Abstract
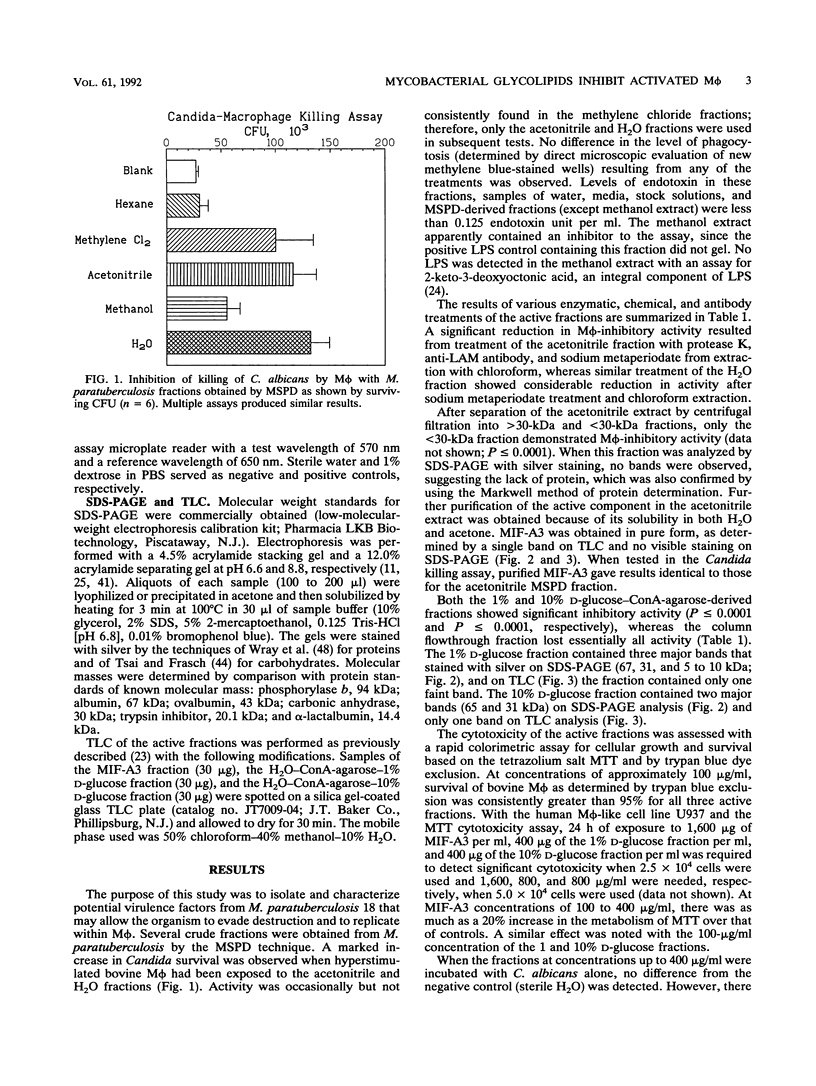
Glycolipid fractions from Mycobacterium avium serovar 2 (Mycobacterium paratuberculosis 18) inhibited the killing of Candida albicans by activated bovine peripheral-blood-derived macrophages. Fractions were derived by using the matrix solid-phase dispersion technique, which is a new method of simultaneous lysis and partial fractionation of components of bacterial cells. Further purification of active fractions was performed by concanavalin A affinity chromatography, centrifugal filtration, and differing solvent solubility. Three different fractions were isolated and partially characterized. Two of these fractions have characteristics typical of glycolipids, and the third fraction has characteristics compatible with a peptidoglycolipid. This peptidoglycolipid fraction has been purified and named MIF-A3.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker S. A., Long A. R., Short C. R. Isolation of drug residues from tissues by solid phase dispersion. J Chromatogr. 1989 Jul 28;475:353–361. doi: 10.1016/s0021-9673(01)89689-8. [DOI] [PubMed] [Google Scholar]
- Brennan P. J. Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S420–S430. doi: 10.1093/clinids/11.supplement_2.s420. [DOI] [PubMed] [Google Scholar]
- Brownback P. E., Barrow W. W. Modified lymphocyte response to mitogens after intraperitoneal injection of glycopeptidolipid antigens from Mycobacterium avium complex. Infect Immun. 1988 May;56(5):1044–1050. doi: 10.1128/iai.56.5.1044-1050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozna J. P., Horan M., Rademacher J. M., Pabst K. M., Pabst M. J. Monocyte responses to sulfatide from Mycobacterium tuberculosis: inhibition of priming for enhanced release of superoxide, associated with increased secretion of interleukin-1 and tumor necrosis factor alpha, and altered protein phosphorylation. Infect Immun. 1991 Aug;59(8):2542–2548. doi: 10.1128/iai.59.8.2542-2548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camphausen R. T., Jones R. L., Brennan P. J. Antigenic relationship between Mycobacterium paratuberculosis and Mycobacterium avium. Am J Vet Res. 1988 Aug;49(8):1307–1310. [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 1984 Jul;74(3):218–262. [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S., Thayer W. R., Jr, Coutu J. A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J Clin Microbiol. 1984 Nov;20(5):966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Coutu J. A. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. J Clin Microbiol. 1986 Sep;24(3):357–363. doi: 10.1128/jcm.24.3.357-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Merkal R. S., Coutu J. A. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1073–1079. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Misaki A. Carbohydrate analysis of concanavalin A-reactive and concanavalin A-nonreactive mycobacterial polysaccharides. Am Rev Respir Dis. 1976 May;113(5):705–706. doi: 10.1164/arrd.1976.113.5.705. [DOI] [PubMed] [Google Scholar]
- Daniel T. M. The antigenicity in guinea pigs and monkeys of three mycobacterial polysaccharides purified by affinity chromatography with concanavalin A. Am Rev Respir Dis. 1975 Jun;111(6):787–793. doi: 10.1164/arrd.1975.111.6.787. [DOI] [PubMed] [Google Scholar]
- Daniel T. M. The purification of mycobacterial polysaccharides with concanavalin A. Am Rev Respir Dis. 1974 Nov;110(5):634–640. doi: 10.1164/arrd.1974.110.5.634. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Todd L. S. The species distribution of three concanavalin A-purified mycobacterial polysaccharides. Am Rev Respir Dis. 1975 Sep;112(3):361–364. doi: 10.1164/arrd.1975.112.3.361. [DOI] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Baccarini M. Heterogeneous activity of immature and mature cells of the murine monocyte-macrophage lineage derived from different anatomical districts against yeast-phase Candida albicans. Infect Immun. 1986 Nov;54(2):477–486. doi: 10.1128/iai.54.2.477-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Fournie J. J., Adams E., Mullins R. J., Basten A. Inhibition of human lymphoproliferative responses by mycobacterial phenolic glycolipids. Infect Immun. 1989 Nov;57(11):3653–3659. doi: 10.1128/iai.57.11.3653-3659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelber R. H., Li F., Cho S. N., Byrd S., Rajagopalan K., Brennan P. J. Serum antibodies to defined carbohydrate antigens during the course of treated leprosy. Int J Lepr Other Mycobact Dis. 1989 Dec;57(4):744–751. [PubMed] [Google Scholar]
- Goren M. B. Immunoreactive substances of mycobacteria. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):50–69. doi: 10.1164/arrd.1982.125.3P2.50. [DOI] [PubMed] [Google Scholar]
- Hines M. E., 2nd, Long A. R., Snider T. G., 3rd, Barker S. A. Lysis and fractionation of Mycobacterium paratuberculosis and Escherichia coli by matrix solid-phase dispersion. Anal Biochem. 1991 Jun;195(2):197–206. doi: 10.1016/0003-2697(91)90317-m. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Rivoire B., Mehra V., Bloom B. R., Brennan P. J. The major native proteins of the leprosy bacillus. J Biol Chem. 1990 Aug 25;265(24):14065–14068. [PubMed] [Google Scholar]
- Jarnagin J. L., Colgrove G. S. Identification of Mycobacterium bovis, using miniaturized thin-layer chromatography and morphologic characteristics. Am J Vet Res. 1986 Nov;47(11):2346–2348. [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long A. R., Hsieh L. C., Malbrough M. S., Short C. R., Barker S. A. Isolation and gas chromatographic determination of chlorsulfuron in milk. J Assoc Off Anal Chem. 1989 Sep-Oct;72(5):813–815. [PubMed] [Google Scholar]
- Lévy-Frébault V. V., Thorel M. F., Varnerot A., Gicquel B. DNA polymorphism in Mycobacterium paratuberculosis, "wood pigeon mycobacteria," and related mycobacteria analyzed by field inversion gel electrophoresis. J Clin Microbiol. 1989 Dec;27(12):2823–2826. doi: 10.1128/jcm.27.12.2823-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R. J., Hermon-Taylor J. Determination of genome size and DNA homology between an unclassified Mycobacterium species isolated from patients with Crohn's disease and other mycobacteria. J Gen Microbiol. 1987 Jan;133(1):211–214. doi: 10.1099/00221287-133-1-211. [DOI] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R., Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987 May;25(5):796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Thompson J., Chiodini R., Hermon-Taylor J. The use of DNA probes identifying restriction-fragment-length polymorphisms to examine the Mycobacterium avium complex. Mol Microbiol. 1987 Nov;1(3):283–291. doi: 10.1111/j.1365-2958.1987.tb01934.x. [DOI] [PubMed] [Google Scholar]
- Middlebrook G., Coleman C. M., Schaefer W. B. SULFOLIPID FROM VIRULENT TUBERCLE BACILLI. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1801–1804. doi: 10.1073/pnas.45.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momotani E., Whipple D. L., Thiermann A. B., Cheville N. F. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet Pathol. 1988 Mar;25(2):131–137. doi: 10.1177/030098588802500205. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Gross J. M., Brozna J. P., Goren M. B. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988 Jan 15;140(2):634–640. [PubMed] [Google Scholar]
- Sibley L. D., Franzblau S. G., Krahenbuhl J. L. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect Immun. 1987 Mar;55(3):680–685. doi: 10.1128/iai.55.3.680-685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Hunter S. W., Brennan P. J., Krahenbuhl J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988 May;56(5):1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect Immun. 1987 Feb;55(2):446–450. doi: 10.1128/iai.55.2.446-450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spargo B. J., Crowe L. M., Ioneda T., Beaman B. L., Crowe J. H. Cord factor (alpha,alpha-trehalose 6,6'-dimycolate) inhibits fusion between phospholipid vesicles. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):737–740. doi: 10.1073/pnas.88.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba H., Crawford J. T., Ellner J. J. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophage-activating factor activity of gamma interferon. Infect Immun. 1989 Jan;57(1):239–244. doi: 10.1128/iai.57.1.239-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Kawasumi H., Takashima T., Tsuyuguchi T., Kishimoto S. Mycobacterium avium-Mycobacterium intracellular complex-induced suppression of T-cell proliferation in vitro by regulation of monocyte accessory cell activity. Infect Immun. 1990 May;58(5):1369–1378. doi: 10.1128/iai.58.5.1369-1378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Clara A. M. A 25-kilodalton fraction from Mycobacterium tuberculosis that inhibits hexose monophosphate shunt activity, lysozyme release, and H2O2 production: reversal by gamma interferon. Infect Immun. 1989 Mar;57(3):864–869. doi: 10.1128/iai.57.3.864-869.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Cohen J. D., Rabson A. R. Gamma interferon reverses inhibition of leukocyte bactericidal activity by a 25-kilodalton fraction from Mycobacterium tuberculosis. Infect Immun. 1987 Nov;55(11):2777–2782. doi: 10.1128/iai.55.11.2777-2782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Zurbrick B. G., Czuprynski C. J. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infect Immun. 1987 Jul;55(7):1588–1593. doi: 10.1128/iai.55.7.1588-1593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]