Abstract
E-series resolvins are antiinflammatory and pro-resolving lipid mediators derived from the ω-3 polyunsaturated fatty acid eicosapentaenoic acid (EPA) that actively clear inflammation to promote tissue homeostasis. Aspirin, in addition to exerting antithrombotic actions, also triggers the biosynthesis of these specialized pro-resolving mediators. Here, we used metabolomic profiling to investigate the biosynthesis of E-series resolvins with specific chiral chemistry in serum from human subjects and present evidence for new 18S series resolvins. Aspirin increased endogenous formation of 18S-hydroxyeicosapentaenoate (18S-HEPE) compared with 18R-HEPE, a known resolvin precursor. Human recombinant 5-lipoxygenase used both enantiomers as substrates, and recombinant LTA4 hydrolase (LTA4H) converted chiral 5S(6)-epoxide–containing intermediates to resolvin E1 and 18S-resolvin E1 (RvE1 and 18S-RvE1, respectively). 18S-RvE1 bound to the leukocyte GPCRs ChemR23 and BLT1 with increased affinity and potency compared with the R-epimer, but was more rapidly inactivated than RvE1 by dehydrogenase. Like RvE1, 18S-RvE1 enhanced macrophage phagocytosis of zymosan, E. coli, and apoptotic neutrophils and reduced both neutrophil infiltration and proinflammatory cytokines in murine peritonitis. These results demonstrate two parallel stereospecific pathways in the biosynthesis of E-series resolvins, 18R- and 18S-, which are antiinflammatory, pro-resolving, and non-phlogistic and may contribute to the beneficial actions of aspirin and ω-3 polyunsaturated fatty acids.
Introduction
Acute inflammation is a self-limited protective response against invading microbes or tissue injury and is regulated in a timely manner to restore homeostasis (1). The ideal outcome, or resolution, is a highly coordinated active process, and specialized pro-resolving mediators (SPMs) found in the resolution phase actively stimulate the tissue to regain homeostasis (2). Excessive, uncontrolled inflammation is now considered a central component in many chronic diseases, such as cardiovascular diseases (3), metabolic disorders (4), and cancers (5).
In this regard, antiinflammation and pro-resolution pharmacopeia is of utmost interest (6). Aspirin (acetylsalicylic acid [ASA]), as an example, is known to dampen proinflammatory signals and more recently has been demonstrated to jump-start resolution of acute inflammation (7). Acetylation of COX-2 by aspirin transforms its enzyme activity from a cyclooxygenase to a 15-lipoxygenase–like (15-LOX–like) enzyme (8), synthesizing 15R-hydroxyeicosatetraenoate (15R-HETE) instead of PGH2 from arachidonic acid (AA). In addition to aspirin blocking prostaglandin biosynthesis, giving some antiinflammatory properties, 15R-HETE becomes a precursor to potent 15-epi-lipoxins, which are antiinflammatory and pro-resolving mediators generated by transcellular biosynthesis. These aspirin-triggered lipoxins (ATLs) (9) bear R configuration at carbon 15, which becomes epimeric to the original 15-lipoxygenase–initiated lipoxin biosynthesis pathway with carbon 15 of S configuration (10). The ATLs are more resistant to metabolic inactivation than lipoxins (11) and also possess antiinflammatory and pro-resolving actions in a wide range of inflammatory diseases, such as dermal inflammation (12), periodontitis (13), and inflammatory angiogenesis (14).
In addition to AA-derived lipoxins and ATLs, bioactive SPMs are also generated from essential ω-3 (n-3) polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (2). EPA and DHA are important in the human diet, mounting inflammatory responses and their outcomes (reviewed in refs. 15, 16). The SPMs including the resolvin, protectin, and maresin families together constitute a novel genus of endogenous mediators that exert potent antiinflammatory and pro-resolution actions in many experimental diseases (reviewed in ref. 17).
As with epimeric lipoxin biosynthesis from aspirin-treated, COX-2–expressing cells, aspirin-triggered D-series resolvins (AT-RvDs) were also identified (18). With regard to EPA-derived E-series resolvins, the stereochemical structure of endogenous RvE1 was assigned and confirmed via total organic synthesis (19), and the E-series resolvin biosynthesis mechanism has been partially elucidated (20). The contribution of aspirin in E-series resolvin biosynthesis and identity of downstream enzymes generating EPA-derived (E-series) resolvins in a stereospecific and regiospecific manner remain of interest.
Here, we report a comprehensive biosynthetic pathway of novel E-series resolvins. Using chiral lipid-mediator metabolomics, we analyzed samples from healthy human subjects taking aspirin and EPA and identified 18S-hydroxyeicosapentaenoate (18S-HEPE) as a precursor to E series resolvins. This 18S-HEPE was further converted to epimeric RvE1 and RvE2 by human recombinant 5-LOX and LTA4 hydrolase (LTA4H), known as proinflammatory LTB4-synthesizing enzymes. To investigate the molecular mechanism of action for 18S-E series resolvins, we compared ligand-receptor interactions and metabolic inactivation of 18S-RvE1 with RvE1. Moreover, the pathophysiologic actions of 18S-E series resolvins were assessed in E. coli murine peritonitis and in vitro with phagocytosis of E. coli and apoptotic polymorphonuclear leukocytes (PMNs) by human macrophages.
Results
Chiral lipidomics of endogenous E-series resolvin precursors and contribution of COX-2.
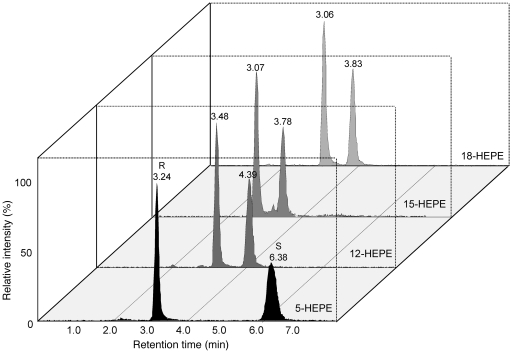
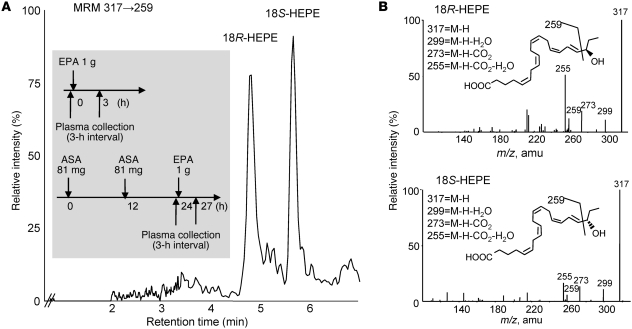
In order to elucidate the biosynthesis and functions of new E-series resolvins, we designed chiral lipidomic profiling of the resolvin precursors, which were generated in vivo and biogenically. Reverse-phase-based chiral HPLC-MS/MS lipidomics achieved baseline separation of all tested stereoisomer pairs (5-, 12-, 15-, and 18R/S-HEPEs) derived from EPA (Figure 1). In order to profile E-series resolvin precursor production in humans, we assessed sera from healthy human peripheral blood (Figure 2A, inset) by both conventional (for high-sensitivity quantitation) and chiral LC-MS/MS (to determine enantiomer ratios). Chiral lipidomic profiling produced a baseline separation of 18R-HEPE and 18S-HEPE from human sera (Figure 2A). The two enantiomers had indistinguishable tandem mass spectra, with fragments at m/z 317 (M-H), 299 (M-H-H2O), 273 (M-H-CO2), and 255 (M-H-CO2-H2O), as well as the diagnostic ion at m/z 291 (Figure 2B).
Figure 1. Chiral lipidomic separation and quantitation of isobaric monohydroxy-EPEs.
In order to detect and quantify each positional isomer without ambiguity, an MRM method was established. Signature daughter ions for each standard HEPE (parent m/z, 317) were as follows: 18-HEPE, 259; 15-HEPE, 219; 12-HEPE, 179; 5-HEPE, 115. For each enantiomer pair, the R isomer was eluted before S isomers. Each signature ion for each species was unique; only two (R and S isomers) peaks are present on each extracted ion chromatogram.
Figure 2. Aspirin enhances endogenous production of novel E-series resolvin precursors.
(A) Chiral HPLC-MS/MS analysis of E-series resolvin precursor stereoisomers from human serum. Healthy human sera, treated with or without aspirin, were collected (see inset) and 18S- and 18R-HEPE were separated by reverse-phase chiral column without derivatization and quantified by MRM, specific transition at 317→259. Results are from human subjects given aspirin; representative chromatogram of n = 3. (B) Tandem mass spectra and structural assignment of 18R-HEPE and 18S-HEPE from human serum.
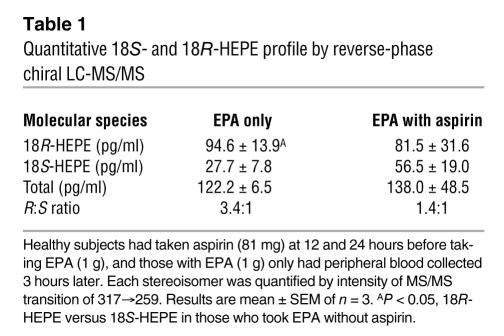
Without EPA supplementation and aspirin, baseline serum 18-HEPE levels remained low (26.4 ± 5.0 pg/ml) for healthy subjects. When 1 g of EPA was ingested, sera collected 3 hours later showed elevated levels of 18-HEPE. Quantitative lipidomic profiling using chiral HPLC indicated that the 18R-HEPE isomer was dominant to 18S-HEPE in all human plasma samples when only EPA was taken. In contrast, sera of human subjects who took aspirin before EPA administration had more 18S-HEPE (27.7 ± 7.8 pg/ml with EPA vs. 56.5 ± 19.0 pg/ml with EPA and aspirin; see Table 1). These results suggested that aspirin might promote 18S-HEPE production as well as 18R-HEPE from ingested EPA. Hence, we tested whether human recombinant COX-2 was involved in this process. Of interest, aspirin-treated human COX-2 preferentially synthesized 15R-HEPE and 18S-HEPE, which were identified and quantified using chiral LC-MS/MS (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI42545DS1). These results were consistent with those of murine air pouch exudate lipidomic analysis, where aspirin treatment both increased total 18-HEPE biosynthesis (~6 fold) and shifted the R/S ratio in the direction of S (R/S ratio of 1.5:1 to 1:1; Supplemental Figure 1B). Together, these indicate that aspirin-treated COX-2 is responsible for production of both 18S- and 18R-HEPE from EPA.
Table 1 .
Quantitative 18S- and 18R-HEPE profile by reverse-phase chiral LC-MS/MS
Biosynthesis of 18S E-series resolvins: role of 5-LOX.
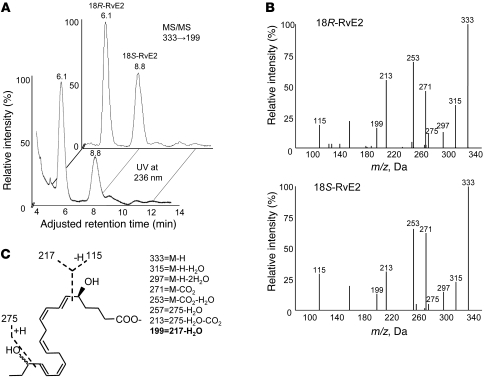
Next, we assessed potential substrate preferences with 18R- and 18S-HEPE for conversion to E-series resolvin. The biosynthesis experiments involved competitive conversion of 18-HEPE with human recombinant 5-LOX. For assessment of the potential for stereospecific preference, racemic mixtures of 18R- and 18S-HEPE were incubated with 5-LOX in the presence of required 5-LOX activators (20), followed by reduction of 5S-hydroperoxide to RvE2 and analysis by both conventional and chiral LC-MS/MS. Two 5-LOX products from racemic 18-HEPE were identified and assigned as 5,18-dihydroxyEPE by UV chromatogram at 236 nm and selective ion monitoring at m/z 333→199 (Figure 3). Both peaks (retention time, 6.1 and 8.8 minutes) had indistinguishable fragments, at m/z 333 (M-H), 315 (M-H-H2O), 297 (M-H-2H2O), 271 (M-H-CO2), and 253 (M-H-2H2O-CO2), along with signature ions at m/z 275, 257 (275-H2O) and 199 (217-H2O). Stereochemistry at carbon 18 was further confirmed by matching with biogenic RvE2 synthesized from stereochemically pure 18R- or 18S-HEPE. These results directly demonstrate that 18S-HEPE is utilized as a substrate of isolated human 5-LOX, which produces a hydroperoxy intermediate in 18S-resolvin biosynthesis.
Figure 3. Human 5-LOX converts both 18R- and 18S-HEPE to direct precursors of RvE2.
(A) Chiral separation of 18S- and 18R-RvE2 after 5-LOX incubation and reduction. Diastereomeric mixture of RvE2 was isolated by conventional RP-HPLC separation and further resolved by chiral HPLC. (B) Tandem mass spectra of 18R-RvE2 and 18S-RvE2 and (C) their MS/MS assignment. 18R- and 18S-RvE2 had indistinguishable MS/MS spectra.
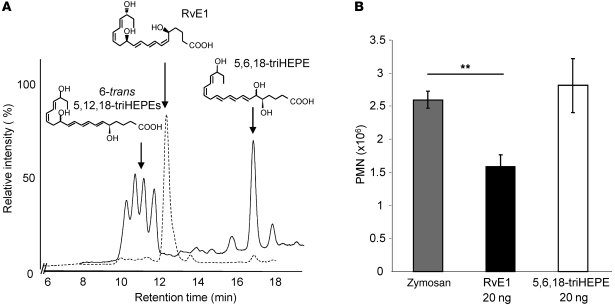
Human 5-LOX can further convert the 5S-hydroperoxy,18-hydroxyeicosapentaenoate intermediate to a 5S(6)-epoxide–containing product (20), which becomes the direct upstream precursor of RvE1. Hydrolysis of this allylic triene epoxide is the key step for RvE1 biosynthesis in order to form the 6Z,8E,10E-triene conjugation present in RvE1 and is required for its bioactivity (19). Without enzymatic activity, this 5(6)-epoxide intermediate was converted not to RvE1 but rather to either 6E,8E,10E-isomers or 5,6,18-trihydroxyeicosapentaenoate by nonenzymatic hydrolysis (20) (Figure 4A). None of these products (solid line, quartet around 11 minutes and single peak at 16.8 minutes) matched the retention time of RvE1 (dotted line, single peak at 12.1 minutes).
Figure 4. Correct stereochemistry of RvE1 is essential for its bioactivity.
(A) RvE1 matching study with synthetic standard. Nonenzymatic hydrolysis products such as 6-trans 5,12,18-triHEPE or 5,6,18-triHEPE (peaks shown with solid line) have retention times different from the synthetic RvE1-spiked run (peak shown with dashed line). (B) Direct comparison of RvE1 and 5,6,18-triHEPE in zymosan-induced 2-hour murine peritonitis model. Equal doses (20 ng/mouse) of synthetic RvE1 and 5,6,18-triHEPE were administered via tail vein immediately before intraperitoneal injection of zymosan A. The cell number in the peritoneum was enumerated, and PMN numbers were compared after differential counting. Results are mean ± SEM. **P < 0.05 compared with vehicle.
Contribution of LTA4H in RvE biosynthesis: bioactions of nonenzymatic hydrolysis products.
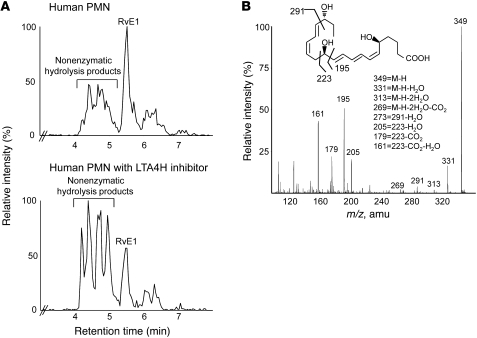
The specific stereochemical structure of RvE1 is crucial for both ligand-receptor interactions and bioaction. Synthetic isomers of RvE1 have different chromatographic properties, and Δ6,14-trans RvE1 is significantly less active than RvE1 (19). We assessed the bioactivity of other nonenzymatic hydrolysis products identified from these incubations, such as 5,6,18-triHEPE. When directly compared with RvE1 at the same 20-ng/mouse doses in murine peritonitis, the isomer 5,6,18-triHEPE did not significantly reduce neutrophil infiltration, whereas RvE1 reduced neutrophilic infiltration (39.9% ± 5.1%, Figure 4B). These results indicate that the specific triene structure is crucial for RvE1 bioactions. Thus, the conjugated addition-type hydrolysis of the 5S(6)-epoxy-6Z,8E,10E-triene to 5S,12R-dihydroxy-6Z,8E,10E-triene is a key step of RvE1 biosynthesis. Because of the structural similarity between the 5S(6)-epoxy-resolvin intermediate and LTA4, we hypothesized that a specific enzymatic reaction, namely a hydrolase, is required, as in LTB4 biosynthesis (21). Thus, we next assessed the role and contribution of LTA4H in the biosynthesis of RvE1. This was addressed in several ways: First, since human PMNs biosynthesize RvE1 and RvE2 from 18-HEPE (20), RvE1 production by PMNs was tested in the presence of the LTA4H inhibitor bestatin (22). When incubated with bestatin, RvE1 biosynthesis by isolated human PMNs was decreased by 67.3% ± 6.2% (Figure 5A). Of interest, a combinatorial incubation product of two recombinant enzymes, 5-LOX and LTA4H, was assigned as RvE1 by matching with synthetic standard. Retention time and diagnostic ions of m/z 291, 205, and 195 were confirmed from combinatorial incubation products (Figure 5B). In contrast, incubation with only 5-LOX generated nearly undetectable amounts of RvE1. Instead, 6-trans–containing isomers were dominant. This RvE1 biosynthesis by enzyme combination was almost completely blocked in the presence of bestatin in the incubation mixture. These results indicate that LTA4H contributes to RvE1 biosynthesis.
Figure 5. LTA4H in RvE1 biosynthesis.
(A) Activated human PMNs were incubated with 18-HEPE, in the presence or absence of LTA4H inhibitor bestatin, and representative LC-MS/MS chromatograms, transition of 349→205 monitored, are shown. Treating PMNs with hydrolase inhibitor (250 μM bestatin) reduced RvE1 synthesis by 67.3% ± 6.2% (mean ± SEM, n = 3; P < 0.05 compared with control). (B) LC-MS/MS spectrum of RvE1 from 5-LOX (25 U) and LTA4H (2 μg) combinatorial incubation. Retention time of this product matched with synthetic RvE1. Statistically significant amounts of RvE1 were not produced with 5-LOX alone or with bestatin-treated combinatorial incubations.
Ligand-receptor interaction and metabolic inactivation of 18S-RvE1 with RvE1 pathway.
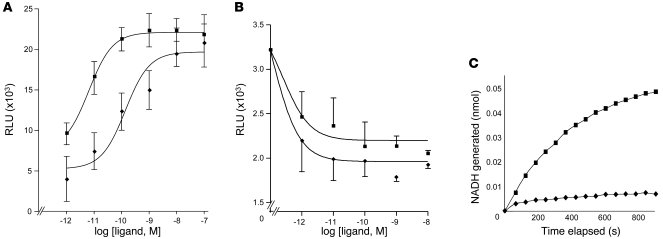
We next assessed the actions and inactivation of both resolvins. RvE1 interacts with at least two different GPCRs present on leukocytes, ChemR23 (19) and BLT1 (23), so the binding affinity of RvE1 and the 18S-epimer to the recombinant GPCR expressed in CHO cells was directly compared. Using a β-arrestin overexpression system to specifically examine targeted GPCRs, we found that 18S-RvE1 displayed an EC50 of 6.33 × 10–12 M derived from fitted dose-response curves, which was lower than that obtained with RvE1 (1.37 × 10–10 M, Figure 6A) in ChemR23-overexpressing cells. 18S-RvE1 also antagonized LTB4-mediated BLT1 activation with higher potency and efficacy than RvE1 (Figure 6B). These results suggest that 18S-RvE1 can share the same site(s) of action as RvE1 with apparently higher affinity in vitro. In contrast to higher efficacy at the molecular level, 18S-RvE1 was more rapidly converted to inactive 18-oxo-RvE1 by NAD-dependent dehydrogenase compared with RvE1 (Figure 6C).
Figure 6. Molecular pathways of 18S-RvE1 actions: ligand-receptor interaction and metabolic inactivation.
(A) ChemR23 activation. Increasing doses of RvE1 (diamonds) or 18S-RvE1 (squares) were incubated with the ChemR23-overexpressing reporter system, followed by β-gal substrate incubation. Luminescence was measured to obtain the RvE1 EC50, 1.37 × 10–10 M, and 18S-RvE1 EC50, 6.33 × 10–12 M, from fitted dose-response curves. (B) BLT1 antagonism. BLT1-overexpressing cells were incubated with different concentrations of 18S-RvE1 or RvE1, followed by 30 nM LTB4, and further incubated for 30 minutes. Results are mean ± SEM; n ≥ 3. P < 0.05, RvE1 versus 18S-RvE1. (C) Comparison between RvE1 and 18S-RvE1 by 15-PGDH–mediated oxidation (diamonds: RvE1, squares: 18S-RvE1) addressed in the presence of NAD+ as a cofactor. NADH was monitored as a readout of dehydrogenation. Results shown are representative of n = 3 with similar trends.
Bioactions of 18S-E series resolvins.
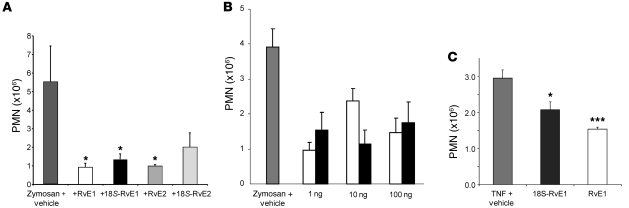
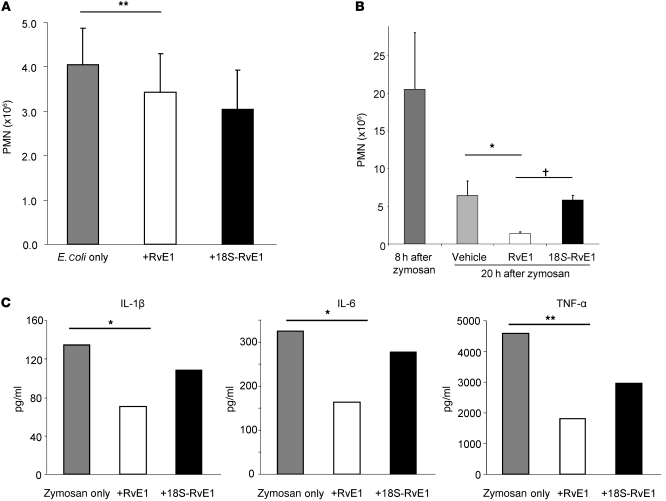
In order to fully understand the bioactions of 18S-E series resolvins, we compared chemically and biogenically prepared 18S-RvE1, RvE1, 18S-RvE2, and RvE2. In order to assess the in vivo actions of 18S E-series resolvins, we carried out direct comparisons between 18R- and 18S-isomers in zymosan-initiated acute murine peritonitis. 18S-RvE1 reduced PMN infiltration at a potency similar to that of RvE1 and RvE2, whereas 18S-RvE2 showed only a trend toward reducing neutrophil infiltration (Figure 7A).
Figure 7. Direct comparison of the antiinflammatory actions of 18S and 18R E-series resolvins.
(A) Direct comparison of action at equal doses of resolvins (20 ng/mouse) in zymosan-induced murine peritonitis at 2 hours. (B) Comparison of 18S-RvE1 (black bars) and RvE1 (white bars) action at 4 hours. (C) 18S-RvE1 decreased PMN infiltration in the TNF-α–induced murine dorsal air pouch. Results are mean ± SEM; n ≥ 3. *P < 0.05, ***P < 0.001 compared with vehicle-treated mice.
We further confirmed bioactions of 18S-RvE1 in two different settings: First, using doses from 1 to 100 ng, 18S-RvE1 was compared with RvE1 in murine peritonitis. At all doses, the 18S-epimer showed efficacy similar to that of RvE1 (Figure 7B). Second, the murine dorsal air pouch was also examined to assess the counterregulation of TNF-α–mediated neutrophil infiltration in vivo; at the same 20-ng doses, 18S-RvE1 displayed potency similar to that of RvE1 in this system (Figure 7C, 30% vs. 48% decrease, P > 0.05 between these isomers).
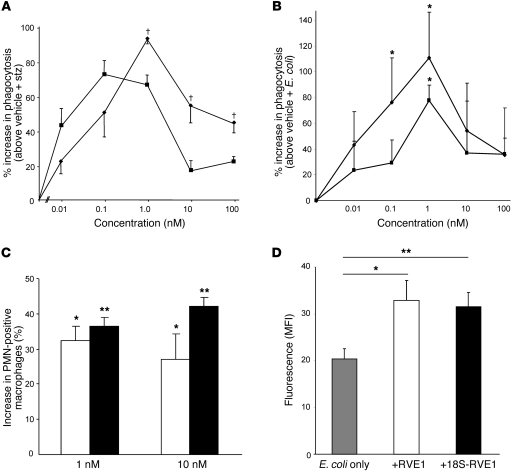
In addition to reducing PMNs, RvE1 also stimulates resolution by enhancing phagocytosis and clearance of invaded microbes, apoptotic neutrophils, and other cellular debris (2, 7, 24). To assess these macrophage-directed pro-resolving bioactions of 18S resolvins, 18S-RvE1 was directly compared with RvE1-enhanced zymosan and live E. coli phagocytosis using murine resident macrophages. The 18S-RvE1 gave a bell-shaped dose-response curve similar to RvE1 (Figure 8A). At lower concentrations, 18S-RvE1 was essentially equipotent to RvE1; at concentrations greater than 1 nM, RvE1 was more potent than 18S-RvE1. Similar results were obtained for the phagocytosis of live E. coli: both RvE1 and 18S-RvE1 enhanced phagocytosis, showing maximal increases at 1 nM (Figure 8B). In addition, 18S-RvE1 and RvE1 enhanced phagocytosis of apoptotic PMNs by human macrophages (Figure 8C).
Figure 8. Pro-resolving actions of 18S-RvE1.
Enhancement of (A) zymosan particle and (B) live E. coli phagocytosis: Increasing doses of RvE1 (diamonds) and 18S-RvE1 (squares) were incubated with murine macrophages, followed by addition of FITC-labeled zymosan or fluorescent E. coli. Only particles or cells completely phagocytosed by macrophages remained fluorescent and were counted. (C) Enhancement of apoptotic PMN phagocytosis by human macrophages. Two doses of RvE1 (white bars) or 18S-RvE1 (black bars) were tested. Results are mean ± SEM; n ≥ 3. *P < 0.05, **P < 0.01 compared with vehicle treatment; †P < 0.05, RvE1 versus 18S-RvE1. (D) Enhancement of microbial clearance by peritoneal resident macrophages. 18S-RvE1 or RvE1 was intraperitoneally administered immediately (100 ng) before fluorescent E. coli injection and each peritoneum lavaged 30 minutes later to measure phagocytosed bacteria by macrophages. Results are mean ± SEM; n = 6–8 for each treatment. *P < 0.05, **P < 0.01 compared with vehicle treatment.
These results were supported by in vivo results with E. coli–initiated peritonitis. Enhancement of bacterial clearance was tested at two different time points and with two routes of administration during the inflammatory time course. First, both RvE1 and 18S-RvE1 directly delivered to peritoneum enhanced phagocytosis of E. coli by resident macrophages (18S-RvE1: 61.4% vs. RvE1: 55.0%, Figure 8D). When RvE1 or 18S-RvE1 was given i.v. 3 hours after peritoneal injection of E. coli, both products reduced neutrophilic infiltration in the resolution phase (Figure 9A), with only RvE1 producing a significant reduction. This difference in activity between RvE1 and 18S-RvE1 was more apparent at longer time points in resolution; namely, when RvE1 was given at the maximal time point of PMN infiltration (i.e., 8 hours after zymosan injection), further decreases in PMN numbers were found 12 hours later. In contrast, 18S-RvE1 only modestly expedited (~10%, P > 0.05) removal of PMNs in the peritoneum, whereas RvE1 significantly reduced (~78%, P < 0.01) the number of remaining PMNs (Figure 9B). RvE1 also reduced proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, produced by zymosan-stimulated murine resident macrophages (Figure 9C). With E. coli peritonitis, RvE1 regulated inflammation and reduced proinflammatory cytokines in vitro and in vivo (Supplemental Figure 2).
Figure 9. Transient bioactions of 18S-RvE1.
(A) Regulation of PMN infiltration during E. coli peritonitis by 18S-RvE1 and RvE1. 18S-RvE1 or RvE1 (100 ng) was administered via tail vein 3 hours after E. coli injection to peritoneum. **P < 0.01 compared with vehicle-treated. (B) Enhancement of neutrophil removal from site of inflammation: Vehicle, 100 ng RvE1, or 18S-RvE1 was injected intraperitoneally at 8 hours after zymosan A injection, and PMNs in the peritoneum were enumerated 12 hours later (20 hours after zymosan injection). Each point is the average from 3 mice with the same treatment on the same day. Results in A and B are mean ± SEM. †P < 0.05, 18S-RvE1 versus RvE1; *P < 0.05 compared with vehicle-treated. (C) Regulation of zymosan-stimulated mouse macrophage cytokine production by RvE1 and 18S-RvE1. Cell supernatants were collected and centrifuged for multiplex cytokine quantitation. Results are averages of duplicated measurements; *P < 0.05, **P < 0.01 compared with cells exposed to zymosan alone.
Discussion
SPMs, such as lipoxins, resolvins, and protectins, have been identified in inflammatory exudates during the resolution phase of self-limited acute responses (refs. 18, 25 and reviewed in refs. 2, 7). Temporal regulation from proinflammatory mediators to SPMs, or “class-switching,” was originally identified in an animal model (26) and is now recognized in humans and in diseases associated with inflammation resolution (27–29).
Along with having distinct biosynthetic pathways and actions, SPMs form a novel molecular genus derived from PUFAs, especially n-3 PUFA such as DHA and EPA (2). In this regard, the production and actions of SPMs can explain some of the beneficial actions of fish oils in a wide range of inflammatory diseases such as cardiovascular diseases (30), as shown in some human clinical studies (31, 32).
Aspirin, the first chemically synthesized drug still widely used today, is now also gaining interest for its ability to trigger generation of SPMs. For example, ATL is generated in vivo from AA in healthy human subjects taking low-dose aspirin (33). As demonstrated in acute inflammation models (34, 35), ATL is at least partially responsible for anti-neutrophilic and subsequent antiinflammatory, pro-resolving actions in humans of low-dose (75–81 mg) aspirin (29, 33). These anti-neutrophilic recruitment and pro-resolving doses are significantly lower than the classical and generally used anti-rheumatic and anti-inflammatory doses (325–650 mg range) (36). Aspirin-treated human cyclooxygenase also generates precursors of epimeric resolvins from ω-3 fatty acids, which can explain some beneficial actions of ω-3 PUFAs in some of the inflammation-associated diseases. In view of the contributions of both ω-3 PUFAs and aspirin to SPM generation, we present evidence herein for: (a) an aspirin-triggered precursor 18S-HEPE derived from EPA; (b) parallel biosynthetic pathways of E-series resolvins; and (c) the bioactions of epimeric E-series resolvins.
Endogenous lipid mediators are highly stereospecific in their formation and bioactivities (37); bioactions and metabolic inactivation of epimeric SPMs, for example LXA4 and 15-epi-LXA4, are also distinct (11). Therefore, stereospecific and high-sensitivity lipidomic profiling is indispensable in order to study aspirin-triggered resolvin biosynthesis. We set up a derivatization-free procedure using reverse-phase chiral HPLC compatible with MS/MS, which enables stereoselective, high-sensitivity quantitation of SPM precursors from biological samples. 18S- and 18R-HEPE, precursors for E-series resolvins, gave essentially baseline separation and quantitation along with other positional HEPE isomers demonstrated in Figure 1.
When compared with EPA alone, more 18S-HEPE (56.5 pg/ml or 178 pM, compared with 27.7 pg/ml or 87 pM in the EPA-only group) was found in those taking aspirin and EPA (Table 1). EPA is present as a free carboxylic acid form in human blood as early as 3 hours after oral administration (38), and circulating n-3 PUFAs rapidly appear at the site of inflammation in murine peritonitis via protein extravasation and consequential edema formation to serve as resolvin precursors (39). Subsequent experiments with isolated recombinant enzyme confirmed that acetylated COX-2 can produce 18S-HEPE from EPA as one of the major products (Supplemental Figure 1). Expression of human COX-2 is very specific in certain tissues at basal level, and it is widely expressed in human endothelium (40) and human brain (41). During its absorption and distribution, orally taken aspirin can interact and acetylate endothelial COX-2, blocking prostacyclin biosynthesis (42, 43). Considering the large surface area of the human vasculature (~1,000 m2; whole body estimate) (44), the EPA present in blood can be in contact with endothelial cells and converted to 18S-HEPE by acetylated COX-2 for further biosynthesis to 18S E-series resolvins via transcellular mechanisms with leukocytes (19, 45). Of interest, hypoxic human microvessel endothelial cells extensively take up and convert EPA to HEPE (25, 45).
E-series resolvins are generated during the resolution phase of acute inflammation, with leukocyte 5-lipoxygenase playing a pivotal temporal role in the endogenous pathway of these molecules (46). Identification of the precursors of E-series resolvins in human serum was further confirmed by elucidating downstream transformation of precursors to bioactive RvE1 and RvE2. These results indicate that both 18R- and 18S-HEPE are substrates for human 5-LOX, which confirms that 18S-HEPE is not only present in vivo but also transformed to bioactive resolvins. Formation of 5(6)-epoxide–containing intermediates from the 5-hydroperoxy precursors is also mediated by 5-LOX, which is similar to LTA4 production in leukotriene biosynthesis (21). Stereospecific hydrolysis to cis, trans, trans-triene requires activity of enzymes subsequent to the 5-LOX step, which is also the case with LTB4 (47). Human PMNs can biosynthesize RvE1 (45). Importantly, incubation of human 5-LOX with 18-HEPE produces only 5,12,18-triHEPEs (likely to be 6-trans) and 5,6,18-triHEPEs (vicinal diols) from 5(6)-epoxide intermediates (Figure 4A). Since none of these products matched with synthetic resolvin E1, nor were they as bioactive as RvE1 in vivo (Figure 4B), these results indicate that an additional leukocyte enzyme is required to produce RvE1.
Based on structural similarity between LTA4 and the 5(6)-epoxide intermediate, further experiments were designed to assess whether LTA4H contributes to human PMN biosynthesis of RvE1, and whether RvE1 biosynthesis can be reconstituted by combinatorial incubation of 5-LOX and LTA4H. In incubations containing recombinant human LTA4H, RvE1 was indeed produced. In this regard, LTA4H (48) is described as a “gatekeeper of LTB4 synthesis” (49); hence, it has been widely studied as a potential target of anti-LTB4 therapies. These results suggest that LTA4H not only is an important enzyme in the LTB4 biosynthesis pathway along with 5-LOX, but also serves a pivotal role in the production of antiinflammatory and pro-resolving mediators, namely, E-series resolvins. The levels of these local mediators thus depend on the tissue environment and substrate (e.g., AA and 18-HEPE) availability.
In order to confirm whether 18S-RvE1 also shares the same molecular pathway with RvE1, we investigated receptor-ligand interaction and metabolic inactivation. 18S-RvE1 transduced downstream signals via the GPCR ChemR23 more efficaciously (pEC50, 9.86 for RvE1 compared with pEC50, 11.20 for 18S-RvE1). Here too, 18S-RvE1 antagonized LTB4-mediated BLT1 activation with better potency than RvE1 (Figure 6B). Of interest, 18S-RvE1 was more rapidly converted to 18-oxo-RvE1 by NAD-dependent dehydrogenase than RvE1 (Figure 6C). Therefore, 18S-RvE1 may have a lower effective concentration in vivo because of greater rapid local metabolic inactivation, which can be offset by its higher ligand-receptor efficacy. This high efficacy, namely low pM of 18S-RvE1, supports the importance of the increase in 18S-HEPE levels with EPA plus aspirin (see Table 1). Considering that only a portion of 18S-HEPE could be transformed to 18S E-series resolvins, this value appears to be a biologically relevant increase that translates into the functional impact of E-series resolvins.
We assessed antiinflammatory and pro-resolving bioactions of 18S-RvE1 and RvE1 in several in vitro systems and experimental murine diseases. When directly compared at equimolar doses, 18S-RvE1 blocked PMN infiltration to the same extent as RvE1 in two different inflammation models, acute peritonitis and TNF-α–mediated PMN infiltration (Figure 7). The 18S-RvE1 also enhanced macrophage phagocytosis of zymosan particles as well as increasing phagocytosis of live E. coli (Figure 8, A and B). Both 18S-RvE1 and RvE1 gave bell-shaped dose-response curves with maximum enhancement at approximately 1 nM. These pro-resolving actions were further supported by results obtained for the uptake of apoptotic PMNs by human macrophages (Figure 8C).
E. coli–initiated murine peritonitis is considered a pathophysiologically relevant infection/inflammation. 18S-RvE1 and RvE1 were each antiinflammatory and also enhanced phagocytosis and bacterial clearance; both resolvins expedited uptake of live bacteria by resident macrophages (Figure 8D) and reduced PMNs (Figure 9A).
18S-RvE1 only modestly enhanced removal of neutrophils at the apex of zymosan-induced PMN infiltration, whereas RvE1 significantly reduced PMN numbers (Figure 9B). We observed this striking difference among the tested in vivo and in vitro systems, which likely reflects physiological stability and local metabolism, in that 18S-RvE1 is more rapidly metabolized and thus inactivated by dehydrogenation than RvE1 (Figure 6C). This dehydrogenase is highly expressed in monocytes/macrophages of the peritoneum (50), and increased stability of R-isomers against enzymatic inactivation mechanisms such as 15-PGDH–mediated oxidation is in line with the stereoselectivity and results obtained for lipoxins and other resolvins (11, 51). Hence, the 18S-RvE1 can be metabolically inactivated when exposed to cultured primary macrophages or in vivo for an extended period. Therefore, although 18S-RvE1 and RvE1 share the same signaling pathways via ChemR23 and BLT1 and inactivation by dehydrogenase, 18S-RvE1 can act in more transient fashion compared with RvE1.
These antiinflammatory, antimicrobial, and pro-resolving actions of 18S-RvE1 and RvE1 appear to be related to the regulation of intracellular signaling pathways. Both E-series and D-series resolvins control cytokine expression in a zymosan-induced peritonitis and cecal ligation and puncture model in mice that causes polymicrobial sepsis (24, 52, 53). These actions of resolvins were further supported by results of in vitro and in vivo studies of the regulation of cytokine production by E series resolvins, wherein RvE1 reduced TNF-α, IL-1β, and IL-6, demonstrating its non-phlogistic actions during inflammation resolution, increasing microbial containment and clearance.
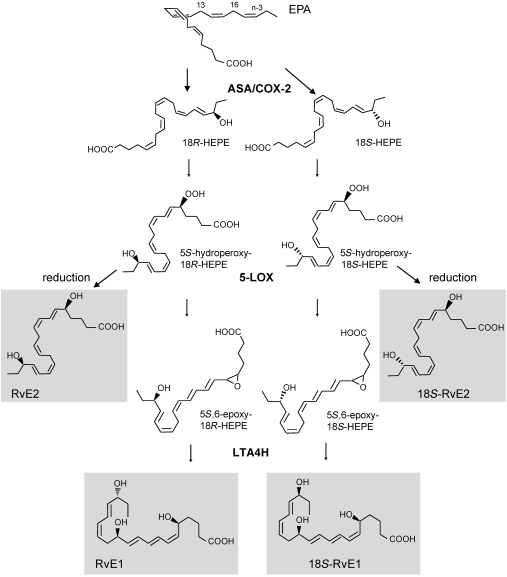
Taken together, these results provide evidence for dual stereoselective biosynthetic pathways in the production of E-series resolvins, namely 18R- and 18S-RvE1 and RvE2. EPA is transformed to E-series resolvins by two separate routes (Figure 10); the precursor 18S-HEPE described herein is also a substrate for 5-LOX, the product of which can be reduced to 18S-RvE2 or further converted by hydrolysis to 18S-RvE1. LTA4H function is critical in the enzymatic hydrolysis of 5S(6)-epoxide–containing RvE1 intermediates, catalyzing the formation of a conjugated triene unit in a cis, trans, trans-triene present in both RvE1 and 18S-RvE1. Here, we found that the 18S-resolvins are a class of bioactive epimers of E-series resolvins, likely with tighter regulatory mechanisms for in vivo production and their local metabolic inactivation. These findings also suggest a potential beneficial impact of combined therapy with low-dose aspirin and n-3 PUFAs that could be relevant in human health and merits future clinical evaluation.
Figure 10. Dual biosynthetic pathways of 18S and 18R E-series resolvins.
18R- and 18S-HEPE are endogenously generated in a stereoselective manner by enzymes such as aspirin-treated COX-2 and subsequently converted to epimeric E-series resolvins by a shared biosynthetic route. The stereochemistry of the epoxide intermediate shown is tentative, and those of RvE1, RvE2, and their 18S forms are established. See text for details.
Methods
Materials.
The following reagents and columns were used: fish oil capsules (generic brand); recombinant human COX-2 and LTA4H and lipid mediators for enzymatic incubation and mass spectrometry standards from Cayman Chemical; hematin, phenol, and other chemicals from Sigma-Aldrich; solid-phase extraction (SPE) columns from Waters; Eclipse Plus C18, Chiralpak AD-RH, and Luna C18(2) columns from Agilent, Chiral Technology, and Phenomenex, respectively.
Blood collection.
Peripheral blood from healthy human donors who declined medication for a minimum of 2 weeks was used (protocol 88-02642 approved by the Partners Human Research Committee, Boston, Massachusetts, USA; written informed consent was obtained from the donors). Venous blood (10 ml) was collected from each donor before and 3 hours after they took fish oil capsules (containing 1 g EPA). This EPA dose was used in the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) trial previously (19, 31). For aspirin experiments, each volunteer that took aspirin (81 mg, twice at 24 hours and 12 hours before) donated 10 ml blood, then immediately took a fish oil capsule and donated a second 10 ml of blood 3 hours later. Collected blood samples were left to clot in 10-ml BD Vacutainers (catalog 367820, 16 × 100 mm). Serum was separated by centrifugation and 2 ml methanol/acetonitrile containing 1 ng 5S-d8-HETE added as an internal standard. Samples were stored at –20°C for approximately 2 hours to precipitate serum proteins and preserved at –80°C until extraction. Upon extraction, precipitated protein was separated by centrifugation (2,000 g, 15 minutes) and supernatant was prepared for extraction.
Sample extraction.
All samples for LC-MS/MS analysis were extracted with SPE columns. Briefly, columns were equilibrated with 1× column volume of methanol and 2× volume ddH2O. Sample supernatants were diluted with 10× volume of ddH2O, acidified (to pH ~3.5), and immediately loaded onto an SPE column. After loading, columns were washed with 1× volume of neutral ddH2O and hexane. Samples were eluted with 1.5× volume methyl formate and dried by Speedvac or nitrogen stream. Dried samples were resuspended in methanol/water for LC-MS/MS analysis.
Mediator lipidomics/chiral lipidomics.
Extracted samples were analyzed by a LC-UV-MS/MS system (QTrap 3200, Applied Biosystems) equipped with an Agilent HP1100 binary pump and diode-array detector (DAD). For conventional mediator lipidomics, an Agilent Eclipse Plus C18 column (50 mm × 4.6 mm × 1.8 μm) was used with a gradient of methanol/water/acetic acid of 60:40:0.01 (v/v/v) to 100:0:0.01 at 0.4-ml/min flow rate. For chiral lipidomic analysis, a Chiralpak AD-RH column (150 mm × 2.1 mm × 5 μm) was used with a gradient of acetonitrile/water/acetic acid of 70:30:0.05 (v/v/v) to 100:0:0.05 at 0.2-ml/min flow rate, or with isocratic methanol/water/acetic acid 95:5:0.01 (v/v/v) at 0.15 ml/min. To monitor isobaric monoHEPE, the multiple reaction monitoring (MRM) method was developed with signature ion fragments for each molecule.
COX-2 product profile.
Ten units of human recombinant COX-2 (Cayman Chemical) was incubated with aspirin (500 μM) for 30 minutes at 37°C. Enzyme was filtered with a Microcon column (Fisher) to remove unreacted aspirin and was resuspended in PBS. Next, 500 μM phenol and 1 μM hematin were added immediately before incubation with EPA (15 μM). The reaction was terminated 30 minutes after EPA addition by 2 volumes of ice-cold methanol, and samples were stored at –80°C until extraction.
Reverse-phase and chiral separation of 18R- and 18S-isomers.
The Agilent HP1100 system equipped with binary pump and DAD was used for lipid mediator isolation. Samples were separated with the Luna C18(2) column (150 mm × 2.1 mm × 5 μm) and a gradient of methanol/water/acetic acid of 55:45:0.01 (v/v/v) to 100:0:0.01. For chiral separation, a Chiralpak AD-RH column (150 mm × 2.1 mm × 5 μm) was used with a gradient of methanol/water/acetic acid of 95:5:0.01 (v/v/v) to 100:0:0.01.
Human PMN isolation and incubations.
Fresh human PMNs from deidentified donors were isolated from leukocytes obtained from the Children’s Hospital Blood Bank and incubated as in ref. 45. Isolated PMNs (50 × 106/ml) were incubated with bestatin (250 μM) for 30 minutes, then activated with 5 μM calcium ionophore A21387 for 3 minutes. 18-HEPE (25 μM) was added and incubated for 45 minutes; then the incubation was stopped with 2× volumes of cold methanol and extracted.
Human recombinant 5-LOX incubations.
18-HEPE was incubated with human recombinant 5-LOX, as described in ref. 20. Human recombinant 5-LOX (25 U) was incubated with 18R/S-HEPE (50 μM) at 37°C in the presence of 2 mM CaCl2, 1 mM ATP, and 5 μM 13-HpODE as activators. After 30 minutes, the reaction was stopped with 2 volumes of cold MeOH, and hydroperoxide intermediates were reduced with an excess amount of sodium borohydride.
Combinatorial incubation.
Human recombinant 5-LOX and LTA4H combinatorial incubations were carried out with 18-HEPE. Briefly, 5 nmol (final concentration, 100 μM) of 18-HEPE was incubated with human recombinant 5-LOX (10 U) and LTA4H (1 μg) in pH 7.4 phosphate buffer in the presence of activators and in the presence/absence of bestatin (250 μM). Additional hr5-LOX (5 U) and LTA4H (0.5 μg) were added at 7-minute intervals 4 times. Incubations were stopped with 2× volume cold methanol and extracted as above.
Total organic synthesis of 18S-RvE1.
18S-RvE1 was chemically synthesized from stereochemically determined precursors as reported in ref. 19 with stereochemically distinguished starting material.
Ligand-receptor interaction monitoring with Chemr23 and BLT1 β-arrestin systems.
Activation of ChemR23 and antagonism of BLT1 receptor were carried out using a new GPCR β-arrestin system (54, 55). With the ChemR23-overexpressing system, cells were plated (10,000/well) 24 hours prior to experiments and incubated with tested compounds (Figure 8A) in serum-free medium (1 hour, 37°C). With the BLT1-overexpressing system, plated cells were incubated first with different doses of compounds for 1 hour, followed by 30 nM BLT for 90 minutes. Cells were further incubated with β-gal substrate (PathHunter EFC Detection Kit, DiscoveRx) for 90 minutes, and ligand-dependent receptor activation was assessed by luminescence using a plate reader (EnVision, PerkinElmer).
Metabolic inactivation by dehydrogenation.
The alcohol group at carbon 18 is crucial to E-series resolvin function, as reported previously (56). To assess whether stereochemistry of the carbon 18 affects metabolic conversion and subsequent inactivation, we compared the time course of conversion with human recombinant 15-PGDH in the presence of NAD+.
Murine peritonitis.
In accordance with Harvard Medical Area Standing Committee on Animals (Boston, Massachusetts, USA) protocol no. 02570, peritonitis was initiated by injecting zymosan into 6- to 8-week-old male FVB mice (Charles River Laboratories). For direct comparison, vehicle or 20 ng of each compound was administered via tail vein injection. For dose-responses, 1, 10, or 100 ng of RvE1 or 18S-RvE1 was injected. Zymosan A (Sigma-Aldrich) was injected intraperitoneally immediately following compound administration. Mice were sacrificed 2 or 4 hours after zymosan injection with an overdose of isoflurane. To assess enhancement of PMN removal, 100 ng RvE1 or 18S-RvE1 was administered 8 hours after zymosan A was injected intraperitoneally, and mice were sacrificed 12 hours after injection of either RvE1 or 18S-RvE1. For microbial peritonitis, E. coli was grown, enumerated, and prepared at the log phase and diluted in saline for peritoneal delivery. Either 107 (early phagocytosis) or 106 CFU (18 hour resolution model) of E. coli was injected. For resident macrophage phagocytosis, 100 ng of 18S-RvE1 or RvE1 was directly injected into peritoneum and lavaged 30 minutes later; for the resolution model, the same doses of 18S-RvE1 or RvE1 were administered via tail vein, and peritonea were lavaged at 18 hours after E. coli delivery.
Differential counting and flow cytometry of exudate leukocytes.
Peritoneal lavages were immediately collected and leukocytes enumerated under a light microscope. Differential leukocyte counts were carried out using light microscopy with modified Wright-Giemsa staining (Sigma-Aldrich) or FACS analysis. For FACS analysis, aliquots (0.5 × 106 cells) were incubated with anti-CD16/32 to block nonspecific binding to Fcγ receptors (5 minutes, 4°C), followed by anti-Gr1 and anti-CD11b Ab or isotype IgG controls (30 minutes, 4°C). Cells were washed and analyzed on a FACSCanto II (BD) cytometer. Data were analyzed with FlowJo (TreeStar Inc).
Murine dorsal air pouch.
Six-day murine dorsal air pouches were raised as in ref. 57. For comparison of antiinflammatory actions between RvE1 and 18S-RvE1, 20 ng of individual compounds was injected via tail vein, immediately followed by injection of 100 ng mouse recombinant TNF-α to air pouch. Four hours after TNF-α injection, mice were sacrificed and pouches were lavaged to enumerate infiltrated neutrophils.
Murine macrophage phagocytosis assay.
Zymosan phagocytosis was carried out as described in ref. 56. Briefly, resident macrophages were collected from the peritonea of naive mice and incubated on a 24-well plate (105 cells/well) in PBS+/+ at 37°C. RvE1 or 18S-RvE1 was added to the wells at indicated concentrations, and cells were incubated in the dark for 15 minutes at 37°C. FITC-labeled, opsonized zymosan (stz) was then added and incubated with the cells for 30 minutes. For E. coli phagocytosis assays, murine resident macrophages were prepared, plated, and treated with RvE1 or 18S-RvE1 as above, then fluorescent E. coli (2.5 × 106 CFU) was added and samples further incubated for 60 minutes. Phagocytosis was stopped by aspirating supernatant, and extracellular fluorescence was quenched by the addition of trypan blue and washing with PBS+/+. Plates were read using a PerkinElmer VICTOR3 plate reader, and fluorescence was measured for each well.
Human macrophage phagocytosis.
Human PBMCs and PMNs were isolated by density-gradient Ficoll-Histopaque isolation as in ref. 18. Isolated PBMCs were washed 3 times to remove platelets, plated on the plastic for 2 hours, washed twice with PBS, then cultured for 7 days in RPMI-1640 medium supplemented with 10% FCS and 10 ng/ml GM-CSF. At day 7, macrophages were detached with PBS-EDTA, washed, and plated in a 12-well plate (5 × 105/well). PMNs from the same donor were isolated and resuspended in RPMI-1640 medium supplemented with 10% FCS for 24 hours at 37°C to induce apoptosis. The next day (day 8), vehicle or a different concentration of 18S-RvE1 or RvE1 was added 15 minutes before addition of CFDA-stained (1:2,500, 30 minutes) apoptotic PMNs (1.5 × 106/well) to each well. At 60 minutes after apoptotic PMN exposure, macrophages were washed with PBS, detached, and analyzed by FACS to determine ratio of nonfluorescent/fluorescent macrophage population.
Cytokine analysis.
Cell culture supernatants or peritoneal lavages were centrifuged to remove the cellular component and kept at –80°C until quantified with Searchlight cytokine analysis (Aushon Bioscience).
Statistics.
All results in the Figures and text are expressed as mean ± SEM of n ≥ 3. Statistical significance was determined by 2-tailed Student’s t test; a P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Mary H. Small for skillful manuscript preparation, Makoto Arita for samples of synthetic RvE2, Sriram Krishnamoorthy for help with the receptor and murine experiments, and Khalil Nasser and Timothy H. Porter for technical assistance. This study was supported in part by the NIH grants DK074448 and GM38765.
Footnotes
Conflict of interest: The results reported herein were obtained by the authors prior to licensing of patents for clinical development. Charles N. Serhan is inventor on patents assigned to Brigham and Women’s Hospital and Partners HealthCare on the composition of matter, uses, and clinical development of antiinflammatory and pro-resolving lipid mediators. These are licensed for clinical development. Charles N. Serhan retains founder stock in Resolvyx Pharmaceuticals.
Citation for this article: J Clin Invest. 2011;121(2):569–581. doi:10.1172/JCI42545.
References
- 1. Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS.Robbins and Cotran Pathologic Basis of Disease . 7th ed. Philadelphia, Pennsylvania, USA: Elsevier Saunders; 2005. [Google Scholar]
- 2. Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(suppl 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Caterina R, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J Clin Invest. 2003;112(6):945–955. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawrence T. Inflammation and cancer: a failure of resolution? Trends Pharmacol Sci. 2007;28(4):162–165. doi: 10.1016/j.tips.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 6. Patrono C, Baigent C. Low-dose aspirin, coxibs, and other NSAIDS: a clinical mosaic emerges. Mol Interv. 2009;9(1):31–39. doi: 10.1124/mi.9.1.8. [DOI] [PubMed] [Google Scholar]
- 7. Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 8. Holtzman MJ, Turk J, Shornick LP. Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J Biol Chem. 1992;267(30):21438–21445. [PubMed] [Google Scholar]
- 9. Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92(21):9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA. 1984;81(17):5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serhan CN, et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34(44):14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 12. Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. . J Clin Invest. 1998;101(4):819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A4 analogues inhibit leukocyte recruitment to Porphyromonas gingivalia: a role for cyclooxygenase-2 and lipoxins in periodontal disease. . Biochemistry. 2000;39(16):4761–4768. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- 14. Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A4 and lipoxin A4. . J Pharmacol Exp Ther. 2002;300(2):385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- 15. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 16. Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 17. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):249–261. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Serhan CN, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arita M, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201(5):713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tjonahen E, et al. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13(11):1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 21. Shimizu T, Radmark O, Samuelsson B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc Natl Acad Sci U S A. 1984;81(3):689–693. doi: 10.1073/pnas.81.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mueller MJ, Samuelsson B, Haeggstrom JZ. Chemical modification of leukotriene A4 hydrolase. Indications for essential tyrosyl and arginyl residues at the active site. Biochemistry. 1995;34(11):3536–3543. doi: 10.1021/bi00011a007. [DOI] [PubMed] [Google Scholar]
- 23. Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. . J Immunol. 2007;178(6):3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 24. Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(7146):869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Serhan CN, Clish CB, Brannon J, Colgan SP, Gronert K, Chiang N. Anti-microinflammatory lipid signals generated from dietary N-3 fatty acids via cyclooxygenase-2 and transcellular processing: a novel mechanism for NSAID and N-3 PUFA therapeutic actions. J Physiol Pharmacol. 2000;51(4 pt 1):643–654. [PubMed] [Google Scholar]
- 26. Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 27. Wu S-H, Liao P-Y, Yin P-L, Zhang Y-M, Dong L. Elevated expressions of 15-lipoxygenase and lipoxin A4 in children with acute poststreptococcal glomerulonephritis. . Am J Pathol. 2009;174(1):115–122. doi: 10.2353/ajpath.2009.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu S-H, Liao P-Y, Yin P-L, Zhang Y-M, Dong L. Inverse temporal changes of lipoxin A4 and leukotrienes in children with Henoch-Schönlein purpura. . Prostaglandins Leukot Essent Fatty Acids. 2009;80(4):177–183. doi: 10.1016/j.plefa.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 29. Morris T, et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J Immunol. 2009;183(3):2089–2096. doi: 10.4049/jimmunol.0900477. [DOI] [PubMed] [Google Scholar]
- 30. Lee JH, O’Keefe JH, Lavie CJ, Harris WS. Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat Rev Cardiol. 2009;6(12):753–758. doi: 10.1038/nrcardio.2009.188. [DOI] [PubMed] [Google Scholar]
- 31. Lancet. 1999;354(9177):447–455. doi: 10.1016/S0140-6736(99)07072-5. [DOI] [PubMed] [Google Scholar]
- 32. Yokoyama M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 33. Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci U S A. 2004;101(42):15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiang N, Takano T, Clish CB, Petasis NA, Tai H-H, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudates: development of a specific 15-epi-LXA4 ELISA. . J Pharmacol Exp Ther. 1998;287(2):779–790. [PubMed] [Google Scholar]
- 35. Paul-Clark MJ, van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. . J Exp Med. 2004;200(1):69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weissmann G. Aspirin. Sci Am. 1991;264(1):84–90. doi: 10.1038/scientificamerican0191-84. [DOI] [PubMed] [Google Scholar]
- 37. Ramwell PW, Shaw JE, Corey EJ, Andersen N. Biological activity of synthetic prostaglandins. Nature. 1969;221(5187):1251–1253. doi: 10.1038/2211251b0. [DOI] [PubMed] [Google Scholar]
- 38. Surette ME, Koumenis IL, Edens MB, Tramposch KM, Chilton FH. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin Ther. 2003;25(3):948–971. doi: 10.1016/S0149-2918(03)80116-9. [DOI] [PubMed] [Google Scholar]
- 39. Kasuga K, et al. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181(12):8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flower RJ. The development of COX2 inhibitors. Nat Rev Drug Discov. 2003;2(3):179–191. doi: 10.1038/nrd1034. [DOI] [PubMed] [Google Scholar]
- 42. McAdam BF, et al. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. . J Clin Invest. 2000;105(10):1473–1482. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Praticò D, Tillmann C, Zhang Z-B, Li H, FitzGerald GA. Acceleration of atherogenesis by COX-1-dependent prostanoid formation in low density lipoprotein receptor knockout mice. Proc Natl Acad Sci U S A. 2001;98(6):3358–3363. doi: 10.1073/pnas.061607398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pugsley MK, Tabrizchi R. The vascular system. An overview of structure and function. J Pharmacol Toxicol Methods. 2000;44(2):333–340. doi: 10.1016/S1056-8719(00)00125-8. [DOI] [PubMed] [Google Scholar]
- 45. Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Souza PM, Newson J, Gilroy DW. Targeting lipoxygenases with care. Chem Biol. 2006;13(11):1121–1122. doi: 10.1016/j.chembiol.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 47. Thunnissen MMGM, Nordlund P, Haeggström J. Crystal structure of human leukotriene A4 hydrolase, a bifunctional enzyme in inflammation. . Nature Struct Biol. 2001;8(2):131–135. doi: 10.1038/84117. [DOI] [PubMed] [Google Scholar]
- 48. Funk CD, et al. Molecular cloning and amino acid sequence of leukotriene A4 hydrolase. Proc Natl Acad Sci U S A. 1987;84(19):6677–6681. doi: 10.1073/pnas.84.19.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haeggstrom JZ. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J Biol Chem. 2004;279(49):50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- 50. Serhan CN, Fiore S, Brezinski DA, Lynch S. Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. . Biochemistry. 1993;32(25):6313–6319. doi: 10.1021/bi00076a002. [DOI] [PubMed] [Google Scholar]
- 51. Sun YP, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282(13):9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 52. Bannenberg GL, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174(7):4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 53. Spite M, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Olson KR, Eglen RM. Beta galactosidase complementation: a cell-based luminescent assay platform for drug discovery. Assay Drug Dev Technol. 2007;5(1):137–144. doi: 10.1089/adt.2006.052. [DOI] [PubMed] [Google Scholar]
- 55. Krishnamoorthy S, et al. Resolvin D1 binds human phagocytes with evidence for pro-resolving receptors. Proc Natl Acad Sci U S A. 2010;107(4):1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180(5):3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 57. Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci U S A. 1999;96(14):8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.