Abstract
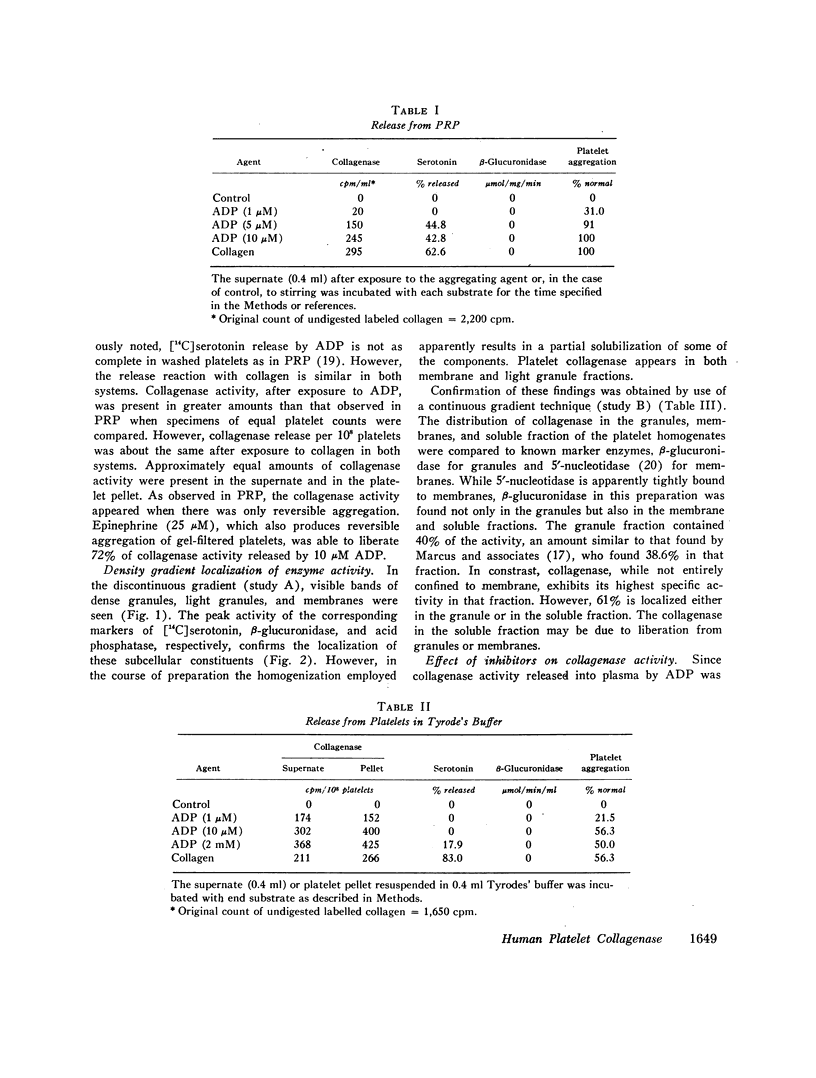
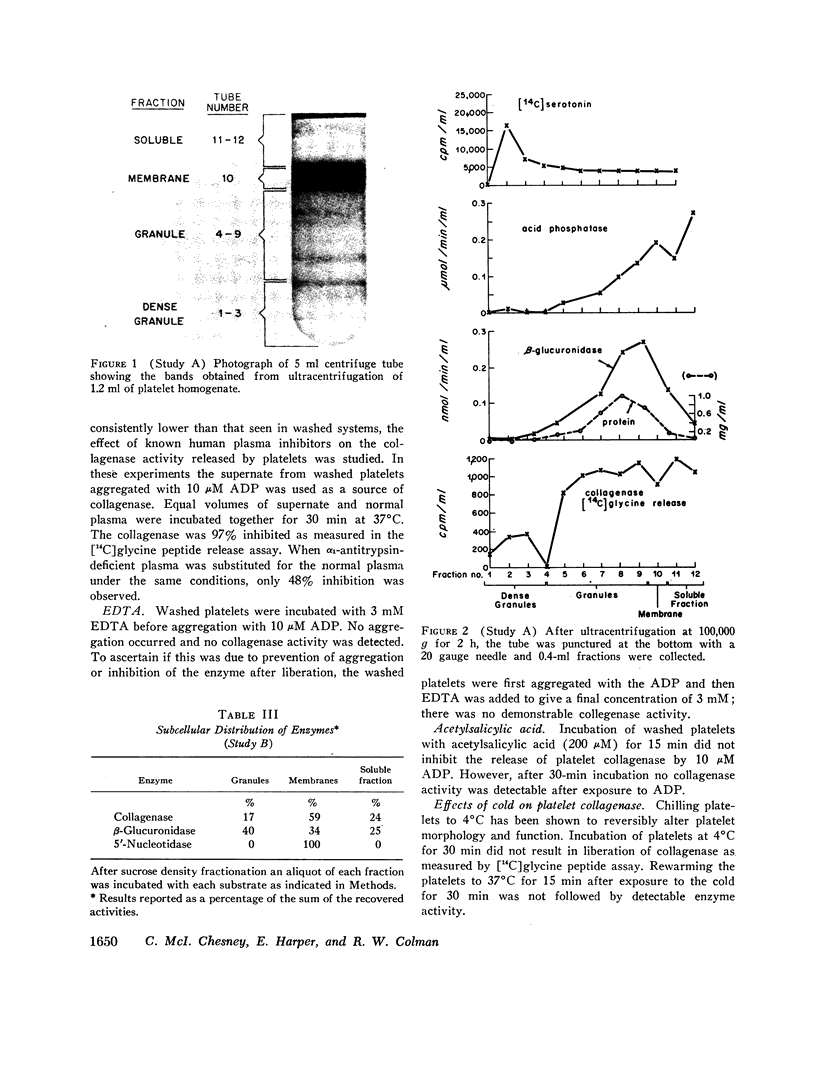
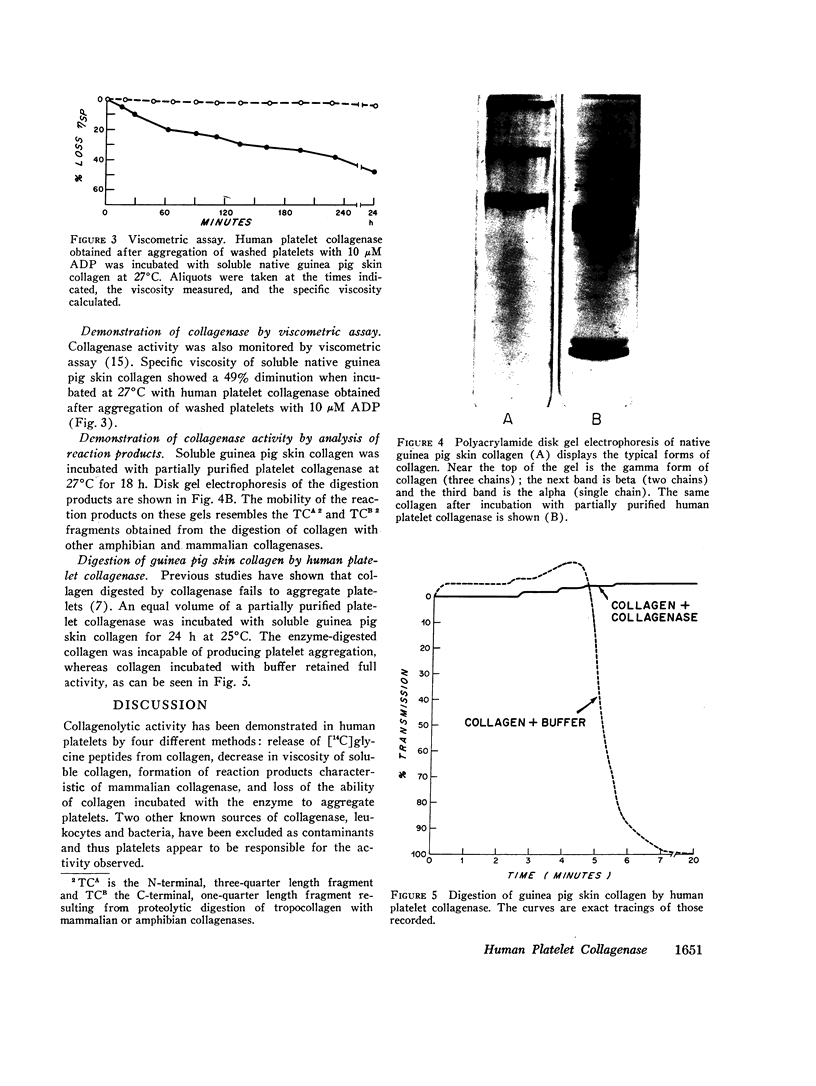
The presence of proteolytic enzymes such as cathepsin and elastase in platelets and the important role of collagen in platelet aggregation suggested that collagenase might be present in platelets. Epinephrine, ADP, and collagen liberate collagenase from platelets in plasma as measured by the hydrolysis of [14C]glycine-labeled collagen fibrils. The collagenase activity appeared in an early phase of platelet aggregation and was not a part of the release reaction. However, only 50% of the total collagenase could be liberated by the aggregating agents used. Sucrose density gradient analysis of platelet homogenates using appropriate sub-cellular markers indicated that collagenase appeared in both the granule and membrane fractions. Gel-filtered platelets failed to show collagenase activity before exposure to aggregating agents but released more collagenolytic activity than was found in platelet-rich plasma. This observation was explained by the finding that collagenolytic activity was inhibited by normal human plasma. One of the inhibitors is α1-antitrypsin as demonstrated by decreased inhibition in plasma from a patient with homozygous α1-antitrypsin deficiency. Platelet collagenase activity could also be demonstrated by its ability to decrease the viscosity of collagen solutions and to produce collagen fragments similar to those produced by other mammalian collagenases on disk gel electrophoresis. The observation that partially purified platelet collagenase could destroy the platelet-aggregating activity of collagen suggests that the enzyme might function in a negative feedback mechanism limiting thrombus formation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. I., Weller C. A., Wassermann H. E. Collagenolytic activity of alkali-burned corneas. Arch Ophthalmol. 1969 Mar;81(3):370–373. doi: 10.1001/archopht.1969.00990010372015. [DOI] [PubMed] [Google Scholar]
- Chesney C. M., Harper E., Colman R. W. Critical role of the carbohydrate side chains of collagen in platelet aggregation. J Clin Invest. 1972 Oct;51(10):2693–2701. doi: 10.1172/JCI107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON T. F., PURDOM M. Serum 5-nucleotidase. J Clin Pathol. 1954 Nov;7(4):341–343. doi: 10.1136/jcp.7.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A. Z., Bloch K. J., Sakai T. Inhibition of human skin collagenase by human serum. J Lab Clin Med. 1970 Feb;75(2):258–263. [PubMed] [Google Scholar]
- Eisen A. Z., Henderson K. O., Jeffrey J. J., Bradshaw R. A. A collagenolytic protease from the hepatopancreas of the fiddler crab, Uca pugilator. Purification and properties. Biochemistry. 1973 Apr 24;12(9):1814–1822. doi: 10.1021/bi00733a024. [DOI] [PubMed] [Google Scholar]
- HARPER E., SEIFTER S., HOSPELHORN V. D. EVIDENCE FOR SUBUNITS IN BACTERIAL COLLAGENASE. Biochem Biophys Res Commun. 1965 Feb 17;18:627–632. doi: 10.1016/0006-291x(65)90802-8. [DOI] [PubMed] [Google Scholar]
- Harbury C. B., Hershgold J. E., Schrier S. L. Requirements for aggregation of washed human platelets suspended in buffered salt solutions. Thromb Diath Haemorrh. 1972 Aug 31;28(1):2–13. [PubMed] [Google Scholar]
- Harper E., Bloch K. J., Gross J. The zymogen of tadpole collagenase. Biochemistry. 1971 Aug 3;10(16):3035–3041. doi: 10.1021/bi00792a008. [DOI] [PubMed] [Google Scholar]
- Harper E., Gross J. Separation of collagenase and peptidase activities of tadpole tissues in culture. Biochim Biophys Acta. 1970 Feb 11;198(2):286–292. doi: 10.1016/0005-2744(70)90061-6. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, DiBona D. R., Krane S. M. Collagenases in human synovial fluid. J Clin Invest. 1969 Nov;48(11):2104–2113. doi: 10.1172/JCI106177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerushalmy Z., Zucker M. B. Some effects of fibrinogen degradation products (FDP) on blood platelets. Thromb Diath Haemorrh. 1966 May 15;15(3):413–419. [PubMed] [Google Scholar]
- Kassell B., Kay J. Zymogens of proteolytic enzymes. Science. 1973 Jun 8;180(4090):1022–1027. doi: 10.1126/science.180.4090.1022. [DOI] [PubMed] [Google Scholar]
- Kocsis J. J., Hernandovich J., Silver M. J., Smith J. B., Ingerman C. Duration of inhibition of platelet prostaglandin formation and aggregation by ingested aspirin or indomethacin. Prostaglandins. 1973 Feb;3(2):141–144. doi: 10.1016/0090-6980(73)90081-6. [DOI] [PubMed] [Google Scholar]
- Kruze D., Wojtecka E. Activation of leucocyte collagenase proenzyme by rheumatoid synovial fluid. Biochim Biophys Acta. 1972 Dec 28;285(2):436–446. doi: 10.1016/0005-2795(72)90330-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Lian J., Burleigh M. C. Role of granulocyte collagenase in collagen degradation. Am J Pathol. 1972 Sep;68(3):565–578. [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Zucker-Franklin D., Safier L. B., Ullman H. L. Studies on human platelet granules and membranes. J Clin Invest. 1966 Jan;45(1):14–28. doi: 10.1172/JCI105318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Harwood J. L., Coleman R., Hawthorne J. N. Characteristics of rat liver phosphatidylinositol kinase and its presence in the plasma membrane. Biochim Biophys Acta. 1967 Dec 5;144(3):649–658. doi: 10.1016/0005-2760(67)90053-7. [DOI] [PubMed] [Google Scholar]
- NAGAI Y., GROSS J., PIEZ K. A. DISC ELECTROPHORESIS OF COLLAGEN COMPONENTS. Ann N Y Acad Sci. 1964 Dec 28;121:494–500. doi: 10.1111/j.1749-6632.1964.tb14221.x. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Weksler B., Ferris B. Increased vascular permeability produced by human platelet granule cationic extract. J Clin Invest. 1970 Feb;49(2):274–281. doi: 10.1172/JCI106237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Robert B., Legrand Y., Pignaud G., Caen J., Robert L. Activité élastinolytique associée aux plaquettes sanguines. Pathol Biol (Paris) 1969 Jun-Jul;17(11):615–622. [PubMed] [Google Scholar]
- Takahashi S. Isolation of a collagenolytic enzyme from Mycobacterium tuberculosis. J Biochem. 1967 Feb;61(2):258–259. doi: 10.1093/oxfordjournals.jbchem.a128538. [DOI] [PubMed] [Google Scholar]
- Tangen O., Berman H. J., Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [PubMed] [Google Scholar]
- Vaes G. Multiple steps in the activation of the inactive precursor of bone collagenase by trypsin. FEBS Lett. 1972 Dec 1;28(2):198–200. doi: 10.1016/0014-5793(72)80711-7. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Coupal C. E. Platelet-dependent generation of chemotactic activity in serum. J Exp Med. 1973 Jun 1;137(6):1419–1430. doi: 10.1084/jem.137.6.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Nachman R. L. Rabbit platelet bactericidal protein. J Exp Med. 1971 Nov 1;134(5):1114–1130. doi: 10.1084/jem.134.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G. Effects of ethylenediamine tetracetic acid (EDTA) on platelet structure. Scand J Haematol. 1968;5(4):241–254. doi: 10.1111/j.1600-0609.1968.tb01743.x. [DOI] [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., LeRoy E. C. Aggregation of platelets by collagen. J Clin Invest. 1968 Dec;47(12):2616–2621. doi: 10.1172/JCI105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. B., Peterson J. Inhibition of adenosine diphosphate-induced secondary aggregation and other platelet functions by acetylsalicylic acid ingestion. Proc Soc Exp Biol Med. 1968 Feb;127(2):547–551. doi: 10.3181/00379727-127-32737. [DOI] [PubMed] [Google Scholar]
- Zucker M. B. Proteolytic inhibitors, contact and other variables in the release reaction of human platelets. Thromb Diath Haemorrh. 1972 Dec 31;28(3):393–407. [PubMed] [Google Scholar]




