Abstract
Several lines of research have illustrated that negative environments can precipitate psychopathology, particularly in the context of relatively increased biological risk, while social resources can buffer the effects of these environments. However, little research has examined how social resources might buffer proximal biological risk for psychopathology or the neurobiological pathways through which such buffering may be mediated. Here we report that the expression of trait anxiety as a function of threat-related amygdala reactivity is moderated by perceived social support, a resource for coping with adversity. A significant positive correlation between amygdala reactivity and trait anxiety was evident in individuals reporting below-average levels of support but not in those reporting average or above-average levels. These results were consistent across multiple measures of trait anxiety and were specific to anxiety in that they did not extend to measures of broad negative or positive affect. Our findings illuminate a biological pathway, namely moderation of amygdala-related anxiety, through which social support may confer resilience to psychopathology. Moreover, our results indicate that links between neural reactivity and behavior are not static but rather may be contingent on social resources.
Keywords: Anxiety, Social Support, Amygdala, Neuroscience, Neuroimaging, Psychopathology
1. Introduction
A substantial literature highlights the critical role of stressful or adverse circumstances in precipitating psychopathology, particularly in relation to individual differences in personality, brain function, and genetic predisposition (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010; Caspi et al., 2003; Costello et al., 2002; Monroe & Simons, 1991). In general, epidemiological studies have reported increased risk for psychopathology, particularly mood and anxiety disorders, in individuals having encountered a variety of adverse or stressful situations including childhood maltreatment, violent crime, divorce, unemployment, and medical illness (Hammen, 2005; Kendler, Karkowski, & Prescott, 1999; Monroe & Simons, 1991). In parallel, human neuroimaging studies have revealed that increased sensitivity of neural circuitries, especially the amygdala, to threat and stress may mediate this risk (Price & Drevets, 2010).
Reactivity of the amygdala to threat-related cues such as emotional faces has been linked to trait anxiety (Fakra et al., 2009), anxiety disorders (Phan, Fitzgerald, Nathan, & Tancer, 2006), and depression (Monk et al., 2008; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002), and therefore represents a well researched biological risk factor for negative psychological outcomes. The link between trait anxiety and amygdala reactivity is particularly robust and seen across many different studies and approaches (Dickie & Armony, 2008; Etkin et al., 2004; Fakra et al., 2009; Haas, Omura, Constable, & Canli, 2007; Hariri, 2009; Killgore & Yurgelun-Todd, 2005; Most, Chun, Johnson, & Kiehl, 2006; Ray et al., 2005; Stein, Simmons, Feinstein, & Paulus, 2007). Thus this research suggests that variability in anxiety, an individual’s behavioral sensitivity to threat and stress, reflects in part both the underlying reactivity of these neural circuitries as well as the risk for psychopathology associated with stress and adversity (Hariri, 2009).
Partly in response to the work emphasizing the harmful psychological effects of negative environments, resiliency research has explored factors that may buffer against negative environments and biological risk leading to positive outcomes even in unfavorable environments (Cohen & Hoberman, 1983; Masten, 2001; Masten & Coatsworth, 1998; Rutter, 2006). One such buffering factor is social support, which typically reflects people in an individual’s life (family, friends, neighbors, community members) that are available in times of need and can provide resources such as emotional support (someone to communicate with), companionship (someone to spend time and share activities with), and instrumental aid (financial and material resources; Cohen & Wills, 1985). Social support has been shown to buffer the relation between negative life events and depressive symptomatology (Cohen, McGowan, Fooskas, & Rose, 1984; Cohen & Hoberman, 1983), and has been associated broadly with well-being and health, both directly and as a buffer against stressful circumstances (Turner, 1981). Interestingly, perceptions of social support are often more powerful in predicting the buffering effects of this support than objective measures (Cohen et al., 1984). For example, in a review of the stress and social support literature, Cohen and Willis (1985) concluded that studies assessing perceptions of social support found evidence for the “buffering” effect of social support (social support predicted more positive outcomes only during times of stress), while those using more objective measures of social support generally found only a main effect of social support (the support predicted positive outcomes regardless of levels of stress). Thus, individuals’ subjective appraisal of their social support may be particularly important in evaluating the effects of social support as a moderator between biological risk and health related outcomes.
In the current study we asked if perceived social support, a resource for coping with adversity, moderates the link between amygdala reactivity to threat-related cues and the expression of trait anxiety, a well-established personality risk factor for psychopathology (Kendler, Kuhn, & Prescott, 2004; Lahey, 2009). To do so, we used blood oxygen level-dependent functional MRI (BOLD fMRI) to assess threat-related amygdala reactivity in 103 healthy adults. Social support was measured using the Interpersonal Support Evaluation List, a self-report measure of perceived availability of potential social resources including material aid, as well as individuals with whom one can interact and share experiences (Cohen & Hoberman, 1983; Cohen & Wills, 1985). Multiple indices of personality, mood, and affect as well as an index of recent negative life experiences were also assessed via self-report to examine the specificity of links between social support, amygdala reactivity, and trait anxiety.
2. Materials and Methods
2.1 Participants
103 participants (45% male; mean age=44.5 years; SD=6.8; range: 31-54 years) were recruited from a larger community sample of 1379 middle-aged volunteers who were in good general health and free of the major medical or psychiatric illness (Fakra et al., 2009; Manuck et al., 2010). Written informed consent according to the guidelines of the University of Pittsburgh’s institutional review board was provided by all subjects before their participation in the neuroimaging subcomponent of the larger project. All participants included in these analyses were in good general health and free of the following: (1) medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease, or a lifetime history of psychotic symptoms; (2) use of psychotropic, glucocorticoid, or cardiovascular (e.g., antihypertensive or antiarrhythmic) medication; (3) conditions that affect cerebral blood flow and metabolism (e.g., hypertension); and (4) any current DSM-IV Axis I disorder as assessed by the nonpatient version of the Structured Clinical Interview for DSM-IV. Participants reported their race as follows: 88% reported being European-American, 7% reported being African American, and 4% reported being other races (Asian-American, Multi-racial, or other).
2.2 Amygdala reactivity paradigm
We used a well-characterized and widely used fMRI challenge paradigm which elicits robust bilateral amygdala reactivity to threat-related cues (Bigos et al., 2008; Brown et al., 2005; Fakra et al., 2009; Fisher et al., 2009; Fisher, Meltzer, Ziolko, Price, & Hariri, 2006; Hariri et al., 2009; Manuck, Brown, Forbes, & Hariri, 2007; Manuck et al., 2010). The paradigm consists of 4 blocks of a perceptual face processing task interleaved with 5 blocks of a sensorimotor control task. During face processing blocks, participants view a trio of faces (expressing either anger or fear) and select 1 of 2 faces (bottom) that is identical to a target face (top). Angry and fearful facial expressions can represent honest indicators of an ecologically valid threat and have likely been associated with negative outcomes in the past. In this context, we interpret the amygdala activation elicited by our task as being threat-related. Each face-processing block consists of 6 images, balanced for gender and target affect (angry or fearful), all of which were derived from a standard set of pictures of facial affect (Ekman & Friesen, 1976). During the sensorimotor control blocks, participants view a trio of simple geometric shapes (circles and vertical and horizontal ellipses) and select 1 of 2 shapes (bottom) that are identical to a target shape (top). Each sensorimotor control block consists of 6 different shape trios. All blocks are preceded by a brief instruction (“match faces” or “match shapes”) that lasts 2 seconds. In the face-processing blocks, each of the 6 face trios is presented for 4 seconds with a variable inter-stimulus interval (ISI) of 2 to 6 seconds (mean = 4 seconds), for a total block length of 48 seconds. In the sensorimotor control block, each of the 6 shape trios is presented for 4 seconds with a fixed inter-stimulus interval of 2 seconds, for a total block length of 36 seconds. Total task time is 390 seconds.
2.3 BOLD acquisition parameters
Each participant underwent scanning with a Siemens 3-T MAGNETOM Allegra (Siemens AG, Erlangen, Germany), which was developed specifically for advanced brain imaging applications and is characterized by increased T2* sensitivity and fast gradients (slew rate, 400 T/m/s), which minimize echospacing, thereby reducing echoplanar imaging geometric distortions and improving image quality. Blood oxygen level-dependent (BOLD) functional images were acquired with a gradient-echo EPI sequence (TR/TE=2000/25 milliseconds, FOV=20 cm, matrix=64×64), covering 34 interleaved axial slices (3mm) on a Siemens 3T Allegra scanner developed specifically for advanced brain imaging applications. All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before collecting fMRI data for each participant, we acquired a reference echoplanar imaging scan, which we visually inspected for artifacts (e.g., ghosting) and good signal across the entire volume of acquisition, including the amygdala. Additionally, an autoshimming procedure was conducted before the acquisition of BOLD data in each participant to minimize field inhomogeneities.
2.4 Image Processing and analysis
Whole-brain image analysis was completed using the general linear model of SPM2 (http://www.fil.ion.ucl.ac.uk/spm). Images for each participant were realigned to the first volume in the time series to correct for head motion, spatially normalized into standard stereotactic space (Montreal Neurological Institute template) using a 12-parameter affine model, and smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter (6mm FWHM). Voxelwise signal intensities were ratio-normalized to the whole-brain global mean.
Following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate condition-specific (faces>shapes) contrast images for each individual, which were used in second-level random effects models accounting for scan-to-scan and participant-to-participant variability to determine mean condition-specific regional responses using one-sample t-tests (voxel threshold = p<0.05, FDR-corrected; cluster threshold ≥ 10 contiguous voxels).
2.4.1 Amygdala Regions of Interest
BOLD contrast estimates were extracted from functional clusters exhibiting a main effect of task using the above threshold within anatomically defined amygdala regions of interest (ROIs). Due to structural and functional heterogeneity of amygdala nuclei implicated in the processing of threat-related cues we independently examined the ventral and dorsal amygdala, which encompass the amygdala’s principal input and output regions, respectively (Manuck et al., 2010). We constructed hemisphere-specific ROIs using Marsbar (version 0.41) for the ventral amygdala, which encompassed the basolateral complex, and for the dorsal amygdala, which encompassed the central nucleus as well as the sublenticular extended amygdala and nucleus baysalis of Meynert. The ventral amygdala ROIs were anchored by MNI coordinates x = ± 21, y = −3, z = −23, with widths of 14mm, 6mm, and 6mm along the x-, y-, and z-axes, respectively. The total volume of the ventral amygdala ROI was 1024mm3 in each hemisphere. The dorsal amygdala ROI was anchored by the MNI coordinates x = ± 21, y = −4, z = −13, with widths of 14mm, 8mm and 10mm along the x-, y-, and z-axes, respectively. The total volume of the dorsal amygdala ROI was 1920mm3 in each hemisphere. The reported widths reflect the total for the ROI along each axis and are centered on the MNI coordinate anchoring each axis (i.e., with x = 21 and width = 14mm, the range of coordinates included along that axis of the ROI are from x = 14 to x = 28). The posterior extent of both the dorsal and ventral amygdala was carefully defined to exclude the hippocampus.
2.5 Self-Report Measures
The Interpersonal Support Evaluation List (ISEL) measures the perceived availability of social support through self-report (Cohen & Hoberman, 1983). This measure assesses availability of support across four domains: availability of material aid, availability of someone to talk to about one’s problems, availability of a positive comparison when comparing one’s self to others, and availability of people one can do things with.
The Revised NEO Personality Inventory (NEO-PI-R) contains the trait Neuroticism (N) characterized by emotional distress, negative affect, and lack of emotional stability. The N1 subscale of Neuroticism measures Anxiety. Those high on “Anxiety” in this scale are characterized by anxiousness, fearfulness, worrying, and feelings of nervousness. The trait Extraversion (E) is characterized by sociability and tendency to experience positive emotions (Costa & McCrae, 1992, 1995).
The Temperament and Character Inventory (TCI) contains the trait Harm Avoidance (HA) characterized by excessive worrying, pessimism and shyness. The H1 subscale of HA measures Anticipatory Worry (Cloninger, Przybeck, Svrakic, & Wetzel, 1994). Those high on “Anticipatory Worry” express apprehension, uncertainty and fear about future events and exhibit a pervading sense of pessimism (Cloninger, Svrakic, & Przybeck, 1993).
The State-Trait Anxiety Inventory (STAI) contains a Trait Anxiety scale (STAI-T) measuring general and long standing feelings of apprehension, tension, nervousness, and worry (Spielberger, 1983).
The Positive Affect Negative Affect Schedule (PANAS) measures independent constructs of Positive and Negative Affect as both personality states and traits (Watson, Clark, & Tellegen, 1988).
2.6 Statistical Analyses
Interactions were analyzed using PASW (v.18, SPSS Inc.) according to the guidelines of Preacher et al. (Preacher, Curran, & Bauer, 2006). The main effects of amygdala reactivity, social support, and the amygdala reactivity-social support interaction term were entered into a regression predicting anxiety while controlling for gender. Each regression was run for all combinations of amygdala reactivity (left versus right and dorsal versus ventral) and anxiety (N1, HA, H1, STAI-T, latent anxiety construct). Identical analyses were conducted with measures of negative affect (neuroticism, negative affect) and positive affect (extraversion, positive affect) as an outcome. Our latent trait anxiety construct was composed of N1, H1, and STAI-T. This latent construct was constructed using a confirmatory factor analysis in Mplus (v.5), all factors demonstrated high loadings (all >.79), and factor scores were extracted and used to test subsequent interactions.
3. Results
Consistent with prior reports (Manuck et al., 2010), there was robust bilateral activation within our anatomically defined ventral and dorsal amygdala ROIs.
Social support was correlated with measures of trait anxiety (r = −.26 to −.48, p < .01), but not with measures of amygdala reactivity (r = −.09 to −.13, p > .15). In interaction analyses, social support predicted anxiety and moderated amygdala-related anxiety (Table 1). Moreover, these results were consistent across multiple measures of anxiety and highly specific to anxiety. In those reporting less than average levels of social support, amygdala reactivity was positively and significantly correlated with measures of anxiety. However, in those reporting average or above average levels of support there was no correlation or non-significant negative correlation between these variables. The results were in the same direction for dorsal and ventral regions of the amygdala but much stronger in the dorsal regions (interaction terms predicting latent anxiety: left dorsal β = −.265, p = .005; left ventral β = −.122, p = .18; right dorsal β = −.267, p = .005; right ventral β = −.078, p = .40).
Table 1.
Summary of regressions predicting outcomes with main effects and interaction terms for self-report measures of anxiety, affect and social support, and left dorsal amygdala reactivity while controlling for gender
| Outcome measure | Left Dorsal Amygdala β |
Social Support β |
Interaction Term β |
|---|---|---|---|
| Broad Negative Affect Measures | |||
|
| |||
| NEO-PI-R–Neuroticism | .136 | −.457*** | −.097 |
| PANAS–Negative Affect | .101 | −.323** | −.082 |
|
| |||
| Specific Anxiety Measures | |||
|
| |||
| Latent Construct of Anxiety | .082 | −.355*** | −.265** |
| NEO–N1–anxiety | −.012 | −.225* | .231* |
| TCI–Harm Avoidance | .039 | −.389*** | −.191* |
| TCI–Harm Avoidance H1–anticipatory worry | .158+ | −.300** | −.305*** |
| STAI–Trait Anxiety | .085 | −.438*** | −.156+ |
|
| |||
| Positive Emotion and Affect Measures | |||
|
| |||
| PANAS–Positive Affect | .014 | .505*** | .094 |
| NEO-PI-R–Extraversion | .022 | .461*** | .095 |
p <.10
p<.05
p<.01
p<.001.
While this general pattern of moderation was present across broad measures of negative affect and emotionality (i.e., neuroticism, negative affect), the interaction terms did not reach significance. However, there were strong and significant interactions when analyzing specific subscales within these and other measures that selectively assess anxiety (i.e., anxiety proneness, harm avoidance, anticipatory worry, and trait anxiety). Interestingly, the results were strongest when a latent construct of anxiety was derived from these subscales and used as an outcome (Figure 1). Moreover, within the various measures of anxiety, results were particularly strong for the anticipatory worry scale of harm avoidance. Similar analyses with measures of positive emotion and affect (i.e., extraversion, positive affect) did not produce significant results (Table 1).
Figure 1.
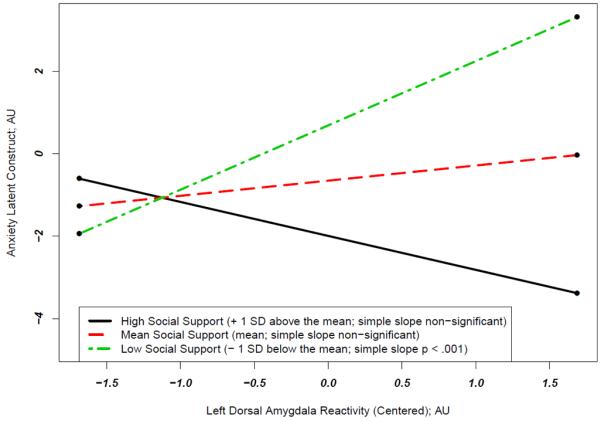
Left dorsal amygdala reactivity differentially predicts a latent construct of anxiety as a function of perceived social support. The significant interaction term (β=−.265, p<.01) and simple slopes indicate that amygdala reactivity has a significant and positive correlation with anxiety at below average levels of social support (at 1 SD below the mean of social support, the simple slope = 1.56, SE = .55, p = .005), but at average or above average (+1SD) levels of social support this relation is not statistically significant (at 1 SD above the mean of social support the simple slope = −.824, SE = .59, p = .17 and at the mean of social support the simple slope = .368, SE = .39, p > .3). Amygdala reactivity did not have a main effect on anxiety (β=.082, p>.3). Social support was negatively associated with anxiety (β=−.364, p<.001). All analyses control for gender. AU=Arbitrary Units; SD=Standard Deviation. The latent construct of anxiety was derived from a confirmatory factor analysis of the three different self-report measures of trait anxiety used in our study and thus reflects the measurement of trait anxiety common to and underlying all three separate measures.
When examining the interaction within the left dorsal amygdala predicting the latent anxiety construct using simple slope calculations, the pattern of results became more evident: amygdala reactivity has a significant and positive correlation with anxiety at below average levels of social support (at 1 SD below the mean of social support, the simple slope = 1.56, SE = .55, p = .005), but at average or above average (+1 SD) levels of social support this relationship is not statistically significant (+1 SD: simple slope = −.824, SE = .59, p = .17; Mean: simple slope = .368, SE = .39, p > .3).
By using interaction methods described above (Preacher et al., 2006), we were able to determine at what values of social support the relationship between left dorsal amygdala reactivity and the latent construct of anxiety became significant. In this regression, there is a significant positive relationship between amygdala reactivity and anxiety for those at or below 0.38 standard deviations below the mean of social support. As 34 of the 103 participants had social support scores that fell below this mark, this positive relationship between amygdala reactivity and anxiety is quite salient, even in this community sample. A statistically significant negative relationship between amygdala reactivity and social support only theoretically emerges for those 1.66 standard deviations above the mean of social support, which is beyond the range of reported social support values (range 6 – 36, mean = 28.5; SD =6.5). Thus, while the line in Figure 1 depicting the relationship between anxiety and amygdala reactivity for those high on social support appears to have a negative slope, this slope is not statistically significant and would not be statistically significant within the present range of data (see Supplemental Figure 1 for a plot of all participants’ scores).
To determine if these interaction effects were specific to amygdala reactivity, we examined several regions of interest in an extended corticolimbic circuitry also engaged by our fMRI paradigm. These regions of interest included orbitofrontal cortex (OFC; BA11), as well as bilateral ventrolateral prefrontal cortex (vlPFC; BA47) and hippocampal formation, and right posterior fusiform gyrus (see Supplemental Methods). Using methods identical to those for amygdala reactivity, we examined if extracted BOLD values from each of these brain regions interacted with social support to predict the latent construct of trait anxiety. We found a significant interaction effect between social support and task-related activation in OFC and right vlPFC. No significant interaction effects were found for the other brain regions, although a trend emerged for left vlPFC (Table 2). For regions exhibiting a significant interaction effect (i.e., OFC and right vlPFC), the graphs of these interactions and simple slope analyses were nearly identical to those for the amygdala: at low levels of social support there is a significant positive relationship between activation and anxiety, but at mean or high levels of social support there is no significant relationship.
Table 2.
Summary of additional corticolimbic regions of interest (ROI) tested for interaction effects with social support. Note that each regression included sex as a covariate, main effects of social support, main effects of the ROI, and the interaction between ROI and social support. Maximum voxel coordinates for each ROI are reported in MNI (ICBM 152) space
| Region of Interest (ROI) | Interaction Term: ROI x Social support (β) |
|---|---|
| Orbitofrontal Cortex (BA11) | |
| X = 2, Y = 56, Z = −14 | −.267** |
|
| |
| Ventrolateral Prefrontal Cortex (BA47) | |
| Right: X = 32, Y = 34, Z = −16 | −.265** |
| Left: X = −26, Y = 12, Z = −20 | −.156+ |
|
| |
| Hippocampal Formation | |
| Right: X = 28, Y = −12, Z = −18 | −.170 |
| Left: X = −28, Y = −14, Z = −14 | −.014 |
|
| |
| Posterior Fusiform Gyrus | |
| X = 40 Y = −46, Z = −20 | .040 |
p <.10
p<.05
p<.01
p<.001
Discussion
Our findings reveal that the behavioral expression of threat-related amygdala reactivity as anxiety is significantly moderated by the perceived availability of social support. Specifically, the commonly observed positive correlation between threat-related amygdala reactivity and anxiety (Dickie & Armony, 2008; Etkin et al., 2004; Fakra et al., 2009; Haas et al., 2007; Hariri, 2009; Killgore & Yurgelun-Todd, 2005; Most et al., 2006; Ray et al., 2005; Stein et al., 2007) was absent in individuals reporting average or above average levels of social support. In contrast, there was a significant positive correlation between amygdala reactivity and anxiety in those reporting below average levels of support.
The moderating role of social support was specific to the link between threat-related amygdala reactivity and the expression of negative affect and emotionality, especially anxiety, and did not extend to positive emotion or affect. Remarkably, this social support contingent effect was strongest for covariation between anxiety and reactivity of bilateral dorsal amygdala regions encompassing the central nucleus, sublenticular extended amygdala and nucleus baysalis of Meynert, which collectively drive behavioral and physiological arousal, two hallmarks of anxiety (Davis, Johnstone, Mazzulla, Oler, & Whalen, 2010). This point is particularly salient when considering that main effects and interaction effects were strongest when examining a latent construct of anxiety and the specific “anticipatory worry” subscale of harm avoidance as outcomes. These results highlight the relationship between anxiety as measured as a latent variable across different scales and the more specific construct of anticipatory worry as they relate to specific regions of the extended amygdala previously linked with hypervigilance or heightened anticipation of threat or harm (Somerville, Whalen, & Kelley, 2010). Consistent with this interpretation, the modulatory effect of social support extended to two prefrontal brain regions, namely the OFC and vlPFC, which exhibit reciprocal structural and functional interactions with the amygdala, and together comprise a core circuitry supporting the generation, integration and regulation of behavioral and physiologic arousal. In contrast, the modulatory effect of social support did not extend to the hippocampal formation or posterior fusiform gyrus, two brain regions also interconnected with the amygdala but contributing to contextual memory consolidation and perceptual acuity, respectively, rather than emotional arousal.
More broadly, our findings suggest the intriguing possibility that behavioral expression of underlying variability in brain function can be shaped by external factors and that biology may not necessarily or uniformly predict behavior. While gene-by-environment interaction research has already demonstrated that biological factors distal to behavior (i.e., genetic polymorphisms) do not uniformly affect behavior in the form of increased risk for psychopathology (Caspi et al., 2002; Caspi et al., 2003), the current study emphasizes that similarly meaningful interactions may occur at the level of more proximate biology (i.e., neural reactivity), and that the link between even such proximate factors and behavior is not only probabilistic but subject to the moderating effects of social context.
While we believe these findings are important both for our understanding of the link between threat-related amygdala reactivity and trait anxiety specifically and for developing models of biological risk and psychopathology more broadly, there are some limitations worth noting. First, our measure of social support represents only subjective perceptions of support. Although some literature indicates that perceptions of these resources are more important than objective measures, particularly in the context of buffering risk for negative outcomes (Cohen et al., 1984), it would have been helpful to have had additional objective measures of social support such as reports from other informants or measures of social network integration. Second, there is some nontrivial overlap in the constructs of amygdala reactivity, perceived social support and trait anxiety in that they all may contain a component related to the appraisal of threat. However, as social support was not correlated with amygdala reactivity, we believe these measures index conceptually different constructs. Moreover, we conducted identical analyses using self-reported recent negative life events (Cohen, Tyrell, & Smith, 1991), which include similar reports of subjective perceptions (e.g., how much each event impacted participants) and appraisal of threat, but did not find significant moderation effects (data available upon request). This implies that the results we present are likely specific to social support. Third, the current study is cross-sectional. Thus it is impossible to determine the direction of effects. While we conceptualize social support as a buffering independent variable, it is possible that differences in perceived social support follow from differences in trait anxiety. For example, those higher on trait anxiety may make less of an effort to acquire social support or may evoke less social support. Hence, this possible evocative gene-environment correlation could support the possibility that differences in social support could be the effect rather than cause of trait anxiety. Future studies that prospectively assess the links between these variables are needed to address this issue. Finally, while a notable strength of our study was the use of a large community sample in which there was continuous variability in trait anxiety and social support, these results need to be replicated across different populations. For example, though clinical populations may confound trait anxiety and social support (i.e., those in the patient group are likely to have lower levels of social support), intervention studies that focus on modifying social support (e.g., interpersonal therapy) may be able to examine the explicit moderating role of social support in these populations with obvious clinical implications.
It is worth noting several additional strengths of our approach such as the substantial sample size for a neuroimaging study, a widely used, well validated, and extensive measure of perceived social support, use of multiple measures of anxiety and comparison to measures of other forms of affect and personality, and a conservative analytic approach. As noted in the methods, we extracted our amygdala reactivity values from functional clusters within anatomical regions of interest based on the main effects of our fMRI challenge paradigm. Hence, we avoided any possibility of correlations that are artificially inflated due to extraction and correlation techniques that capitalize on the same data twice (Viviani, 2010). In sum, our analytic method was quite conservative and yet still demonstrated robust effects across multiple measures of anxiety.
Collectively, our results provide evidence that social support, a resource for coping with adversity, acts to buffer or mitigate the expression of anxiety as a function of an individual’s amygdala response to threat-related cues. Although largely independent lines of inquiry have implicated both biases in the reactivity of neural systems as well as environmental circumstances in the etiology of psychopathology, ours directly examined the potential interactions of these factors. More broadly, we provide critical evidence that predictive links between measures of variability in brain function and behavior are not static but rather may be powerfully shaped by external factors. Thus, the current work not only informs our understanding of specific environmentally moderated biological pathways related to risk for psychopathology, but also our theoretical understanding of how neural reactivity maps onto behavior as a function of environmental context.
Supplementary Material
Acknowledgements
We wish to thank Patrick M. Fisher and Kelley M. Kidwell for help with manuscript preparation. This work was supported by NIH grants HL040962 (to S.B.M.) and MH072837 (to A.R.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Peet E, Manuck SB, Williamson DE, Dahl RE, Ferrell RE, Hariri AR. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol Psychiatry. 2005;10:884–888. doi: 10.1038/sj.mp.4001716. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic Sensitivity to the Environment: The Case of the Serotonin Transporter Gene and Its Implications for Studying Complex Diseases and Traits. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory: A Guide to Its Development and Use. Washington University Center for Psychobiology and Personality; St. Louis: 1994. [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cohen LH, McGowan J, Fooskas S, Rose S. Positive life events and social support and the relationship between life stress and psychological disorder. Am J Community Psychol. 1984;12:567–587. doi: 10.1007/BF00897213. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. J Appl Soc Psychol. 1983;13:99–125. [Google Scholar]
- Cohen S, Tyrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psych Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- Costa PT, Jr., McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Psychological Assessment Resources; Odessa: 1992. [Google Scholar]
- Costa PT, Jr., McCrae RR. Domains and facets: hierarchical personality assessment using the revised NEO personality inventory. J Pers Assess. 1995;64:21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, Biederman J, Goldsmith HH, Kaufman J, Lewinsohn PM. Development and natural history of mood disorders. Biol Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cer Cortex. 2010;20:612. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: interaction between sex and trait anxiety. Psychiat Res-Neuroim. 2008;162:51–57. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Fakra E, Hyde LW, Gorka A, Fisher PM, Munoz KE, Kimak M, Halder I, Ferrell RE, Manuck SB, Hariri AR. Effects of HTR1A C(−1019)G on amygdala reactivity and trait anxiety. Arch Gen Psych. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, Coleman FL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR. Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cereb Cortex. 2009;19:2499–2507. doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Hariri AR. Capacity for 5-HT1A–mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–1363. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Emotional conflict and neuroticism: Personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav Neurosci. 2007;121:249–256. doi: 10.1037/0735-7044.121.2.249. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. In: Nolen-Hoeksema S, editor. Annnual Review of Clinical Psychology. Vol. 1. 2005. pp. 293–319. Annual Reviews. [DOI] [PubMed] [Google Scholar]
- Hariri AR. The Neurobiology of Individual Differences in Complex Behavioral Traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Gorka A, Hyde LW, Kimak M, Halder I, Ducci F, Ferrell RE, Goldman D, Manuck SB. Divergent Effects of Genetic Variation in Endocannabinoid Signaling on Human Threat- and Reward-Related Brain Function. Biol Psychiatry. 2009;66:9–16. doi: 10.1016/j.biopsych.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Lahey BB. Public health significance of neuroticism. Am Psychol. 2009;64:241–256. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Brown SM, Forbes EE, Hariri AR. Temporal stability of individual differences in amygdala reactivity. Am J Psychiatry. 2007;164:1613–1614. doi: 10.1176/appi.ajp.2007.07040609. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Flory JD, Gorka A, Ferrell RE, Hariri AR. Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology. 2010;35:94–104. doi: 10.1016/j.psyneuen.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS. Ordinary magic. Resilience processes in development. Am Psychol. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- Masten AS, Coatsworth JD. The development of competence in favorable and unfavorable environments. Lessons from research on successful children. Am Psychol. 1998;53:205–220. doi: 10.1037//0003-066x.53.2.205. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Most SB, Chun MM, Johnson MR, Kiehl KA. Attentional modulation of the amygdala varies with personality. Neuroimage. 2006;31:934–944. doi: 10.1016/j.neuroimage.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JDE, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cog Affect Behav Neurosci. 2005;5:156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Rutter M. Implications of resilience concepts for scientific understanding. Ann N Y Acad Sci. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory (Form Y) Manual. MindGarden; Redwood City: 1983. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Turner RJ. Social support as a contingency in psychological well-being. Journal of Health and Social Behavior. 1981;22:357–367. [Google Scholar]
- Viviani R. Unbiased ROI selection in neuroimaging studies of individual differences. Neuroimage. 2010;50:184–189. doi: 10.1016/j.neuroimage.2009.10.085. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


