeta(+) T lymphocytes. J Immunol. 2002;168:5514–20. doi: 10.4049/jimmunol.168.11.5514. [DOI] [PubMed] [Google Scholar]
- 28.Bricard G, Cesson V, Devevre E, et al. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NK T cells in intrahepatic malignant tumors. J Immunol. 2009;182:5140–51. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]
- 29.Jing Y, Gravenstein S, Chaganty NR, et al. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NK T cells from human peripheral blood. Exp Gerontol. 2007;42:719–32. doi: 10.1016/j.exger.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Baev DV, Peng XH, Song L, et al. Distinct homeostatic requirements of CD4+ and CD4− subsets of Valpha24-invariant natural killer T cells in humans. Blood. 2004;104:4150–6. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- 31.de Lalla C, Festuccia N, Albrecht I, et al. Innate-like effector differentiation of human invariant NK T cells driven by IL-7. J Immunol. 2008;180:4415–24. doi: 10.4049/jimmunol.180.7.4415. [DOI] [PubMed] [Google Scholar]
- 32.Ranson T, Vosshenrich CA, Corcuff E, et al. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci USA. 2003;100:2663–8. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silk JD, Hermans IF, Gileadi U, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114:1800–11. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teng MW, Westwood JA, Darcy PK, et al. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 35.Hegde S, Fox L, Wang X, Gumperz JE. Autoreactive natural killer T cells: promoting immune protection and immune tolerance through varied interactions with myeloid antigen-presenting cells. Immunology. 2010;130:471–83. doi: 10.1111/j.1365-2567.2010.03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 37.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–6. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan AC, Serwecinska L, Cochrane A, Harrison LC, Godfrey DI, Berzins SP. Immune characterization of an individual with an exceptionally high natural killer T cell frequency and her immediate family. Clin Exp Immunol. 2009;156:238–45. doi: 10.1111/j.1365-2249.2009.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


