Abstract
Both mucosal and systemic immune responses are required for preventing or containing HIV transmission and chronic infection. However, currently described vaccination approaches are largely ineffective in inducing both mucosal and systemic responses. In this study, we found that the ubiquitin-editing enzyme A20 — an inducible feedback inhibitor of the TNFR, RIG-I, and TLR signaling pathways that broadly controls the maturation, cytokine production, and immunostimulatory potency of DCs — restricted systemically immunized DCs to induce both robust mucosal and systemic HIV-specific cellular and humoral responses. Mechanistic studies revealed that A20 regulated DC production of retinoic acid and proinflammatory cytokines, inhibiting the expression of gut-homing receptors on T and B cells. Furthermore, A20-silenced, hyperactivated DCs exhibited an enhanced homing capacity to draining and gut-associated lymphoid tissues (GALTs) after systemic administration. Thus, this study provides insights into the role of A20 in innate immunity. This work may allow the development of an efficient HIV vaccination strategy that is capable of inducing both robust systemic and mucosal anti-HIV cellular and humoral responses.
Introduction
Since transmission of HIV-1 mainly occurs at mucosal surfaces, HIV-1 vaccines should activate the mucosal arm of the immune system to prevent or contain viral transmission and the establishment of chronic infection in gut-associated lymphoid tissues (GALTs) (1–4). Antigen-specific effector T and B cells in the bloodstream recognize mucosal high endothelial venules and enter the mucosa (5, 6). Antigen-specific CTLs can kill HIV-infected cells, while mucosal and systemic antibodies could block HIV transmission by inhibiting HIV transcytosis and neutralizing viral infection (2, 7). However, the host defenses cannot mount cellular and humoral immune responses of sufficient magnitude and breadth to contain HIV infection at the mucosal entrance. Most of the DC subsets in mucosal GALTs transmit HIV to T cells though C-type lectins (8–11), which leads to mucosal T cell depletion during the acute phase of infection regardless of the mucosal or systemic transmission route (12, 13), whereas Langerhans cells, DCs in the epidermis, were reported to specifically express langerin to inhibit HIV transmission (14). Immunization with peripheral antigen delivery usually fails to induce a robust mucosal immune response, and, likewise, mucosal immunization primarily induces poor systemic immune responses, for the route of antigen entry determines the differential acquisition of tissue-specific homing molecules on lymphocytes (2, 15). Hence, there is an urgent need to develop an HIV vaccination strategy that is capable of inducing both robust systemic and mucosal anti-HIV immune responses.
DCs, the most potent of APCs, play critical roles in initiating and regulating innate and adaptive immunity against viral infections by providing proinflammatory cytokines and costimulatory molecules and presenting processed or unprocessed antigens to T and B cells (16). DCs use TLRs to recognize conserved microbial products to activate MAP kinase and NF-κB, resulting in the activation of innate and adaptive immunity (17, 18). In addition to their role in antigen presentation, DCs critically affect the trafficking and tissue homing of the lymphocytes they activate. The homing phenotypes of antigen-specific effector T and B cells are predetermined by APCs activated at the antigen-processing site (19–21). Mucosa-tropism of activated T and B lymphocytes is governed by sequential interactions between intestinal homing receptors, particularly the integrin α4β7 and chemokine receptor CCR9 on activated lymphocytes, and their counter-receptors on endothelial cells (5, 6, 22). Several recent studies indicate that activation of TLR or retinoic acid–inducible gene I (RIG-I) signaling in DCs plays a critical role in promoting the mucosal homing of activated lymphocytes (23, 24). Johansson-Lindbom et al. reported that α4β7+CCR9+ CD8+ T cells were efficiently generated in GALTs in the presence of TLR ligands (23). It was also reported that protective mucosal immunity was induced by systemic administration of an attenuated replication-competent SIV, likely due to the fact that replicating SIV dsRNA activated RIG-I signaling in APCs, promoting the mucosal homing of activated lymphocytes (24).
A20 is a zinc-finger ubiquitin-modifying enzyme and inhibits several key proinflammatory signal transduction pathways of TNF receptor (TNFR), TLR, and RIG-I in a feedback manner (25–30). A20 was originally discovered as a TNF-inducible gene and is an NF-κB target gene whose expression is induced in many types of cells by various stimuli (25, 31). A20 was recently found to inhibit these signaling pathways by ubiquitination or deubiquitination of receptor-interacting protein (RIP), TNFR-associated factor 6 (TRAF6), and other molecules for either promotion of target protein degradation or regulation of the interaction of the target proteins with other signaling molecules (26–29, 32). Because TRAF6 is a common signal component that is shared by all members of the TLR family, A20 uniquely suppresses both MyD88-dependent and MyD88-independent TLR signaling pathways. RIG-I is an intracellular sensor of viral dsRNA and mediates the activation of NF-κB and interferon regulatory factor 3 (IRF3) independent of the TLR pathways (33). It was recently reported that A20 blocked RIG-I–mediated signaling to NF-κB and IRF3 by promoting the degradation of TRIF and other molecules (30). A20-deficient mice develop severe inflammation in multiple organs, are neonatally lethal, and are highly hypersensitive to LPS and TNF (26–28). A20-deficient macrophages display prolonged NF-κB activity (27, 28), and A20-silenced DCs induce robust antitumor immune responses to reject engrafted tumors in immunized mice (34). Recently, Breckpot et al. reported that A20-silenced human DCs had an enhanced capacity to polarize the IFN-γ–producing CD4+ T cell response and to prime the tumor-specific CD8+ T cell response (35). These results indicate that A20 plays a critical role in controlling immunostimulatory potency of APCs. In this study, we investigated the role of A20 in controlling DC-mediated anti-HIV immunity, and demonstrated that A20-silenced DCs possess a superior potency to trigger HIV-specific mucosal and systemic immune responses when being administrated systemically.
Results
Immunization of A20-silenced, bone marrow–derived DCs induces systemic anti-HIV immunity.
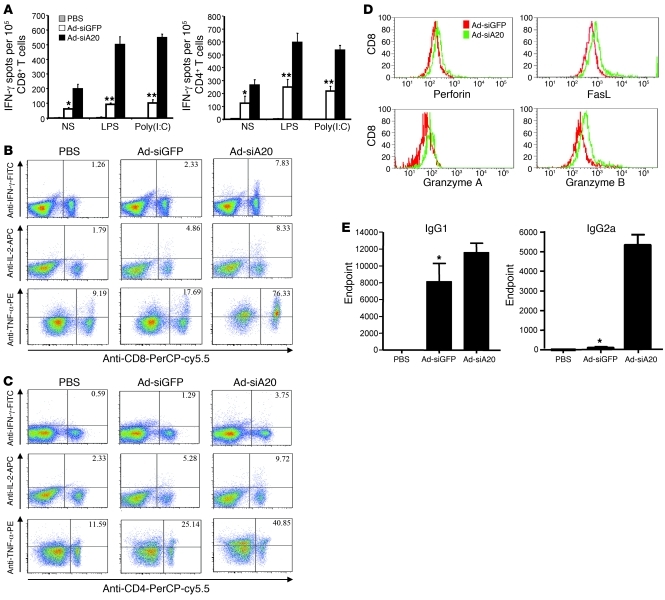
To investigate the role of A20 in HIV antigen presentation by DCs, we generated a recombinant adenoviral vector (Ad-siA20) that expresses siA20 capable of downregulating about 90% of mouse A20 mRNA in transfected cells (34), along with a control Ad vector (Ad-siGFP) (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI42656DS1). Ad vectors were able to efficiently transduce bone marrow–derived DCs (BM-DCs), as determined by flow cytometric assays (Supplemental Figure 1B). A20 expression was significantly downregulated in Ad-siA20–transduced DCs at both mRNA and protein levels (Supplemental Figure 1C). For evaluation of the immunostimulatory potency of A20-silenced DCs, mouse BM-DCs were transduced with Ad-siA20 or Ad-siGFP at an MOI of 500, loaded with recombinant HIV envelope (Env) gp120 proteins, and matured with LPS ex vivo. Groups of mice were then immunized with Ad-transduced DCs or PBS via footpad twice with a week interval. ELISPOT assay showed that Ad-siA20-BM-DCs elicited approximately 1.5-fold stronger gp120-specific CD8+ CTL response and 1-fold stronger gp120-specific CD4+ T cell (Th) response than did the control Ad-siGFP-BM-DCs (Figure 1A). Given that A20 is an inducible feedback regulator of TLR and TNFR-mediated signaling (36), we tested whether in vivo administration of TLR agonists enhances the immunostimulatory potency of Ad-siA20-BM-DCs. Groups of mice were immunized with Ad-transduced DCs or PBS, followed by stimulation with LPS or poly(I:C) in vivo. ELISPOT assays showed that HIV Env–specific CTL and Th responses were significantly enhanced in mice immunized with Ad-siA20-BM-DCs in response to in vivo stimulation of TLR agonists, compared with those in Ad-siGFP-BM-DC mice (Figure 1A). Intracellular cytokine staining (ICS) demonstrated higher percentages of IFN-γ+, IL-2+, or TNF-α+ CD8+ T cells in the draining LNs of Ad-siA20-BM-DC mice compared with those in Ad-siGFP-BM-DC–immunized mice (Figure 1B). Furthermore, the percentages of cytokine-producing CD4+ T cells were also higher in Ad-siA20-BM-DC mice (Figure 1C). We also examined whether Ad-siA20-BM-DC immunization affected the expression of effector molecules in T cells. Figure 1D shows that expression levels of the cytotoxic molecules FasL and granzyme B were increased in CD8+ T cells in the draining LNs of Ad-siA20-BM-DC mice, compared with those from Ad-siGFP-BM-DC mice. However, the expression of effecter molecules such as perforin and granzyme A on Ad-siA20-BM-DCs was only modestly enhanced. Finally, we examined the potency of A20-silenced BM-DCs to induce anti-HIV humoral responses. Figure 1E shows that Ad-siA20-BM-DCs elicited enhanced gp120-specific IgG2a and IgG1 responses compared with the control Ad-siGFP-BM-DCs in the immunized mice. The preferential enhancement of HIV gp120–specific IgG2a responses suggests a Th1-polarized anti-HIV response induced by Ad-siA20-BM-DCs.
Figure 1. Ad-siA20-BM-DCs induce potent systemic HIV gp120–specific immune responses.
Groups of C57BL/6 mice were immunized with HIV gp120 protein–pulsed (100 μg/ml), Ad-transfected BM-DCs (1 × 106 cells/mouse) twice with a week interval, followed by no stimulation or poly(I:C) (50 μg/mouse) or LPS stimulation (30 μg/mouse) (i.p.) daily for 3 consecutive days after each DC immunization. (A) CD8+ T cells or CD4+ T cells isolated from pooled splenocytes of immunized mice (n = 2–3) were subjected to IFN-γ ELISPOT assays. (B and C) ICS of CD8+ T cells (B) and CD4+ T cells (C) from draining LNs of immunized mice was performed after 6–8 hours in vitro restimulation. (D) The expression levels of perforin, granzymes A and B, and FasL in gated CD8+ T cells of pooled draining LNs of immunized mice are shown. (E) Systemic antibody responses enhanced by siA20-BM-DCs. HIV gp120–specific IgG1 and IgG2a subclass titers from the pooled sample of each group (4–6 mice/group) were quantified by capture ELISA. Antibody titers are reported as the mean ± SD of endpoint titers (36). Experiments were repeated 3 times with similar results. *P < 0.05, **P < 0.01, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs.
Systemic immunization with A20-silenced BM-DCs induces mucosal anti-HIV immunity.
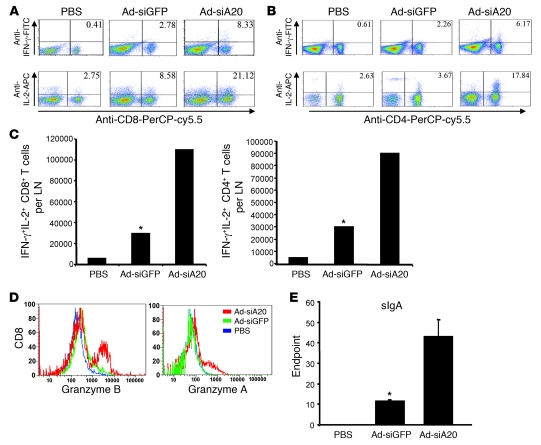
We then tested whether A20-silenced BM-DCs are able to induce HIV-specific CTL and Th responses at intestinal mucosal sites of systemically immunized mice. We isolated mesenteric LNs from mice that were systemically immunized with Ad-transduced BM-DCs via footpad for various immune assays. ICS revealed that higher frequencies of gp120-specific CD8+ T cells expressed IL-2 or IFN-γ in the mesenteric LNs of Ad-siA20-BM-DC mice, compared with those in Ad-siGFP-BM-DC mice (Figure 2A). The percentages of the Th1-polarizing cytokine-producing CD4+ T cells were also increased in the mesenteric LNs of Ad-siA20-BM-DC mice (Figure 2B). Double IFN-γ– and IL-2–secreting CD8+ and CD4+ T cells were preferentially induced in mesenteric LNs derived from Ad-siA20-BM-DC mice (Figure 2C). Furthermore, the mucosal CD8+ T cells activated by Ad-siA20-BM-DCs showed enhanced levels of effector molecules such as granzymes A and B (Figure 2D).
Figure 2. Systemic immunization of Ad-siA20-BM-DCs induces mucosal anti-HIV immune response.
Groups of mice were immunized twice with gp120-pulsed, Ad-transfected BM-DCs (1 × 106 cells/mouse) via footpad, followed by in vivo stimulation with poly(I:C). (A and B) Two weeks later, mesenteric LNs were harvested and pooled, and cell suspensions were then subjected to ICS. (C) The average cell numbers of IFN-γ/IL-2 double-positive CD8+ T cells and CD4+ T cells per mesenteric LN were examined. (D) The expression of effector molecules on gated CD8+ T cells from mesenteric LNs was also examined. (E) Mucosal HIV gp120–specific sIgA titers from the pooled sample of each group (4–6 mice/group) were quantified by capture ELISA. Antibody titers are reported as the mean ± SD of endpoint titers (36). Data from 1 experiment representative of 3 are presented. *P < 0.05, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs.
One of the hallmarks of mucosal immunity is production of secretory IgA (sIgA), which acts to prevent microbial mucosal invasion by neutralizing HIV or blocking HIV transcytosis (1, 2). Accordingly, we examined the production of HIV Env–specific mucosal sIgA in the mice immunized with Ad-siA20-BM-DCs via nonmucosal footpad injection. Figure 2E shows an approximately 4-fold increase in HIV Env–specific sIgA antibody titers in mice systemically immunized with Ad-siA20-BM-DCs, compared with those in Ad-siGFP-BM-DC mice. Hence, these results indicate that A20 silencing endows BM-DCs with the distinctive ability to induce robust mucosal CTL and Th responses and antibody responses against HIV Env, in addition to triggering systemic anti-HIV immunity.
Systemic immunization with A20-silenced BM-DCs induces mucosal anti-HIV immunity equivalent to HIV gp120 intrarectal immunization.
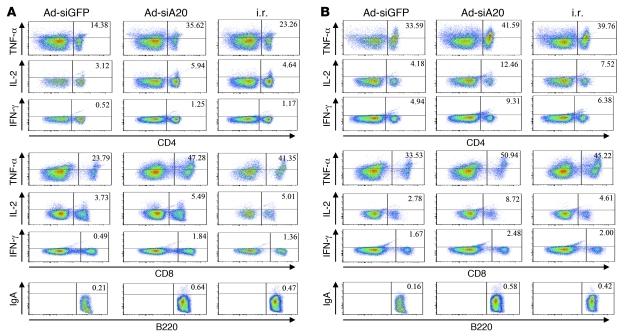
After demonstrating the ability of the systemic immunization with A20-silenced BM-DCs to induce mucosal anti-HIV immunity, we compared the relative potency of the footpad immunization of Ad-siA20-BM-DCs with the commonly used method of intrarectal (i.r.) mucosal immunization with HIV gp120 protein/cholera toxin (CT) in inducing mucosal and systemic immune responses. Groups of mice were immunized with Ad-transfected, HIV gp120–pulsed (10 μg/ml) BM-DCs via footpad or 10 μg HIV gp120 in combination with cholera toxin via i.r. immunization (37, 38). Seven to 10 days after the second immunization, the splenic and mesenteric lymphocytes were isolated for various immune assays. As shown in Figure 3A, comparable or higher frequencies of gp120-specific CD8+ and CD4+ T cells expressed TNF-α, IL-2, and IFN-γ in the mesenteric LNs of Ad-siA20-BM-DC mice, compared with those in i.r. immunized mice. Furthermore, the mesenteric B220+ cells isolated from mice systemically immunized with DCs or from mice i.r. immunized with gp120 secreted a similar level of sIgA, as assessed by IgA surface staining. Although the i.r. immunization with HIV gp120 also activated splenic T cells (37), the frequencies were lower than those triggered by the footpad immunization with Ad-siA20-BM-DCs (Figure 3B). Systemic immunization with Ad-siA20-BM-DCs and i.r. immunization with gp120 induced a similar level of IgA+B220+ splenic B cells (Figure 3B). These results indicate that systemic immunization with Ad-siA20-BM-DCs induces an equivalent mucosal immune response but an enhanced systemic response compared with i.r. mucosal immunization with HIV gp120.
Figure 3. Systemic immunization with Ad-siA20-BM-DCs induces an equivalent mucosal immune response but an enhanced systemic response compared with i.r. immunization with HIV gp120.
Groups of C57BL/6 mice were immunized twice at a 1-week interval with Ad-transduced BM-DCs pulsed with HIV gp120 (10 μg/ml) or with HIV gp120 (10 μg) emulsified in adjuvant by i.r. injection. Seven days after the second immunization, the mice were sacrificed, and the HIV gp120–specific immune response of mesenteric LNs (A) or spleens (B) was analyzed by ICS. Data from 1 experiment representative of 2 are presented.
To investigate whether systemic administration of TLR adjuvant with HIV gp120 antigen also achieves a similar level of mucosal immune responses, we used HIV gp120 (10 μg) emulsified in incomplete Freund’s adjuvant (IFA) (Sigma-Aldrich) in parallel with Ad-siA20-BM-DCs for systemic immunization of C57BL/6 mice, followed by 50 μg of poly(I:C) administration 3 times in all the mice. As shown in Supplemental Figure 2, A and B, significantly higher frequencies of gp120-specific CD8+ and CD4+ T cells that expressed TNF-α, IL-2, and IFN-γ were detected in the mesenteric LNs of Ad-siA20-BM-DC mice, compared with those in gp120/IFA poly(I:C)–immunized mice. Moreover, the Ad-siA20-BM-DC mice also displayed a higher level of secreted IgA compared with gp120/IFA-immunized mice. The result demonstrated the superior ability of systemic Ad-siA20-BM-DC immunization to induce mucosal immune responses.
A20-silenced BM-DCs display enhanced migration to mesenteric LNs.
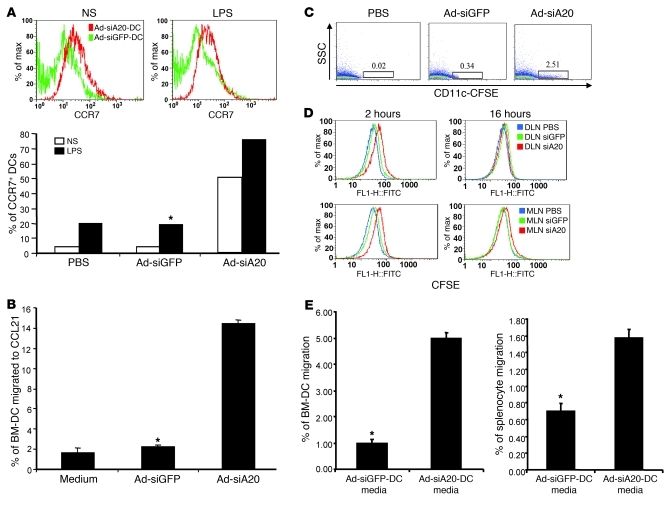
Through flow cytometric assays, we found that the expression of chemokine receptors such as CCR7 was enhanced approximately 5- to 10-fold on Ad-siA20-BM-DCs (Figure 4A). As a consequence, in vitro Transwell migration assays showed that Ad-siA20-BM-DCs had approximately 6-fold-enhanced migration activity in response to CCL21, the ligand of CCR7, compared with Ad-siGFP-BM-DCs (Figure 4B). Consistent with this finding, flow cytometric assays revealed that expression levels of CCR7 were enhanced in Ad-siA20-BM-DC populations that had migrated to the bottom chamber containing CCL21 (data not shown). Given the critical role of CCR7 in mediating DC migration to mesenteric LNs (39), we investigated the effect of A20 silencing on DC migration to mesenteric LNs in the systemically immunized mice. An in vivo migration assay analyzing CFSE-labeled DCs at 16 hours after footpad injection showed that Ad-siA20-BM-DCs more efficiently migrated to the mesenteric LNs compared with Ad-siGFP-BM-DCs (Figure 4C). Dynamic analysis of the footpad-injected, CSFE-labeled DCs revealed that more Ad-siA20-BM-DCs migrated to mesenteric LNs (also to the draining LNs) as fast as 2 hours after injection (Figure 4D). Taken together, these results indicate that A20-silenced DCs more efficiently home to mesenteric LNs after systemic administration, likely due to the enhanced expression of chemokine receptors. In addition, we also tested the ability of Ad-siA20-BM-DCs to attract other immune cells in vitro. Figure 4E shows that the culture media of Ad-siA20-BM-DCs attracted more BM-DCs and splenocytes from naive mice than those of Ad-siGFP-BM-DC, implying that A20-silenced DCs can efficiently attract other immune cells, likely due to the enhanced production of various chemokines (data not shown), for induction of anti-HIV immune responses.
Figure 4. Enhanced migration and chemoattraction of Ad-siA20-BM-DCs.
(A) Enhanced expression of chemokine receptors CCR7 on Ad-siA20-BM-DCs either with or without LPS (100 ng/ml for 18 hours) using flow cytometric analysis; data are from 1 of 3 repeated assays. *P < 0.05, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs. (B) Percentages of migrated Ad-siA20– or Ad-siGFP–transduced BM-DCs in triplicate Transwells following 3 hours of exposure to recombinant CCL21 (100 ng/ml, GeneTex) in vitro; data are from 1 of 3 repeated experiments. *P < 0.05, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs. (C and D) In vivo migration of CFSE-labeled BM-DCs that were transduced with Ad-siA20, Ad-siGFP, or PBS (mock transduction) to mesenteric LNs (MLNs) at 16 hours (C) or to draining LNs (DLNs) or MLNs at different times (D) after footpad injection using flow cytometric assays; data are from 1 of 2 repeated experiments. (E) Enhanced chemoattraction of Ad-siA20-BM-DCs. Percentages of migrated BM-DCs or splenocytes from naive mice following 3 hours exposure to the culture media of Ad-siA20– or Ad-siGFP–transduced BM-DCs; data are from 1 of 2 repeated experiments. *P < 0.05, Ad-siGFP-BM-DC media versus Ad-siA20-BM-DC media.
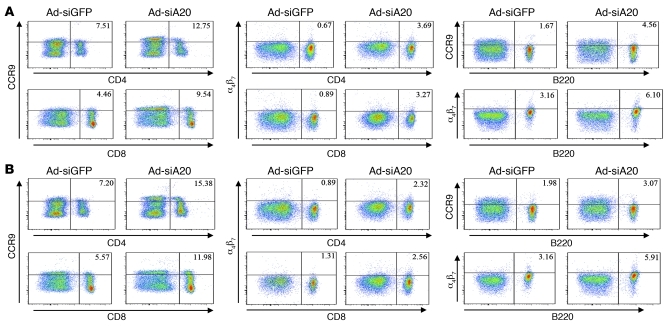
A20-silenced BM-DCs imprint gut-homing receptors on activated T and B cells.
The enhanced mucosal immune responses induced by A20-silenced BM-DCs also prompted us to examine the effect of siA20-DC immunization on the expression of gut-homing receptors, such as α4β7 and CCR9, on T cells and B cells. Figure 5A shows the expression levels of CCR9 were markedly increased on both CD8+ and CD4+ T cells in the mesenteric LNs of mice systemically immunized with Ad-siA20-BM-DCs. Consistent with this observation, expression levels of α4β7 on CD8+ and CD4+ T cells were also increased in the mice systemically immunized with Ad-siA20-BM-DCs. Furthermore, A20-silenced DC immunization also increased expression of both CCR9 and α4β7 on the B220+ B cells in mesenteric LNs. Unexpectedly, CCR9 and α4β7 were also found to be highly expressed on CD4+ and CD8+ T cells, as well as B cells that were derived from the spleen (Figure 5B), and draining LNs (data not shown) of Ad-siA20-BM-DC–immunized mice. Given that Ad-siA20-BM-DC–primed T cells more efficiently localized to mesenteric LNs and other secondary tissues after adoptive transfer into naive mice (Supplemental Figure 3), it is postulated that the lymphocytes activated ex situ by Ad-siA20-BM-DCs (Figure 5B) also contribute to the constitution of the mucosal lymphocyte pool in the immunized mice due to enhanced expression of gut-homing receptors.
Figure 5. Systemic immunization with Ad-siA20-BM-DCs induced gut-homing receptors on lymphocytes.
(A) Expression of CCR9 and α4β7 on gated CD8+ T cells and CD4+ cells, and CCR9 and α4β7 on gated B220+ B cells of pooled mesenteric LNs of mice immunized with gp120-pulsed Ad-siA20-BM-DCs or Ad-siGFP-BM-DCs via nonmucosal footpad administration. (B) Expression of CCR9 and α4β7 on gated CD8+ T cells and CD4+ T cells, and CCR9 and α4β7 on gated B220+ B cells of pooled splenocytes of mice immunized with gp120-pulsed Ad-siA20-BM-DCs or Ad-siGFP-BM-DCs via nonmucosal footpad administration.
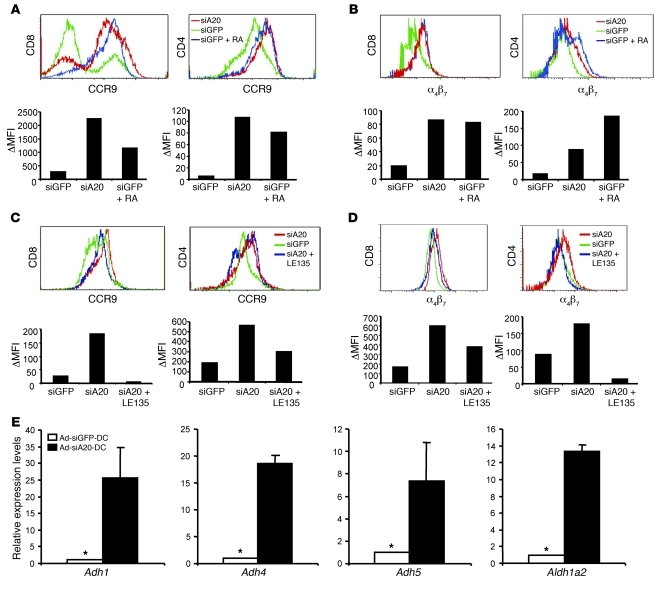
To investigate the mechanism(s) by which Ad-siA20-BM-DCs induce enhanced expression of the gut-homing receptors, we directly examined the effect of siA20-BM-DCs on the expression of gut-homing receptors on activated T cells in vitro. Ad-siA20–transduced BM-DCs and control Ad-siGFP-BM-DCs were pulsed with OT-I or OT-II peptides and then cocultured with CD8+ OT-I or CD4+ OT-II T cells isolated from OT-I or OT-II epitope–specific TCR transgenic mice, respectively. Figure 6A shows that expression levels of CCR9 on both CD8+ OT-I and CD4+ OT-II cells after coculture with Ad-siA20-BM-DCs were substantially higher than those on cells cocultured with control Ad-siGFP-BM-DCs. Similarly, enhanced expression of α4β7 was also observed on both CD8+ OT-I and CD4+ OT-II cells after coculture with Ad-siA20-BM-DCs (Figure 6B), indicating that Ad-siA20-BM-DCs have the distinctive ability to induce the expression of the gut-homing receptors α4β7 and CCR9 on antigen-specific T cells.
Figure 6. Ad-siA20-BM-DCs induced gut-homing receptors on T cells in vitro.
(A and B) Surface expression of CCR9 (A) and α4β7 (B) on CD8+ OT-I or CD4+ OT-II T cells after in vitro coculture with Ad-siA20- or Ad-siGFP-BM-DCs pulsed with H-2Kb/OT-I or OT-II peptide (10 μg/ml) (5 × 104 cells/well) in the presence or absence of RA (Sigma-Aldrich, 100 nM); data are from 1 of 2 repeated experiments. (C and D) Reduced expression of CCR9 (C) and α4β7 (D) by blocking RA receptors. Surface expression of CCR9 and α4β7 on CD8+ OT-I or CD4+ OT-II T cells was examined by flow cytometry after coculture with Ad-siA20- or Ad-siGFP-BM-DCs pulsed with H-2Kb/OT-I or OT-II peptide in the presence or absence of the RA receptor antagonist LE135 (1 μM, Tocris Bioscience). Data from 1 of 2 repeated experiments are presented. (E) Increased expression of RA-producing enzymes by Ad-siA20-BM-DCs. qRT-PCR was used to analyze Ad-siA20-BM-DCs and Ad-siGFP-BM-DCs after stimulation with LPS for 12 hours. The relative mRNA levels of the RA-producing enzymes from 1 of 2 repeated experiments are presented. *P < 0.05, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs.
Recent studies by Iwata et al. and Mora et al. found that GALT-DCs, but not peripheral LN-DCs, produced retinoic acid (RA) to induce the expression of gut-homing receptors (19, 21, 40). Accordingly, we examined whether A20 silencing may allow BM-DCs to produce RA for the induction of gut-homing receptors α4β7 and CCR9. In agreement with the previous reports (19, 21, 40), the addition of RA substantially enhanced the expression of α4β7 and CCR9 on OT-I and OT-II cells (Figure 6, A and B). The blockade of RA receptors by antagonist LE135 (40, 41) substantially reduced the ability of Ad-siA20-BM-DCs to induce the expression of α4β7 and CCR9 on both OT-I and OT-II cells (Figure 6, C and D). However, we noticed that the RA receptor antagonist LE135 had a greater influence on the CCR9 expression of CD8+ T cells than that of CD4+ T cells. Due to the technical difficulty of directly measuring RA concentrations, we examined the mRNA levels of enzymes that are involved in RA synthesis from its precursor vitamin A (retinol) in siA20-BM-DCs by quantitative real-time RT-PCR (qRT-PCR). The main pathway of RA synthesis is dependent upon the intracellular oxidative metabolism of retinol to retinal, which is catalyzed by a subfamily of alcohol dehydrogenases (ADHs) (42), and of retinal to RA, which is then catalyzed by retinal dehydrogenases (RALDHs), of which there are at least 4 isoenzymes. Figure 6E shows that the expression levels of retinal-producing enzymes and RA-producing enzymes were significantly enhanced in Ad-siA20-BM-DCs, compared with those in Ad-siGFP-BM-DCs. Taken together, these results indicate that A20-silenced BM-DCs possess the unique ability to induce the expression of gut-homing receptors on activated T cells, at least partly due to the enhanced production of RA.
A20-silenced DCs preferentially activate CTL and Th, but not Treg, responses.
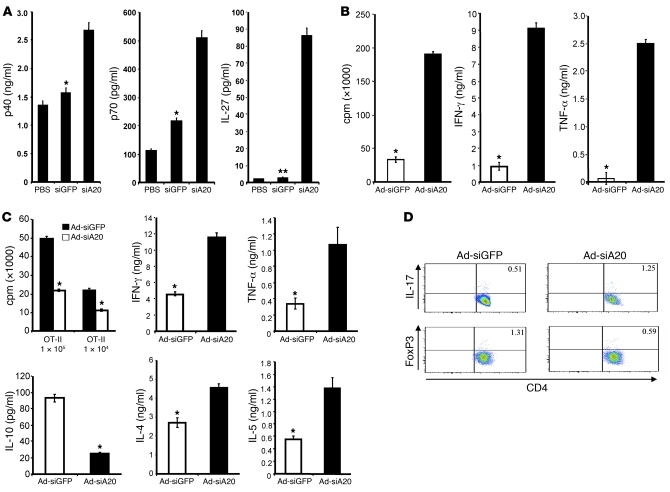
To further assess the underlying mechanism(s) for the enhanced mucosal as well as systemic anti-HIV immunity, we examined the role of A20 in regulating DC maturation and cytokine/chemokine production. In concordance with our previous results (34), FACS analysis showed that Ad-siA20-BM-DCs exhibited significantly higher levels of CD40, CD80, CD86, and MHC class II than Ad-siGFP-BM-DCs, either before or after stimulation with LPS (Supplemental Figure 4). As tipping the balance between the IL-12 family members represents an important mechanism to control antiviral immunity, and mucosal vaccines usually exhibit better immune stimulation when administered concomitantly with exogenous IL-12 or substances that stimulate endogenous IL-12 production (37), we specifically evaluated how A20 controls expression of IL-12 family members in BM-DCs. As shown in Figure 7A, A20-silenced DCs expressed higher levels of IL-12 (500 pg/ml) and IL-27 (80 pg/ml), but undetectable IL-23 (data not shown) compared with Ad-siGFP-BM-DCs, implying a preferential type 1 polarization.
Figure 7. Ad-siA20-BM-DCs had a reduced ability to activate Foxp3+ Tregs.
(A) Enhanced expression of IL-12 and IL-27 by Ad-siA20-BM-DCs after 24 hours of stimulation with LPS in vitro. *P < 0.05, **P < 0.01, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs. (B) Enhanced activation of antigen-specific CD8+ T cells by Ad-siA20-BM-DCs in vitro. CD8+ OT-I T cells (5 × 105 cells) from OT-I transgenic mice were cocultured with Ad-siA20-BM-DCs or Ad-siGFP-BM-DCs (1 × 105 cells) pulsed with 5 μg/ml of OT-I peptide for 5 days. [3H]thymidine incorporation rates and cytokine production of OT-I cells after the depletion of CD11c+ DCs were then examined. Data from 1 representative experiment of 3 are presented. *P < 0.05, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs. (C and D) Enhanced activation of antigen-specific CD4+ Th cells. CD4+ OT-II T cells from OT-II transgenic mice were cocultured with Ad-siA20-BM-DCs or Ad-siGFP-BM-DCs (1 × 105 cells) pulsed with 5 μg/ml OT-II peptide for 5 days. [3H]thymidine incorporation rates and Th1- and Th2-polarizing cytokine production (C) and intracellular IL-17 or Foxp3 expression (D) of OT-II cells after the depletion of CD11c+ DCs were then examined; data from 1 representative experiment of 3 are presented. *P < 0.05, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs.
Consistent with the proinflammatory status of Ad-siA20-BM-DCs, an in vitro priming assay showed that CD8+ OT-I T cells activated by Ad-siA20-BM-DCs proliferated more intensely and produced significantly more IFN-γ and TNF-α than those activated by control Ad-siGFP-BM-DCs (Figure 7B). Ad-siA20-BM-DCs pulsed with OT-II peptides led OT-II cells to have a higher proliferation rate and to produce enhanced levels of both Th1- and Th2-polarizing cytokines such as IFN-γ, TNF-α, IL-4, IL-5, and IL-17, but lower levels of IL-10 and transcription factor FoxP3 (Figure 7, C and D). Despite the reported antiapoptotic role of A20 in TNF-induced apoptosis (26), siA20-BM-DCs and control siGFP-BM-DCs had comparable viability in the presence or absence of a TLR agonist (Supplemental Figure 5A). Ad-siA20-BM-DCs also retained comparable endocytic activity (Supplemental Figure 5B). Collectively, these results indicate that Ad-siA20-BM-DCs possess an inherent potential to induce effector T cell responses.
A20-silenced BM-DCs induce robust anti-HIV immunity in CD4+ T cell–deficient mice.
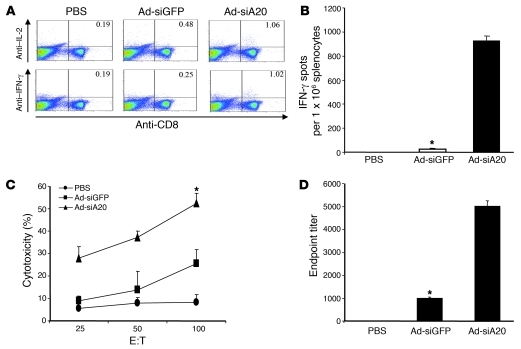
Previous studies demonstrate that activation of TLR and CD40 signaling in DCs could alleviate the need for CD4+ T cells to induce CTL responses (43, 44). Since A20 silencing hyperactivates DCs, which exhibit enhanced expression of costimulatory molecules such as CD40 and proinflammatory cytokines (Supplemental Figure 4 and ref. 34), we investigated the immunostimulatory potency of siA20-BM-DCs to induce anti-HIV immune responses in mice deficient in CD4+ T cells. C57BL/6 mice were treated with anti-CD4 antibodies (GK1.5) (44) to reach 95% CD4+ T cell depletion, as confirmed by flow cytometric assay. CD4+ T cell–depleted mice were immunized twice at a 1-week interval with gp120-pulsed (100 μg/ml), Ad-transfected DCs matured with LPS, followed by in vivo stimulation with poly(I:C) (i.p.) daily for 3 consecutive days after each immunization. Ad-siGFP-BM-DC systemic immunization plus in vivo stimulation with the TLR agonist poly(I:C) was unable to induce HIV Env–specific CTL responses in the mice in which CD4+ T cells were depleted with anti-CD4 antibodies. In contrast, Ad-siA20-BM-DCs effectively induced HIV Env–specific CTL responses in the CD4+ T cell–depleted mice, as demonstrated by ICS and ELISPOT assays (Figure 8, A and B). CD8+ T cells derived from Ad-siA20-BM-DC–immunized, CD4-depleted mice had robust CTL activity against gp120-pulsed syngeneic TC1 cells (Figure 8C). Moreover, anti–HIV gp120 antibodies were also induced in the Ad-siA20-BM-DC–immunized, CD4+-depleted mice (Figure 8D). Thus, A20-silenced BM-DCs have the ability to activate HIV-1 Env–specific CTL responses in the mice deficient in CD4+ T cells.
Figure 8. CD4+ T cell–independent CTL and antibody responses induced by Ad-siA20-BM-DCs.
Groups of C57BL/6 mice (n = 6) were treated with anti-CD4 antibodies (GK1.5, 200 μg/mouse, i.p. injection) 3 days before each immunization (44) and then immunized twice with gp120-pulsed (100 μg/ml), Ad-transfected DCs matured with LPS at a weekly interval, followed by in vivo stimulation with poly(I:C) (i.p., 50 μg/mouse) daily for 3 consecutive days. Splenocytes pooled from immunized mice were subjected to ICS (A), IFN-γ ELISPOT (B), and cytolytic assays against gp120-pulsed syngeneic TC1 cells (36) (C). HIV gp120–specific IgG antibody titers from pooled sera were analyzed by ELISA (D). Data from 1 of 2 repeated experiments are presented. *P < 0.05, Ad-siGFP-BM-DCs versus Ad-siA20-BM-DCs.
Discussion
Our previous study revealed that A20, an inducible feedback inhibitor of the TNFR, RIG-I, and TLR signaling pathways, broadly controls the maturation, cytokine production, and immunostimulatory potency of DCs. A20 functions as an antigen presentation attenuator (APA) to critically regulate tumor antigen presentation by DCs, and subsequently, the magnitude and polarization of adaptive antitumor immune responses. Here, we further revealed that A20-silenced, hyperactivated DCs exhibit a superior capacity to induce both mucosal and systemic anti-HIV cellular/humoral immune responses after systemic immunization. Several studies have reported that CCR7 is a critical signal that determines DC exit from peripheral tissue and access to draining lymphoid tissues (45–47). A recent finding from Jang et al. further indicated that CCR7 is also critically important for DC migration to mesenteric LNs (39). A20-silenced DCs express a high level of CCR7, which endows the DCs with a superior capacity to home to draining and gut-associated LNs after systemic administration to induce potent mucosal and systemic anti-HIV immunity.
This study further uncovers an important role of A20 in controlling the ability of DCs to induce the expression of gut-homing receptors on T and B cells by regulating the production of RA and proinflammatory cytokines. Increasing evidence indicates that the gut-homing phenotypes of antigen-specific effector T and B lymphocytes are predetermined or imprinted by DCs from GALTs such as Peyer’s patches and mesenteric LNs in the presence of TLR ligands (19–21). Whereas naive lymphocytes migrate to secondary lymphoid organs, effector T and B lymphocytes selectively express different adhesion molecules and chemokine receptors that direct their preferential homing for different peripheral tissues such as the intestinal mucosa and inflamed skin (5, 48). During the priming of naive lymphocytes, GALT-DCs preferentially induce the expression of a distinct set of adhesion molecules such as α4β7, whose ligand MAdCAM-1 is expressed on postcapillary venules in the intestinal lamina propria, and chemokine receptor CCR9, whose ligand CCL25 is selectively expressed by intestinal epithelial cells (15, 19–21, 23, 49, 50). GALT-DCs induce the expression of these gut-homing molecules, α4β7 and CCR9, by producing RA and proinflammatory cytokines such as IL-6, while peripheral LN-DCs induce the expression of E- and P-selectin ligands and chemokine receptors CCR4 and CCR10 for T cell homing to skin (19–21, 40, 51). Mora et al. recently found that the environments where DCs reside, not a distinct set of DCs, were responsible for their ability to induce the gut-homing receptors on activated lymphocytes (20). The results of this study demonstrate that A20-silenced BM-DCs effectively induce the expression of gut-homing receptors α4β7 and CCR9 on T/B lymphocytes in mesenteric LNs, as well as in other secondary lymphoid tissues such as draining LNs and spleen. Furthermore, the present study revealed that A20 silencing leads to the activation of RA synthesis genes, as well as a large number of proinflammatory cytokine genes in A20-silenced DCs, as a result of unbridled TNFR, RIG-I, and TLR signaling, which may explain why the systemic immunization of A20-silenced DCs leads to expression of the gut-homing receptors in multiple secondary lymphoid tissues. Further studies are under way to investigate the molecular mechanism(s) by which A20 silencing results in the activation of RA synthesis genes.
A20-silenced DCs efficiently activated antigen-specific lymphocytes for the induction of both robust mucosal and systemic anti-HIV immunity. One of the mechanisms may be that A20-silenced DCs are able to induce expression of different homing receptors to drive migration of the activated effector cells to multiple secondary lymphoid organs, as evidenced by the findings that T cells primed in vitro by A20-silenced DCs migrate to the different secondary lymphoid tissues with enhanced efficiency, and that the T cells activated by A20-silenced DCs also expressed a higher level of skin-homing in addition to gut-homing receptors (data not shown). Although it was reported that peripheral LN-DCs induced the expression of selectin ligands on T cells, which was suppressed by GALT DCs (20), Campbell et al. found that memory T cells coexpressed α4β7 as well as LN-homing CCR7 and L-selectin, which may allow them to home to gut mucosa and peripheral LNs (52). It was also reported that P-selectin ligands play a role in CD4+ T cell homing to the intestinal lamina propria (53). Our results, together with these reports, indicate that A20 silencing programs BM-DCs to acquire the unique ability to induce expression of multiple homing receptors on T cells, and the expression of any homing receptors on activated T cells may not be exclusive.
Another interesting observation of this study is that A20-silenced, hyperactivated DCs are capable of inducing both anti-HIV CTL responses and antibody responses in a CD4+ T cell–deficient host, which implies a possible application of the technique in developing therapeutic HIV vaccines in immunocompromised AIDS patients. CD4+ T cells are known to be the essential helper cells in inducing immune responses. Several recent studies showed the induction of CTL or antibody responses in CD4+ T cell knockout animals. Zheng et al. reported that a DNA or DC vaccine expressing CD40L elicited significant CTL responses in CD4+ T cell–deficient mice (43, 44). Yao et al. found that a hemagglutinin/simian HIV-like particle vaccine induced Th cell–independent CTL and antibody responses (54). Both of these studies have related the bypass of CD4+ T cell requirement in the induction of anti-HIV immune response to the activation of DCs by the incorporated genes. In that regard, A20-silenced DCs exhibit high levels of CD40, CD80, CD86, and other costimulatory molecules and produce copious amounts of proinflammatory molecules in response to TLR stimuli, which may contribute to alleviating the need for CD4+ T cells in induction of anti-HIV CTL and antibody responses in CD4+ T cell–deficient hosts. Developing a therapeutic vaccine to treat HIV represents a challenging task. Lu et al. reported that immunization with virus-pulsed DCs suppressed viral load in HIV-infected patients (55), demonstrating the feasibility of therapeutic vaccines for the control of HIV infection. Letvin et al. also revealed that the magnitude of vaccine-elicited cellular immune responses was associated with the duration of survival of SIV-infected monkeys (56), underscoring the importance of enhancing the potency of anti-HIV immunity to control HIV infection.
In summary, this study provides insights into the role of A20 in developing an efficient HIV vaccination strategy that is capable of inducing both robust systemic and mucosal anti-HIV cellular and humoral responses. However, future studies are needed to test the ability of this A20 inhibition approach, together with use of the conservative, but subdominant protective HIV epitopes or antigens (57–63), in a prophylactic or therapeutic vaccination setting to induce immune responses and protection in animal models of SIV/HIV infections.
Methods
Mice.
C57BL/6, BALB/c mice, H-2Kb/OT-I–TCR (OT-I) transgenic mice, and H-2Kb/OT-II–TCR (OT-II) transgenic mice were purchased from The Jackson Laboratory and maintained in mouse facilities at Baylor College of Medicine and the University of Southern California according to institutional guidelines. This study was approved by the Institutional Animal Care and Use Committees of Baylor College of Medicine and the University of Southern California.
Construction and production of recombinant adenoviral vectors.
An E1- and E3-deleted Ad-Easy system (Quantum Biotechnologies Inc.) was used to construct and generate replication-defective adenoviruses. Synthetic mouse A20 small hairpin RNA oligo duplexes (5′-GATCCCCCAAAGCACTTATTGACAGATTCAAGAGATCTGTCAATAAGTGCTTTGTTTTTGGAAA) with the cloning sites of SpeI and ClaI or control siGFP duplexes were first cloned into a pSuper vector (64). The H1 RNA polymerase promoter-siA20 or siGFP DNA fragments were generated by PCR and cloned into the Pac I–cut Ad-Easy shuttle vector (65). The recombinant replication-deficient Ad-siA20 and Ad-siGFP viruses were then generated according to the manufacturer’s instructions and confirmed by DNA sequencing. Recombinant adenoviruses were produced and titrated in 293 cells using Adeno-X Rapid Titer Kits (BD).
qRT-PCR assays.
The relative expression of mouse A20 was evaluated by quantitative real-time RT-PCR, as described previously (64). Pre-developed primer/probe sets for mouse A20 and 18S ribosomal control (VIC) were purchased from Applied Biosystems (primers for A20, 5′-TTTGCTACGACACTCGGAAC-3′ and 5′-TTCTGAGGATGTTGCTGAGG-3′, and the hybridization probes, 5′-CACCTGCAGCCCGAAGTGGA-3′). The relative expression of RA-producing enzymes was evaluated by qRT-PCR as well, and the primers (PPM04366A, PPM04370A, PPM04391A, PPM28943A, and PPM31491A) were from SuperArray.
Transfection and immunization of BM-DCs.
Mouse BM-DCs were generated by culturing with GM-CSF and IL-4 (64) and were transfected with Ad-siA20 or Ad-siGFP at an MOI of 500 IFU, which routinely yielded a transfection efficiency of about 70% of BM-DCs. The transfected DCs were pulsed with recombinant soluble HIV gp120 proteins (SF162), which were produced and purified from CHO cells and provided by the NIH AIDS Research and Reference Program and Chiron Corp., for 6 hours, followed by ex vivo maturation with LPS (100 ng/ml, Sigma-Aldrich). The DCs were then injected into C57BL/6 or BALB/c mice (The Jackson Laboratory) via footpad. Some of the immunized mice were treated with LPS (30 μg/mouse) or poly(I:C) (50 μg/mouse) intraperitoneally 3 times on days 1, 2, and 3 after DC immunization.
i.r. immunization.
Mice were immunized twice with 10 μg recombinant HIV gp120 in 100 μl endotoxin-free PBS emulsified 1:1 with 10 μg cholera toxin (List Biological Laboratories). For i.r. immunization, mice were sedated with ketamine hydrochloride (10 mg/kg intramuscularly [i.m.]) and placed in a biosafety cabinet in ventral recumbency with hindquarters elevated. The tail was elevated dorsally, and a 3-cc slip-tip syringe was atraumatically inserted into the rectum. The tail was lowered to assure complete delivery.
ELISPOT.
ELISPOT assays of isolated CD8+ and CD4+ T cells and splenocytes were performed as described in our previous studies (36, 64). DCs pulsed with HIV gp120 or an irrelevant protein (ovalbumin; Sigma-Aldrich) were used for T cell stimulation. T cells were isolated from splenocytes by using MACS CD4 (L3T4) or MACS CD8 (Ly-2) MicroBeads (Miltenyi Biotec).
CTL assays.
In vitro CTL activity was assessed by a standard 4-hour 51Cr release assay against labeled target cells as previously described (36). Briefly, splenic T cells obtained from immunized mice were restimulated in vitro with HIV gp120–pulsed (20 μg/ml) syngeneic irradiated splenocytes for 5 days. The effector cells were then mixed at various ratios with 51Cr-labeled syngeneic target cells that were loaded with HIV gp120 antigens for 4 hours. Spontaneous release of 51Cr was determined by incubating the target cells with medium alone, and maximum release was determined by adding Triton X-100 to a final concentration of 5%. The percentage of specific lysis was calculated as follows: 100 × ([experimental release – spontaneous release]/[maximum release – spontaneous release]). Each experiment was performed twice using triplicate samples.
Flow cytometric analysis.
For ICS, splenocytes or lymphocytes were harvested from draining or mesenteric LNs or spleens of immunized mice and cultured with gp120-loaded BM-DCs for 8 hours at 37°C. For the final 6 hours of culture, GolgiPlug (BD Biosciences —Pharmingen) was added to the supernatant. After surface staining with anti-CD8 or -CD4, cells were permeabilized and stained for intracellular cytokines, as previously described (36). For cell surface staining, cells were incubated with antibodies conjugated with FITC, PE, or allophycocyanin (APC) in PBS containing 0.1% NaN3 and 2% FCS. mAbs specific for mouse CD8 (clone 53-6.7), CD11c (HL3), CD40 (3/23), CD80 (16-10A1), CD86 (GL1), CCR9, CCR7, α4β7, MHC-II, and matched isotype controls were purchased from BD Biosciences — Pharmingen or eBioscience. Stained cells were analyzed on a FACSAria II (BD) flow cytometer and FlowJo software (TreeStar Inc.).
Antibody and cytokine ELISA assays.
To determine gp120-specific serum IgG and mucosal sIgA, sera were collected from mice and vaginal lavage was obtained by rinsing the vaginal cavity with PBS. Supernatants of lavage and sera were then used for ELISA assays, as described previously (36, 66). The results are expressed as reciprocal endpoint titers, determined from a scatter plot with OD values on the y axis and dilution-1 on the x axis, for which the x-axis scale was logarithmic. After the data were plotted, a logarithmic curve fit was applied to each individual dilution series, and the point where the curve fit intersects the positive-negative cut-off value was determined. The cutoff value was calculated for each antibody isotype as the mean (+3 SD) of all dilutions from control mouse sera. All samples were tested in triplicate with repeated assays (36). To detect cytokine production in BM-DCs and T cells, the culture supernatants were harvested and detection performed with commercial cytokine kits (BD).
In vitro T cell proliferation assays.
T cells were purified from wild-type mice or OT-I or OT-II transgenic mice (The Jackson Laboratory) using the MACS CD8+ or CD4+ T cell isolation kit (Miltenyi Biotec). For antigenic stimulation of TCR transgenic OT-I or OT-II cells, 5 × 104 purified T cells and 5 × 103 Ad-transfected BM-DCs pulsed with H2-Kb–restricted OT-I (SIINFEKL) or OT-II (ISQAVHAAHAEINEAGR) peptides (64) were placed in each well of a round-bottom 96-well microtiter plate in triplicate in 200 μl RPMI 1640 medium supplemented with 10% FCS, 4 mM l-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin and streptomycin, 10 mM HEPES, and 5 μM 2-ME. After 3–6 days of coculture, the cells in the cocultures were subjected to flow cytometric assays. In some experiments, RA (Sigma-Aldrich) or LE135 (Tocris Bioscience) was added into the cocultures at a final concentration of 100 nM or 1 μM, respectively. To assess T cell proliferation and cytokine production, CD11c+ BM-DCs were depleted by using MACS columns, activated T cells were seeded into plates in the presence of anti-CD3 (2.5 μg/ml) for 16 hours, and culture media were then collected for ELISA analysis of the indicated cytokines (BD Biosciences — Pharmingen). T cell proliferation was assessed by adding 1 μCi [3H]TdR per well for the last 8 hours of culture and measured using a MicroBeta scintillation counter (TopCount NXT, Packard). Triplicate determinations were performed and are representative of repeated experiments.
Adoptive transfer assay.
The isolated OT-II cells from OT-II transgenic mice were cocultured with OT-II peptide–pulsed DCs for 5 days with a DC/T ratio of 1:5. The cocultured OT-II cells (5 × 106) were harvested and labeled with 25 μM CFSE (Molecular Probes, Invitrogen) and transferred into naive C57BL/6 mice by retro-orbital injection. Sixteen hours after the adoptive transfer, migration of the labeled T cells to the mesenteric LNs, draining LNs, or spleen in the recipient mice was analyzed by flow cytometric assay.
In vitro and in vivo migration assay.
In vitro migration assay was performed in Transwell plates (Costar) of 6.5-mm diameter with 5-μm pore filters separating the upper and lower compartments of the Transwells. A total of 500,000 cells in 100 μl assay medium (0.5% BSA/RPMI) was added to the upper compartment, and 600 μl assay medium with or without chemokines or culture medium of Ad-siA20– or Ad-siGFP–transfected BM-DCs was added to the lower compartment in triplicate. After 3 hours incubation, the cells that migrated to the lower compartments were measured. For in vivo migration assays, Ad-transfected BM-DCs were labeled with 25 μM CFSE (Molecular Probes, Invitrogen) for 15 minutes at 37°C, cultured overnight, and then injected into mice via footpads at 4 × 106 BM-DCs per mouse. At different time points, mice were sacrificed and cell suspensions generated from draining and mesenteric LNs were subjected to flow cytometry.
Statistics.
We used the 2-tailed Student’s t test and 95% confidence limits to assess results for statistical significance, defined as P < 0.05. Results are typically presented as mean ± SD.
Supplementary Material
Acknowledgments
We would like to thank Kevin Evel-Kabler, Bingya Liu, Bing Wan, and other members of the laboratory for technical assistance and valuable suggestions. We would also like to thank many colleagues at the Baylor College of Medicine and the University of Southern California for helpful suggestions and assistance. This work was supported by grants from the NIH (R01AI68472, R01AI084811, R01CA090427, and R01CA116677 to S-.Y. Chen, and R01CA100841 and R21AI087185 to X.F. Huang).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(2):739–751. doi:10.1172/JCI42656.
References
- 1. Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9(7):847–852. doi: 10.1038/nm0703-847. [DOI] [PubMed] [Google Scholar]
- 2. Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 3. Douek DC, Kwong PD, Nabel GJ. The rational design of an AIDS vaccine. Cell. 2006;124(4):677–681. doi: 10.1016/j.cell.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4. Letvin NL. Progress toward an HIV vaccine. Annu Rev Med. 2005;56:213–223. doi: 10.1146/annurev.med.54.101601.152349. [DOI] [PubMed] [Google Scholar]
- 5. von Andrian UH, Mackay CR. T-cell function and migration — two sides of the same coin. N Engl J Med. 2000;343(14):1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 6. von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 7. Matoba N, Geyer BC, Kilbourne J, Alfsen A, Bomsel M, Mor TS. Humoral immune responses by prime-boost heterologous route immunizations with CTB-MPR649-684, a mucosal subunit HIV/AIDS vaccine candidate. Vaccine. 2006;24(23):5047–5055. doi: 10.1016/j.vaccine.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 8. Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100(5):587–597. doi: 10.1016/S0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 9. Turville SG, et al. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3(10):975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- 10. Turville SG, et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103(6):2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 11. Veazey RS, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438(7064):99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 12. Veazey RS, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 13. Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Witte L, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13(3):367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 15. Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195(1):135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 17. Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3(2):169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 18. Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 19. Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424(6944):88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 20. Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. . J Exp Med. 2005;201(2):303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 22. Berlin C, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74(1):185–195. doi: 10.1016/0092-8674(93)90305-A. [DOI] [PubMed] [Google Scholar]
- 23. Johansson-Lindbom B, Svensson M, Wurbel M-A, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198(6):963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miller CJ, Abel K. Immune mechanisms associated with protection from vaginal SIV challenge in rhesus monkeys infected with virulence-attenuated SHIV 89.6. J Med Primatol. 2005;34(5–6):271–281. doi: 10.1111/j.1600-0684.2005.00125.x. [DOI] [PubMed] [Google Scholar]
- 25. Opipari AW, Jr, Boguski MS, Dixit VM. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem. 1990;265(25):14705–14708. [PubMed] [Google Scholar]
- 26. Lee EG, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5(10):1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 28. Wertz IE, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 29. Sarma V, et al. Activation of the B-cell surface receptor CD40 induces A20, a novel zinc finger protein that inhibits apoptosis. J Biol Chem. 1995;270(21):12343–12346. doi: 10.1074/jbc.270.21.12343. [DOI] [PubMed] [Google Scholar]
- 30. Lin R, et al. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J Biol Chem. 2006;281(4):2095–2103. doi: 10.1074/jbc.M510326200. [DOI] [PubMed] [Google Scholar]
- 31. Krikos A, Laherty CD, Dixit VM. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem. 1992;267(25):17971–17976. [PubMed] [Google Scholar]
- 32. Saitoh T, et al. A20 is a negative regulator of IFN regulatory factor 3 signaling. J Immunol. 2005;174(3):1507–1512. doi: 10.4049/jimmunol.174.3.1507. [DOI] [PubMed] [Google Scholar]
- 33. Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 34. Song XT, Evel-Kabler K, Shen L, Rollins L, Huang XF, Chen SY. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat Med. 2008;14(3):258–265. doi: 10.1038/nm1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breckpot K, et al. Attenuated expression of A20 markedly increases the efficacy of double-stranded RNA-activated dendritic cells as an anti-cancer vaccine. J Immunol. 2009;182(2):860–870. doi: 10.4049/jimmunol.182.2.860. [DOI] [PubMed] [Google Scholar]
- 36. Song XT, et al. An alternative and effective HIV vaccination approach based on inhibition of antigen presentation attenuators in dendritic cells. PLoS Med. 2006;3(1):e11. doi: 10.1371/journal.pmed.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belyakov IM, et al. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc Natl Acad Sci U S A. 1998;95(4):1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J Immunol. 2007;178(11):7211–7221. doi: 10.4049/jimmunol.178.11.7211. [DOI] [PubMed] [Google Scholar]
- 39. Jang MH, et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol. 2006;176(2):803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 40. Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Hashimoto Y, Agadir A, Kagechika H, Zhang X. Identification of a novel class of retinoic acid receptor beta-selective retinoid antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in human breast cancer cells. J Biol Chem. 1999;274(22):15360–15366. doi: 10.1074/jbc.274.22.15360. [DOI] [PubMed] [Google Scholar]
- 42. Duester G. Families of retinoid dehydrogenases regulating vitamin A function. Production of visual pigment and retinoic acid. Eur J Biochem. 2000;267(14):4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 43. Zheng M, et al. CD4+ T cell-independent vaccination against Pneumocystis carinii in mice. J Clin Invest. 2001;108(10):1469–1474. doi: 10.1172/JCI13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng M, et al. CD4+ T cell-independent DNA vaccination against opportunistic infections. J Clin Invest. 2005;115(12):3536–3544. doi: 10.1172/JCI26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Forster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99(1):23–33. doi: 10.1016/S0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 46. Ohl L, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21(2):279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 47. Gunn MD, et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189(3):451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weninger W, Manjunath N, von Andrian UH. Migration and differentiation of CD8+ T cells. Immunol Rev. 2002;186:221–233. doi: 10.1034/j.1600-065X.2002.18618.x. [DOI] [PubMed] [Google Scholar]
- 49. Svensson M, et al. CCL25 mediates the localization of recently activated CD8{alpha}{beta}+ lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110(8):1113–1121. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lefrancois L, Parker CM, Olson S, Muller W, Wagner N, Puddington L. The role of beta 7 integrins in CD8 T cell trafficking during an antiviral immune response. J Exp Med. 1999;189(10):1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 52. Campbell JJ, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166(2):877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- 53. Haddad W, et al. P-selectin and P-selectin glycoprotein ligand 1 are major determinants for Th1 cell recruitment to nonlymphoid effector sites in the intestinal lamina propria. J Exp Med. 2003;198(3):369–377. doi: 10.1084/jem.20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao Q, Zhang R, Guo L, Li M, Chen C. Th cell-independent immune responses to chimeric hemagglutinin/simian human immunodeficiency virus-like particles vaccine. J Immunol. 2004;173(3):1951–1958. doi: 10.4049/jimmunol.173.3.1951. [DOI] [PubMed] [Google Scholar]
- 55. Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10(12):1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 56. Letvin NL, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312(5779):1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Calarese DA, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci U S A. 2005;102(38):13372–13377. doi: 10.1073/pnas.0505763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fischer W, et al. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med. 2007;13(1):100–106. doi: 10.1038/nm1461. [DOI] [PubMed] [Google Scholar]
- 59. Haynes BF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308(5730):1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 60. Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol. 2006;80(3):1414–1426. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pashov A, Perry M, Dyar M, Chow M, Kieber-Emmons T. Defining carbohydrate antigens as HIV vaccine candidates. Curr Pharm Des. 2007;13(2):185–201. doi: 10.2174/138161207779313678. [DOI] [PubMed] [Google Scholar]
- 62. Weaver EA, et al. Cross-subtype T-cell immune responses induced by a human immunodeficiency virus type 1 group m consensus env immunogen. . J Virol. 2006;80(14):6745–6756. doi: 10.1128/JVI.02484-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhou T, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445(7129):732–737. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22(12):1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 65. Ren W, Strube R, Zhang X, Chen S-Y, Huang XF. Potent tumor-specific immunity induced by an in vivo heat shock protein-suicide gene-based tumor vaccine. Cancer Res. 2004;64(18):6645–6651. doi: 10.1158/0008-5472.CAN-04-1084. [DOI] [PubMed] [Google Scholar]
- 66. Lemiale F, et al. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J Virol. 2003;77(18):10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.