Abstract
The purpose of this study was to determine whether the B subunit of cholera toxin (CtxB) has adjuvant activity over and above serving as a carrier protein for orally administered vaccines. An oligonucleotide that encodes an antigenic determinant (GtfB.1) from the glucosyltransferase B gene (gtfB) of Streptococcus mutans was genetically fused to the 5' terminus of either the CtxB gene (ctxB) or the Escherichia coli alkaline phosphatase gene (phoA). The resulting chimeric proteins were expressed in a phoA mutant strain of E. coli and then purified. The antigenicities of the proteins were confirmed by immunoblotting analysis using antisera specific for GtfB, CtxB, or PhoA. An equimolar amount of peptide on each carrier was administered by gastric intubation to mice three times at 10-day intervals. Antibody titers to the peptide, CtxB, and PhoA (in the serum, intestine, vagina, saliva, and bronchus) were determined by enzyme immunoassay. Antibody to the peptide was detected only in the sera of mice immunized with the peptide fused to CtxB. No antipeptide antibody was detected in mice immunized with the peptide fused to PhoA. The lack of detectable levels of antipeptide antibody in intestinal lavage fluid was attributed to dilution of the sample beyond the sensitivity of the assay. This was confirmed by cultivation of Peyer's patch and mesenteric lymph node tissue from mice orally immunized with the GtfB.1::CtxB chimera. Using this method, antipeptide antibody was detected in the culture fluid. We conclude that CtxB possesses unique properties that allow it to act as more than a simple carrier protein.
Full text
PDF

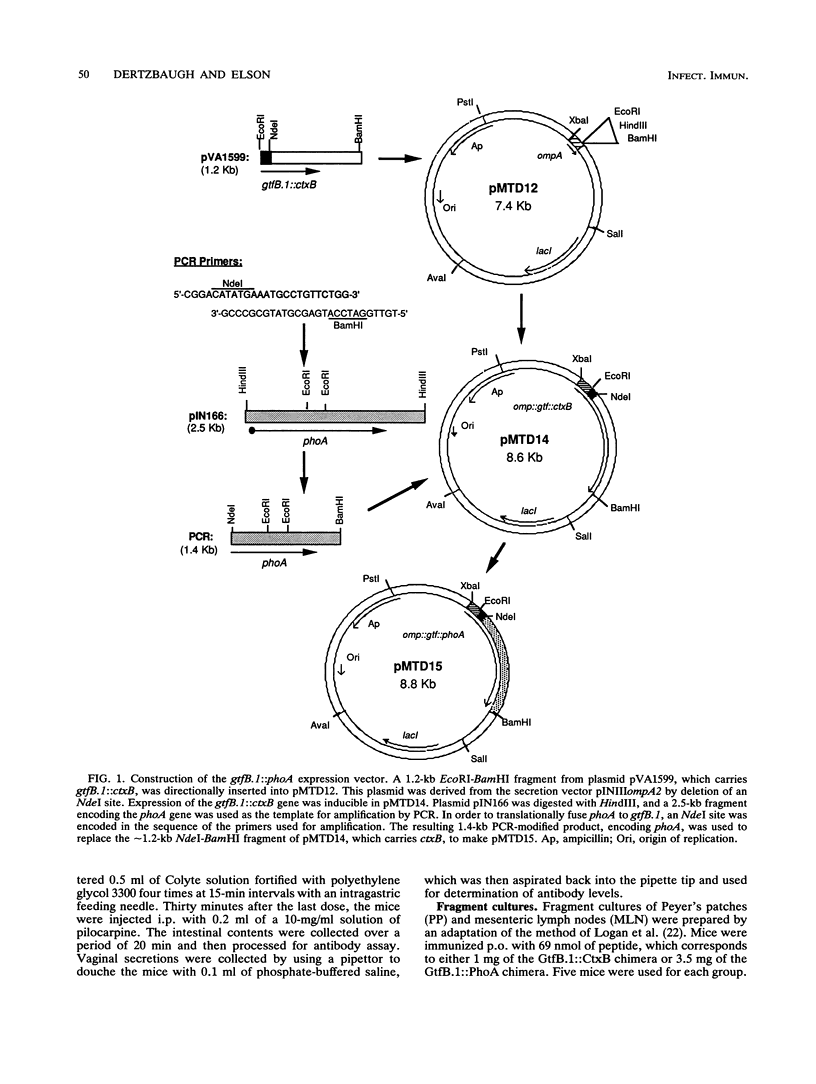


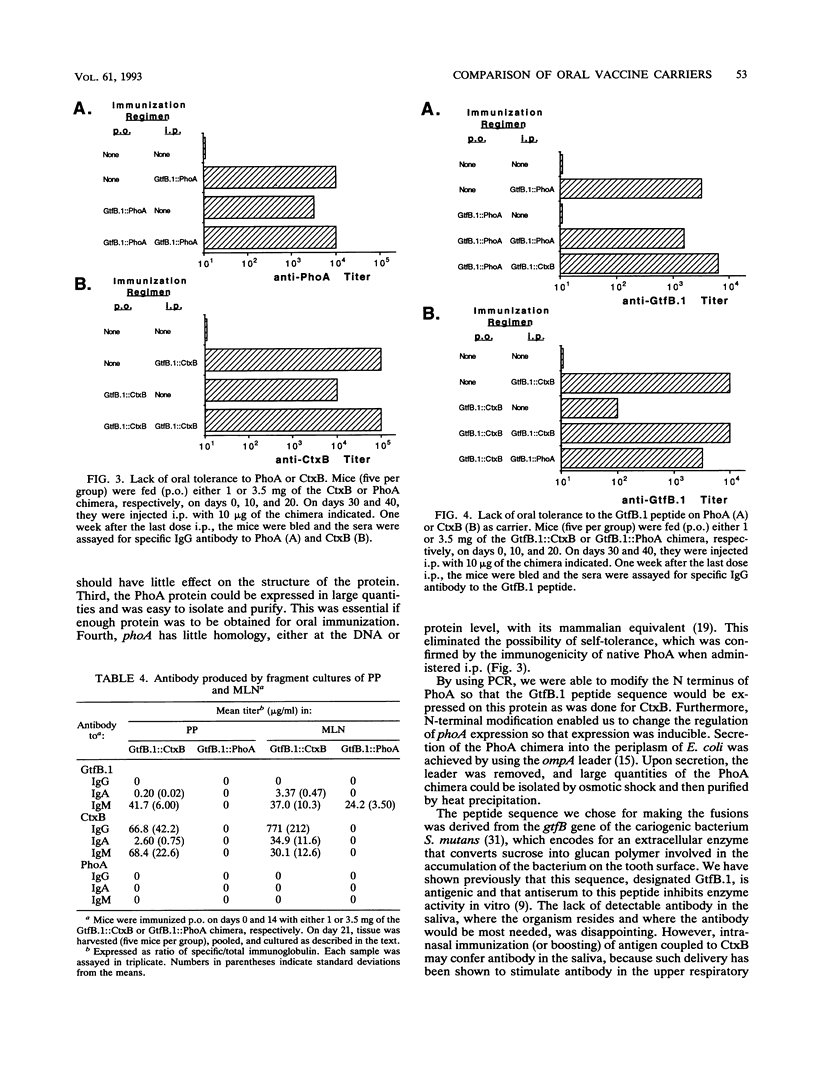


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastassiou E. D., Yamada H., Boumpas D. T., Tsokos G. C., Thyphronitis G., Balow J., Mond J. J. Cholera toxin promotes the proliferation of anti-mu antibody-prestimulated human B cells. Cell Immunol. 1992 Mar;140(1):237–247. doi: 10.1016/0008-8749(92)90190-z. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bromander A., Holmgren J., Lycke N. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J Immunol. 1991 May 1;146(9):2908–2914. [PubMed] [Google Scholar]
- Challacombe S. J., Tomasi T. B., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980 Dec 1;152(6):1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. N., Kuang W. J., Chen E. Y. Nucleotide sequence of the alkaline phosphatase gene of Escherichia coli. Gene. 1986;44(1):121–125. doi: 10.1016/0378-1119(86)90050-8. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Russell M. W., Lycke N., Lindblad M., Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989 Apr;57(4):1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertzbaugh M. T., Macrina F. L. Inhibition of Streptococcus mutans glucosyltransferase activity by antiserum to a subsequence peptide. Infect Immun. 1990 Jun;58(6):1509–1513. doi: 10.1128/iai.58.6.1509-1513.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertzbaugh M. T., Peterson D. L., Macrina F. L. Cholera toxin B-subunit gene fusion: structural and functional analysis of the chimeric protein. Infect Immun. 1990 Jan;58(1):70–79. doi: 10.1128/iai.58.1.70-79.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984 Dec;133(6):2892–2897. [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Elson C. O., Ealding W., Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984 Feb 24;67(1):101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- Ghrayeb J., Kimura H., Takahara M., Hsiung H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984 Oct;3(10):2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye H., Michaelis S., Wright A., Beckwith J. Cloning and restriction mapping of the alkaline phosphatase structural gene (phoA) of Escherichia coli and generation of deletion mutants in vitro. J Bacteriol. 1981 May;146(2):668–675. doi: 10.1128/jb.146.2.668-675.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam W., Clauser E., Kim Y. S., Kan Y. W., Rutter W. J. Cloning, sequencing, and chromosomal localization of human term placental alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8715–8719. doi: 10.1073/pnas.82.24.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang X. P., Lamm M. E., Nedrud J. G. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988 Sep 1;141(5):1495–1501. [PubMed] [Google Scholar]
- Logan A. C., Chow K. P., George A., Weinstein P. D., Cebra J. J. Use of Peyer's patch and lymph node fragment cultures to compare local immune responses to Morganella morganii. Infect Immun. 1991 Mar;59(3):1024–1031. doi: 10.1128/iai.59.3.1024-1031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Severinson E., Strober W. Cholera toxin acts synergistically with IL-4 to promote IgG1 switch differentiation. J Immunol. 1990 Nov 15;145(10):3316–3324. [PubMed] [Google Scholar]
- McGhee J. R., Michalek S. M. Immunobiology of dental caries: microbial aspects and local immunity. Annu Rev Microbiol. 1981;35:595–638. doi: 10.1146/annurev.mi.35.100181.003115. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- Morrison D. A. Transformation in Escherichia coli: cryogenic preservation of competent cells. J Bacteriol. 1977 Oct;132(1):349–351. doi: 10.1128/jb.132.1.349-351.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedrud J. G., Liang X. P., Hague N., Lamm M. E. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987 Nov 15;139(10):3484–3492. [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroza T., Ueda S., Kuramitsu H. K. Sequence analysis of the gtfB gene from Streptococcus mutans. J Bacteriol. 1987 Sep;169(9):4263–4270. doi: 10.1128/jb.169.9.4263-4270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowadski J. M., Handschumacher M. D., Murthy H. M., Foster B. A., Wyckoff H. W. Refined structure of alkaline phosphatase from Escherichia coli at 2.8 A resolution. J Mol Biol. 1985 Nov 20;186(2):417–433. doi: 10.1016/0022-2836(85)90115-9. [DOI] [PubMed] [Google Scholar]
- Tamura S., Samegai Y., Kurata H., Nagamine T., Aizawa C., Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988 Oct;6(5):409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Woogen S. D., Ealding W., Elson C. O. Inhibition of murine lymphocyte proliferation by the B subunit of cholera toxin. J Immunol. 1987 Dec 1;139(11):3764–3770. [PubMed] [Google Scholar]



