Abstract
Two experiments examined the hypothesis that dual systems of stimulus evaluation for categorization can be observed in event-related potentials: one whose duration is indexed by the latency of the P3 component, and a second evident in a later frontal potential. Subjects categorized artificial animals by a “two out of three” rule. Stimuli with two visual features of their own category and one feature of a different category (i.e., near the boundary between categories) elicited very prolonged reaction times as compared to stimuli with three features from a single category. This RT delay was not accompanied by a delayed P3, suggesting that the P3 indexed only a first pass of stimulus evaluation. The near-boundary stimuli elicited more positive potentials than far-boundary stimuli at prefrontal and frontotemporal sites, suggesting that a secondary stage of stimulus evaluation was triggered when detection of single features or simple conjunctions was insufficient to support a correct decision. The frontal potential that was sensitive to categorization difficulty was of opposite polarity to frontal potentials previously observed in manipulations of working memory. The roles of frontal executive processes in categorization and memory tasks are discussed.
Most cognitive theories of categorization posit multiple systems for learning object categories and categorizing objects (Ashby, Alfonso-Reese, Turken & Waldron, 1988; Knowlton, Squire & Gluck, 1994; Reber, Stark & Squire, 1998). One important distinction is between categorization based on logical rules and categorization based on similarity to prototypes or exemplars (Allen & Brooks, 1991; Erickson & Kruschke, 1998; Johansen & Palmeri, 2002; Nosofsky & Palmeri, 2002; Smith, Patalano & Jonides, 1998). Several lines of evidence suggest that rule-based categorization is slower than familiarity-based categorization and places greater demands on working memory and selective attention (Smith et al., 1998). Consistent with this argument is evidence that the two strategies have different neural correlates, with rule-based categorization recruiting prefrontal and parietal cortices to a greater degree (Koenig et al., 2005; Grossman et al., 2002; Patalano, Smith, Jonides & Koeppe, 2001). Although hemodynamic imaging methods have offered some insights into the anatomical correlates of categorization strategies, event-related potentials (ERPs) offer complementary insights into the temporal characteristics of neural activity in addition to coarse-grain information about the brain regions engaged. Below, we review two distinct components of the ERP that appear to reflect temporally and neurally distinct processes during categorization tasks, and then present two experiments designed to dissociate them.
The P3 component and the mental chronometry of categorization
One ERP component that has proven useful in studies of categorization is the P300 or P3. P3 amplitude is larger for rare than frequent events, and it is primarily categorical probability that is relevant – the class to which a stimulus is assigned by experimental instruction. For instance, Kutas, McCarthy, and Donchin (1977) recorded a large parietal P3 to the rare stimulus “John” as compared to the frequent stimulus “Mary” when participants were asked to discriminate male from female names, but an equally large P3 to a variety of male names mixed with a variety of female names that occurred with higher categorical probability. This result indicates that P3 amplitude is not determined by stimulus probability per se, or else the variable-name list would have led to equivalent P3s for each (equally low-probability) name. Johnson and Donchin (1980) similarly observed that – when assigned to the same response category -- two different 33%-probable stimuli elicited the same P3 as a single 67%-probable stimulus in a different block of trials. A study of target stimulus variability by Breton, Ritter, Simson and Vaughan (1988) confirmed the sensitivity of P3 amplitude to categorical but not physical probability. One block of trials was a standard 80/20 oddball paradigm, with responses to a rare target letter and no response to a different single letter. The response requirements and categorical probabilities were the same in another block of trials, but the targets now consisted of 25 different letters. The targets in this second block were thus 20% probable collectively, but less than 1% probable individually. The parietal P3 was larger for rare targets than frequent nontargets in both blocks, but largely unaffected by the variability in physical identity of the target.
Of greater relevance for the current experiments is that the peak latency of the P3 appears to track the time required to assign a stimulus to its experimentally-defined category. For instance, although multiple physically-distinct targets may elicit a P3 of the same amplitude as a single target, P3 latency is prolonged when numerous distinct items must be evaluated for possible membership in the target category (Breton et al., 1988; Kutas et al., 1977). The argument for the utility of P3 latency in studying categorization is nicely summed up by Dien, Spencer and Donchin (2004): “The well-documented inverse relationship between P300 amplitude and stimulus probability means that P300s must be elicited only after the stimulus has been categorized. Thus, the latency of the P300 will vary with stimulus evaluation and categorization time” (pg 665)1.
If dual systems of categorization exist, it is possible that the P3 is driven by one but not the other. Several studies suggest that the amplitude of the P3 is modulated by a central characteristic of similarity-based categorization, namely the amount of feature overlap between the eliciting stimulus and a rare target. Non-targets with salient target features often elicit larger P3s than non-targets with less salient or fewer target features (Anllo-Vento & Hillyard, 1996; Azizian, Freitas, Parvaz & Squires, 2006; Azizian, Freitas, Watson & Squires, 2006; Keneman, Kok & Smulders, 1993; Smid, Jakob & Heinze, 1999; Wijers, Mulder, Okita, Mulder and Scheffers, 1989). Similarity between targets and non-targets also delays the latency of the P3 (e.g., Duncan-Johnson & Kopell, 1981; Senkowski & Hermann, 2002; see Verleger, 1997 for review).
P3 latency typically parallels reaction time across levels of categorization difficulty when the experimental instructions stress accuracy. This relationship can be disrupted when subjects are pressed to respond quickly and accuracy suffers. Under these conditions, incorrect responses are particularly prevalent when fast RTs are accompanied by slow P3s, suggesting that the behavioral responses were emitted before adequate stimulus evaluation – indexed by P3 latency – has taken place (Coles, Gratton, Bashore, Eriksen, & Donchin, 1985; Kutas et al., 1977). Indeed, ERP studies using the Lateralized Readiness Potential to assess motor cortex activity have shown that response preparation can begin in advance of stimulus presentation, leading to very fast responses but with accuracy close to chance (Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988). Clear dissociations between RT and P3 latency have also been observed when RT is prolonged by manipulations that affect response selection rather than stimulus evaluation, as when the word “right” calls for a left-hand response (Duncan-Johnson & Kopell, 1981; Ilan & Polich, 1999; McCarthy & Donchin, 1981), although there has been some dispute as to whether the P3 or a subcomponent of the P3 is also sensitive to response selection (see e.g., Dien et al., 2004; Smulders, Kenemans, Schmidt, & Kok, 1999; Verleger, 1997 for different views and results).
One goal of the current study is to examine more closely the limits of the relationship between P3 latency and categorization time when categorization is governed by a more complex rule than that used in most prior studies. Previous work has been persuasive in showing that when stimulus evaluation involves simple matching of features or conjunctions of features with a mental template, delays in stimulus evaluation correspond to delays in both RT and P3 latency. However, in some of our previous work with complex rules (described below), large reaction time effects have been observed in the absence of P3 latency effects. Those results have suggested that difficult categorization tasks engender qualitative changes in brain activity that are marked by late positive potentials at prefrontal scalp sites that may indicate the need for executive processes prior to categorization but subsequent to the P3. Here, we examine the relationships among RT, P3 latency and frontal potentials across different levels of categorization difficulty
Categorization and frontal ERPs
In one prior experiment, we asked participants to classify artificial creatures composed of multiple parts (head, arms, legs, torso, antennae) as belonging to category A, category B, or neither (Folstein & Van Petten, 2004). Correct responses were governed by a “three out of five” rule, such that creatures with at least three A-type body parts were members of category A, at least three B-type body parts belonged to Category B, and those with less than three A or B parts were neither A's nor B's. Via a combination of instructional manipulation and post-hoc Bayesian analyses, participants were split into three strategy groups: those who largely followed the three-out-of-five rule (showing a sharp cutoff between stimuli with 1 or 2 versus 3 or 4 features of a given category), those who relied predominantly on a single stimulus feature, and those following an additive strategy (such that the proportion of “A” responses linearly tracked the number of “A” features). These different strategies led to very different mean RTs, ranging from 1075 ms for the simplest single-feature strategy to 1791 ms for those who followed the three-out-of-five rule. In contrast, all three groups showed clear P3 components at parietal scalp sites, with very similar peak latencies hovering around 600 ms.
The same study did, however, show substantial ERP differences that were linked to categorization strategy. Both the rule-governed and additive-strategy groups generated much larger positive waves than the single-feaure group; this difference was of long duration (did not return to baseline over the 1300 ms recording epoch) and maximal at prefrontal scalp sites. The sensitivity of this late frontal positivity to the number of features employed in a categorization strategy suggested that it might index executive processes served by prefrontal cortex. In contrast, the relatively early latency of the P3 with respect to reaction time (the P3 peak preceded mean RT by more than 1100 ms for the Rule group) suggests that it instead reflected an initial first-pass of stimulus evaluation, during an assessment of individual features or simple conjunctions only.
Although our initial observation of a frontal contribution to categorization was based on between-subject differences in strategy, behavioral research suggests that multiple strategies can be engaged simultaneously, in particular that similarity-based strategies can intrude during rule application although they are detrimental to performance (Allen & Brooks, 1991; Hahn, Prata-Sala, Pothos & Brumby, 2010; Regehr & Brooks, 1993). In a recent ERP experiment, we observed both prolonged P3 latencies and larger frontal positive waves for stimuli that were more difficult to categorize as compared to easier stimuli, in a single group of subjects instructed in a two-feature rule. Overall, our prior studies have led us to a dual-system account of 1) a relatively fast process of matching single features (and perhaps two-feature conjunctions) to the diagnostic features of a category, with the speed of this process indexed by P3 latency, and 2) a slower process engaged when stimuli are less readily categorized on the basis of similarity to other exemplars or when the number of relevant features exceeds two, and indexed by the amplitude of ERPs at frontal scalp sites.
The present study
The dual-system hypothesis is pursued here in two experiments designed to more clearly dissociate P3 latency from the frontal positivity by allowing conditions with the same behavioral responses to be compared to one another, in the same subjects. If the use of complex categorization rules recruits a qualitatively different system than the simpler target detection tasks that modulate P3 latency, then changes in difficulty within this system should not modulate P3 latency, but instead modulate the frontal positivity.
Figure 1 schematizes the design of both experiments. Participants were asked to assign a large number of cartoon creatures to one of three response categories labeled Mogs, Nibs, and Others. In Experiment 1, these response categories were equiprobable (33.3% each), such that no category could be considered “deviant” according to the assigned categorization rule. However, stimulus variation within the assigned response categories was used to create conditions with higher or lower levels of predicted categorization difficulty, and higher or lower levels of hypothesized perceptual novelty. Creatures were composed of three rule-relevant features: global body shape (fish-like, horse-like, plant-like, or humanoid), color (purple, red, blue, and green), and body markings (stripes, spots, a single star, or brick pattern). Two response categories – Mogs and Nibs – had a central prototype, for instance purple striped fish or red spotted horses. However, participants were trained on a “two-out-of-three” rule, such that an alien with two Mog and one Nib features should be assigned to the Mog category. These stimuli with two features consistent with their own category and one feature consistent with a different category defined the Near Boundary condition as their feature composition places them near the boundary between the Mog and Nib categories. In contrast, prototypical stimuli with all three features of their own category defined the Far Boundary condition. Two other conditions were included to evaluate the impact of perceptual similarity between the test-phase stimuli and the stimuli used during training in the categorization rule. Participants were instructed (and trained with feedback) that creatures with no Mog or Nib features should be classified as Others. One variety of Other occurred frequently during the training phase (Common Other) and should thus be familiar during the test phase, whereas the Novel Other stimuli were comprised of features (body shape, color, markings) that occurred less frequently in the training phase and thus predicted to be less familiar at test.
Figure 1.
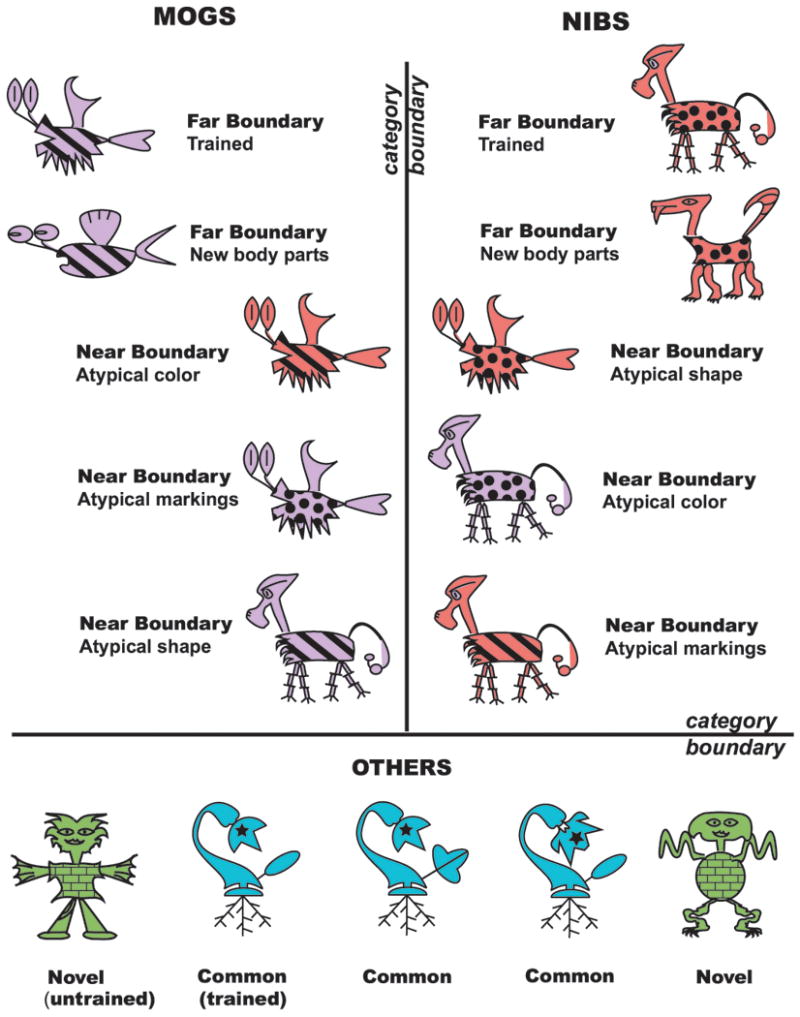
Examples of the test phase stimuli for both experiments. “Trained” refers to presentation during the preceding training phase. Far Boundary stimuli with new body parts not presented during the training phase were used only in Experiment 1.
The Near Boundary stimuli should, of course, prove more difficult to categorize than the Far Boundary, eliciting lower accuracy and slower decisions. Of interest are the differences in brain electrical activity that accompany these behavioral effects. In addition to the P3 and late frontal positivity, we analyze the frontocentral N2 because it has been associated with response conflict (Nieuwenhuis, Yeung, van den Wildenberg, & Ridderinkhof., 2003). One might imagine that the presence of features associated with different categories in the Near Boundary condition may thus elicit a larger N2 than the Far Boundary condition. However, the presence of an enhanced N2 is not an unambiguous sign of response conflict. Frontocentral N2s are also observed in cases when stimuli deviate considerably from a perceptual template, as when unique novel stimuli are interspersed in a sequence that otherwise consists of two stimuli (Courchesne, Hillyard, & Galambos, 1975). Some authors have suggested that frontal N2s are triggered in the absence of perceptual fluency or by difficulty in object recognition (Schendan & Maher, 2009; Ferrari, Bradley, Codispoti, & Lang, 2009). The relationship between the “conflict N2” and “novelty N2” is deeply unclear, and we have suggested that these are functionally distinct ERP signatures of different cognitive processes (Folstein & Van Petten, 2008). For the current paradigm, we predict a larger N2 for the Novel Other condition than the Common Other condition because stimuli in the Novel Other condition are individually infrequent and perceptually discrepant from all other conditions. Characterization of the predicted “novelty N2” difference between the Novel Other and Common Other conditions will aid in assigning a functional role to any possible N2 difference in the core comparison between the Near and Far Boundary conditions.
Experiment 1
Methods
Participants
Sixteen healthy young adults (10 men, 6 women, mean age of 25 years) were paid for their participation after providing informed consent. All were free of neurological or psychiatric disorders by self-report, reported no medications likely to affect the central nervous system, and were right handed. They had a mean of 17 years of formal education. Participants were questioned about their color vision during the initial recruitment, and tested with the Ishihara plates at the end of the experiment. An additional five individuals participated, but generated unusable data: one due to color blindness, two because more than 50% of trials were contaminated by eyeblink or movement artifact, and two whose accuracies in the Near Boundary condition (63% and 50%) were more than two standard deviations below the mean of the other participants.
Stimuli
Stimuli were 3026 cartoon creatures that spanned approximately 3.7 by 3.0 degrees of visual angle (Figure 1). Each alien was composed of three dimensions of color, global shape, and markings; the features filling these dimensions determined category membership. A global shape was created by combining body parts from an extensive library so that, for instance, multiple horse-like shapes could be formed from a variety of horse-heads, horse-legs, etc. These parts were: head, body, legs, and tail for horses; head, body, legs, and arms for humanoids; eye-stalks, body, dorsal fin, and tail fin for fish; and flower, stem, leaf, and root for plants. The identity of individual parts was irrelevant to the categorization rule. Variation among individual stimuli belonging to the same category was introduced to reduce the likelihood that participants could simply memorize individual stimuli and learn stimulus-response associations rather than categorization rules. The within-category variability also approximates that of natural categories, for which every cat one encounters is unique yet shares some general properties. The impact of frequency of exposure to particular body parts was also assessed here, as described below under “Test Phase”.
Instruction and Training phases
The experiment was conducted in a 2 to 3.25 hour session consisting of instructions, categorization training with feedback, and finally a test phase without feedback in which behavioral and ERP data were collected. Participants were verbally instructed in the rule that Mogs consisted of at least two Mog attributes of global body shape, color and markings, Nibs consisted of at least two Nib attributes, and Others were those lacking two Mog or two Nib features. Sample stimuli were used to illustrate the rule-relevant features and participants encouraged to name the features aloud. Sample stimuli included all varieties of the stimuli that would appear in the training phase: 1) prototypical or Far Boundary Mogs containing the three Mog features for that subject (e.g., purple striped fish) with global body shapes formed from two versions of each body part, 2) Far Boundary Nibs (e.g., red spotted horses), also formed from two versions of each body part; 3) all three varieties of Near Boundary Mogs, composed from the same specific body parts as the Far Boundary cases (e.g., red striped fish, purple spotted fish, purple striped horse, see Figure 1), 4) all three varieties of Near Boundary Nibs, and 5) the Common Other stimuli for that participant, which had no features in common with either Mogs or Nibs (e.g., blue plant with brick pattern markings). After the instructions, participants used key presses to categorize 60 Far boundary stimuli (half Mogs and half Nibs), 60 Near Boundary stimuli (half Mogs and half Nibs) and 60 Common Others and received accuracy feedback after each trial.
Test phase
Test phase trials included all of the exemplars from the training phase: 1) 100 Old Far Boundary trials (50 Mog, 50 Nib), 2) 60 Near Boundary trials (half correctly categorized as Mogs and half as Nibs); and 3) 60 Common Others. Two additional conditions occurred in the test phase. New Far Boundary stimuli included three Mog or three Nib features just as in the Old Far Boundary condition, but the individual stimuli were assembled from body parts not viewed during training. A Mog created from new parts might, for instance, have different shaped fins than trained Far Boundary Mogs, although still being a purple striped fish. The “novelty” of the New Far Boundary stimuli is thus irrelevant to the categorization rule, as their rule-relevant features of global shape, color and markings are shared with Old Far Boundary stimuli. The test phase included 4) 60 New Far Boundary trials (half Mog, half Nib) that were all different from one another. The New Far Boundary condition is included as one control over the potential impact of perceptual novelty during the test phase. For the rule-relevant features of global shape, color and markings, the Near Boundary exemplars are necessarily more numerous and varied than Far Boundary exemplars, because they can differ from the prototypical feature combination in three different ways – lacking either the global shape, color or markings of their category. Comparison of a small number of Far Boundary exemplars to a more varied set of Near Boundary exemplars is thus potentially confounded by raw perceptual novelty rather than by the desired variation in categorization difficulty. Because the New Far Boundary condition incorporates perceptual variability that is irrelevant to the categorization rule, comparison between the Old and New Far Boundary conditions serves as one check on the potential impact of raw perceptual novelty.
Novel Others had a fourth body shape, color and marking pattern that did not overlap with Mogs, Nibs, or Common Others and called for a response of “Other”. The novelty of the Novel Others is relevant to the categorization rule, as it is expressed in the rule-relevant features. The 5) 50 trials in the Novel Other condition included 50 individual stimuli to maximize their perceptual novelty. For instance, although all might be green humanoids with stars, a variety of body parts (e.g., different humanoid arms) were used to assemble the humanoids. Comparison of the Common and Novel Others allows a different means of controlling and assessing the impact of perceptual novelty because neither the individual body parts nor the rule-relevant features of the Novel Others were experienced during the training phase, but the rule-relevant features need to be attended during the test phase in order to arrive at the correct categorization decision. Table 1 lists the frequency with which each stimulus feature occurred in the test phase, and in the training and test phases combined1.
Table 1. Exemplar and Feature Frequency.
| TEST PHASE | TRAINING PLUS TEST PHASES | ||||||
|---|---|---|---|---|---|---|---|
| Condition | Exemplars | Trials | Exemplar frequency | Relevant feature frequency | Specific body part frequency | Relevant feature frequency | Specific body part frequency |
| Experiment 1 | |||||||
| Old Far Boundary | 10 | 100 | 3.03% | 33.3% | 12.1% | 33.3% | 13.7% |
| New Far Boundary | 60 | 60 | 0.30% | 33.3% | 1.8% | 33.3% | 1.2% |
| Near Boundary | 60 | 60 | 0.30% | 33.3% | 12.1% | 33.3% | 13.7% |
| Common Other | 10 | 60 | 1.82% | 18.2% | 9.1% | 23.5% | 11.8% |
| Novel Other | 50 | 50 | 0.30% | 15.2% | 3.0% | 9.8% | 2.0% |
| Experiment 2 | |||||||
| Far Boundary | 10 | 100 | 2.86% | 22.9% | 11.4% | 27.7% | 13.7% |
| Near Boundary | 60 | 60 | 0.29% | 22.9% | 11.4% | 27.7% | 13.7% |
| Common Other | 10 | 140 | 4.00% | 40.0% | 20.0% | 34.0% | 17.0% |
| Novel Other | 50 | 50 | 0.29% | 14.3% | 7.1% | 10.5% | 5.3% |
Note. The frequency of an exemplar or feature is the percentage of trials in which it occurs. Features relevant to the categorization rule are global body shape (horse-like, humanoid, fish-like, plant-like), color, and body markings. Specific body parts (e.g. which particular horse-head was used to create a horse-like shape) are irrelevant to the categorization rule. Note that for the Near and Far Boundary stimuli, relevant-feature frequency is equivalent to the frequency of the correct response category (i.e., 33% of the stimuli should be labeled as Mogs, 33% as Nibs in Experiment 1). Common and Novel Others were assigned to the same response category, so that their relevant-feature frequency sums to the probability of the “Other” response category (33% in Experiment 1, 54% in Experiment 2).
Across participants, all global shapes occurred equally often in all conditions such that, for instance, fish-like stimuli were equally likely to be assigned to the Mog, Nib, and Other response categories and, within the Other category, to be Common Others or Novel Others. Similarly, each of the four colors and four types of markings were rotated across Mog, Nib, Novel Other and Common Other exemplars. Finally, each global body shape was paired with each color and marking with equal probability across the 16 participants.
Stimulus duration in the test phase was 600 ms with an interstimulus interval of 5000 ms. Participants were instructed to respond as quickly as possible while maintaining a high level of accuracy, and to blink, if necessary, well after their button press response. Trials requiring Mog, Nib, and Other button presses occurred with equal probability (110 trials each). To maximize the possibility of response conflict associated with Near Boundary stimuli, the Mog and Nib buttons were always on the index or middle fingers of opposite hands. Across participants, the Other button was equally likely to be on either hand and finger.
Electrophysiological Methods
The electroencephalogram (EEG) was recorded from 29 tin electrodes in an elastic cap (Electrocap International, Eaton, OH). Seven electrodes spanned the midline of the scalp from prefrontal to occipital (Fpz, Fz, Fcz, Cz, Cpz, Pz, Oz). Lateral sites included seven pairs near the midline (Fp1/2, F3/4, Fc3/4, C3′/4′ – placed 4 cm lateral to Cz, Cp3/4, P3/4, and O1/2), and four pairs that spanned inferior frontal and temporal scalp (F7/8, Ft7/8, Tp7/8, T5/6). An electrode below the right eye (Le) was used to detect the vertical electrooculogram (EOG), but as typical, also detected EEG. Scalp and Le electrodes were referenced to the left mastoid during the recording, and digitally re-referenced to the mean of the right and left mastoids offline. Electrodes lateral to the external canthi of the two eyes were used to record the horizontal EOG and were referenced to each other (right active, left reference). The EEG was amplified by a Grass Model 12 polygraph (Grass, West Warwick, RI) with half-amplitude cutoffs of 0.01 and 100 Hz, and digitized at 250 Hz. Trials contaminated by eye movements and blinks, amplifier saturation, or movement artifact were rejected. Event related potentials were averaged for 200 ms preceding stimulus onset and 1100 ms after onset. ERPs timelocked to the behavioral responses (button presses) were also formed, as described in the Results.
Data Analysis
For all dependent measures, three contrasts are of interest, namely the differences between 1) Old Far Boundary and Near Boundary conditions (boundary effect), 2) Common Other and Novel Other (perceptual novelty in rule-relevant dimensions) and 3) Old Far Boundary and New Far Boundary (perceptual novelty in the rule-irrelevant dimensions of individual body parts). These were evaluated by separate t-tests or ANOVAs. When F-ratios have more than one degree of freedom in the denominator, the Huhyn-Feldt correction for nonsphericity of variance is applied; reported are the original df and the ε correction factor. Measurement strategies for the ERP components of interest – N2, P3, late frontal positivity – are described in the Results.
Results
Accuracy
Accuracy was above 99% in all conditions (se 0.2 to 0.3) except for the Near Boundary condition which had significantly lower accuracy of 90% (se 1.6; Near Boundary vs. all other conditions: ts(15) > 4.50, ps < .0005).
Reaction time
Figure 2, top, shows that mean reaction time to Near Boundary stimuli was slower than all other conditions (ts(15) > 8.00, ps < .0005). An ANOVA performed on reaction times to all conditions other than Near Boundary still revealed a main effect of condition (F(3,45) = 7.23, p < .05, ε = .378). Follow-up paired t-tests revealed that RTs to both Other conditions were faster than both Old Far Boundary and New Far Boundary conditions (ts(15) > 2.00, ps < .05). Reaction times for Novel Others did not differ from Common Others (t(15) = 1.46, p > .1); RTs to New Far Boundary stimuli were slightly slower than RTs to Old Far Boundary stimuli (t(15) = 2.63, p < .05). Overall, the behavioral results indicate that the Near Boundary condition elicited slower and less accurate responses than the other conditions, whereas the impact of perceptual novelty --assessed in the contrasts between Common Other and Novel Other, and between Old and New Far Boundary -- was modest.
Figure 2.
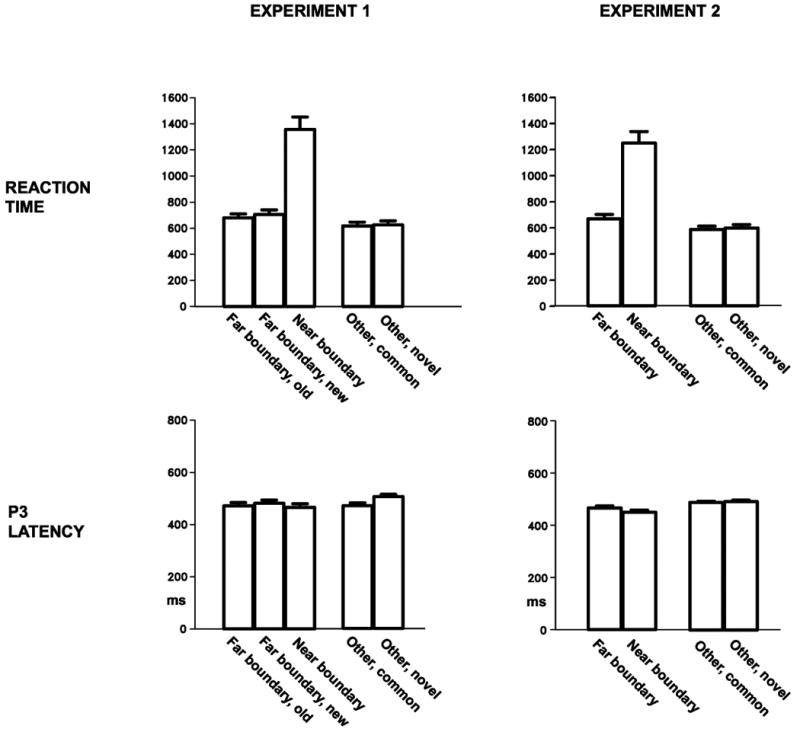
Mean reaction times and P3 peak latencies. Error bars represent standard errors.
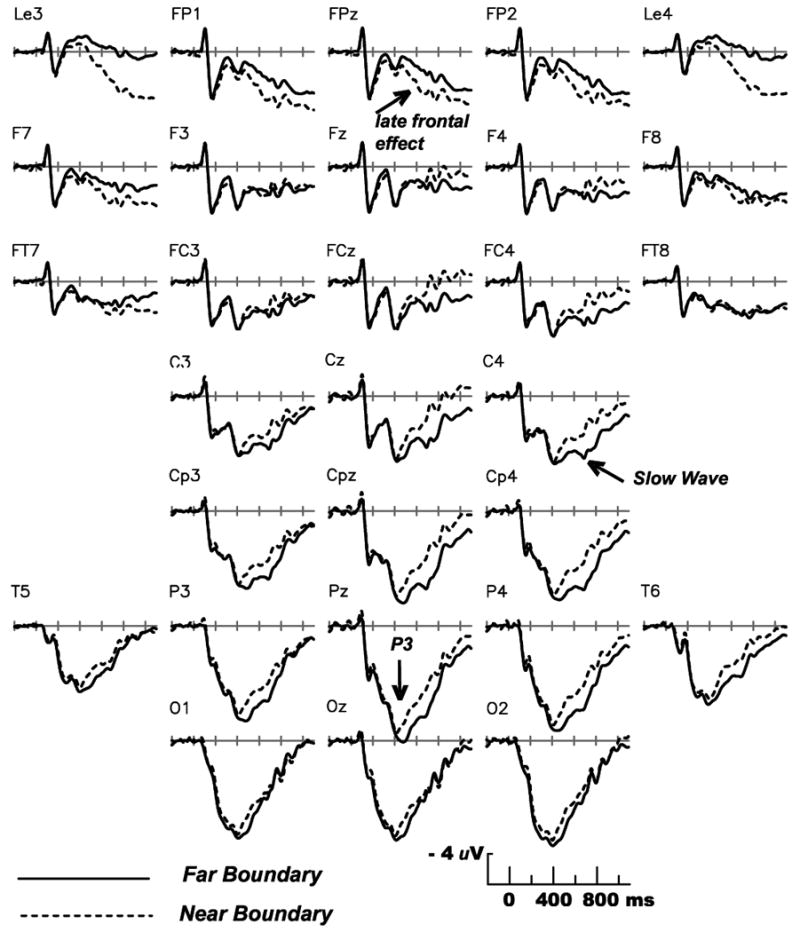
N2
The left side of Figure 3 shows little difference in the ERPs elicited by the Common Other versus Novel Other stimuli (the midline scalp sites shown are representative of all sites). The anterior N2 was defined as the mean amplitude from 190 to 370 ms at prefrontal, frontal, frontocentral and central scalp sites Fp1, Fpz, Fp2, F3, Fz, F4, Fc3, Fcz, Fc4, C3′, Cz, C4′ where this component is typically visible. Amplitudes were entered into ANOVAs with factors of Condition (2 levels), anterior-to-posterior location (4 levels), and laterality (left/midline/right, 3 levels). The predicted enhancement of the N2 by task-relevant perceptual novelty (Common Other versus Novel Other) was not obtained (F < 1). In contrast, Figure 4 shows that a very small but significant difference in N2 amplitude was observed between the Near and Far Boundary conditions with Near Boundary stimuli eliciting a larger N2 (F(1,15) = 5.50, p < .05).
Figure 3.
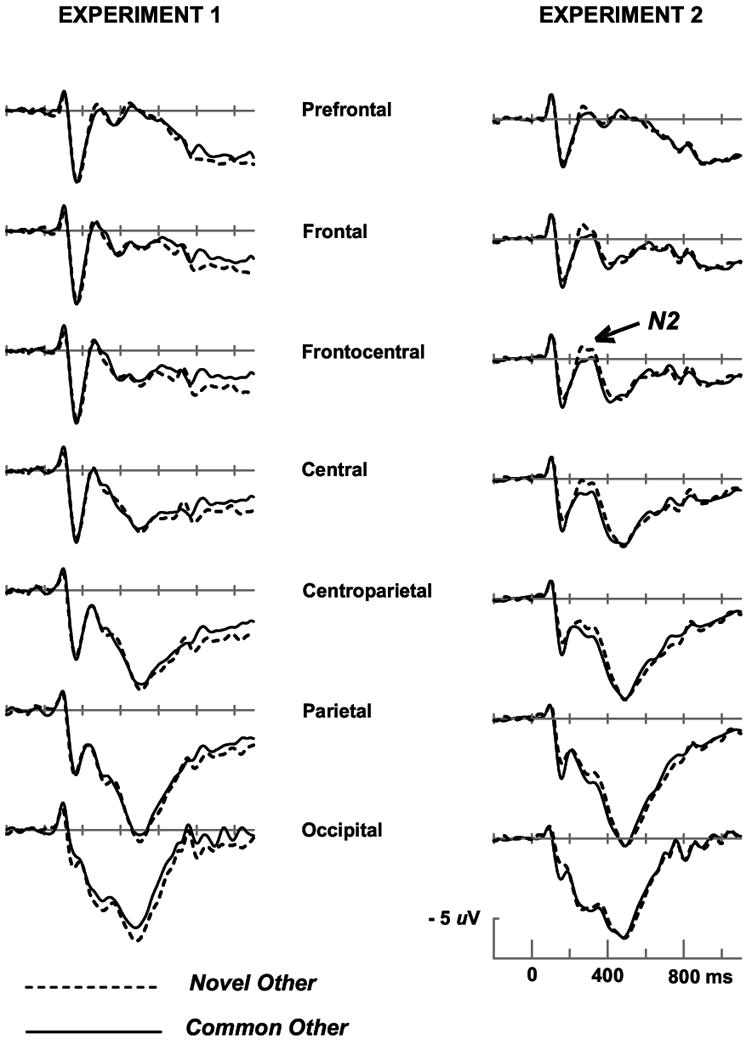
Grand average ERPs from midline scalp sites in Experiment 1. Time zero marks stimulus onset.
Figure 4.
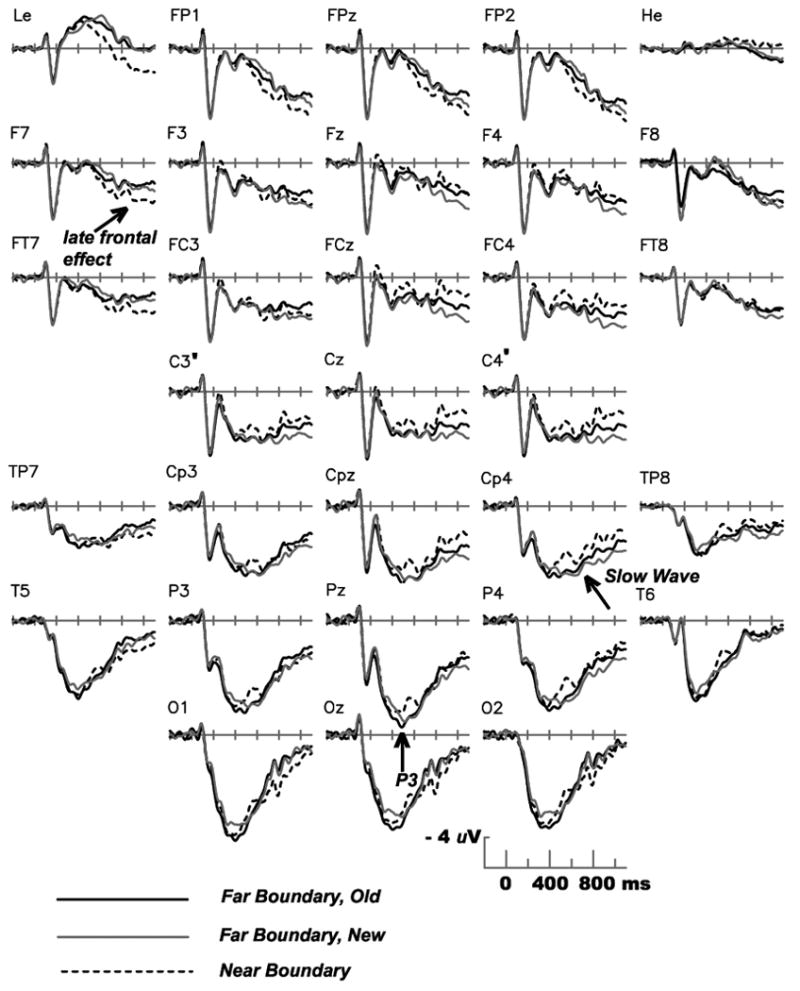
Grand average ERPs from all scalp sites for Experiment 1. Time zero marks stimulus onset.
P3 amplitude
Figures 3 and 4 show that all conditions elicited a large positive wave with maximum amplitude at the midline parietal site Pz, peaking at about 500 ms after stimulus onset. The waveshape, large amplitude and scalp distribution are as expected for a P3 component. The same figures also show that all conditions elicited P3s of similar amplitude, as expected given that the assigned response categories were equiprobable. Peak amplitude of the P3 was defined for each participant as the most positive amplitude between 300 and 700 ms at centroparietal and parietal sites Cp3, Cpz, Cp4, P3, Pz, P4 where this component is typically largest. The data were first passed through a 10 Hz low pass filter to avoid spurious identification of high-frequency noise as the P3 peak. Amplitudes were entered into ANOVAs with factors of Condition (2 levels), anterior-to-posterior location (2 levels), and laterality (left/midline/right). No significant effects of condition were observed in any of the three contrasts between pairs of conditions (Near Boundary vs. Old Far Boundary, Common Other vs. Novel Other, Old Far Boundary vs. New Far Boundary, all (F(1,15) < 1.8).
P3 latency
Peak latency of the P3 was defined as time of most positive voltage between 300 and 700 ms after stimulus presentation at the centroparietal and parietal sites, after applying the low pass filter above. Mean latencies are shown in Figure 2, bottom left. Novelty had little effect on P3 latency: no significant difference was observed between Old Far Boundary and New Far Boundary (F(1,15) = 1.81), or between Common Others and Novel Others (F(1,15) = 3.35, p = .09). The remaining comparisons revealed dissociations between P3 latency and reaction time. First, even though reaction times to Novel Others were faster than both Near Boundary and Old Far Boundary stimuli, P3 latency was significantly slower (Fs(1,15) > 4.50, ps < .05). A more central finding for the current hypotheses is that although Near Boundary stimuli elicited much slower behavioral responses than Far Boundary stimuli, the two conditions did not differ in P3 latency (F < 1).
Late epoch: Late frontal positivity and centroparietal Slow Wave
Figure 4 shows that after the peak of the P3, the Near Boundary condition elicited more positive ERPs than the both of the Far Boundary conditions at anterior sites, as predicted. Over the more posterior scalp, an unanticipated effect was evident – less positive potentials for the Near Boundary condition. The topography of positive and negative boundary effects over different scalp regions is illustrated in Figure 5, which also shows that the anterior effect had a leftward asymmetry whereas the posterior effect had a rightward asymmetry. As described in the General Discussion, the general morphology and scalp distribution of the more posterior effect closely resembles that of an ERP originally dubbed the Slow Wave, and we adopt that nomenclature here.
Figure 5.
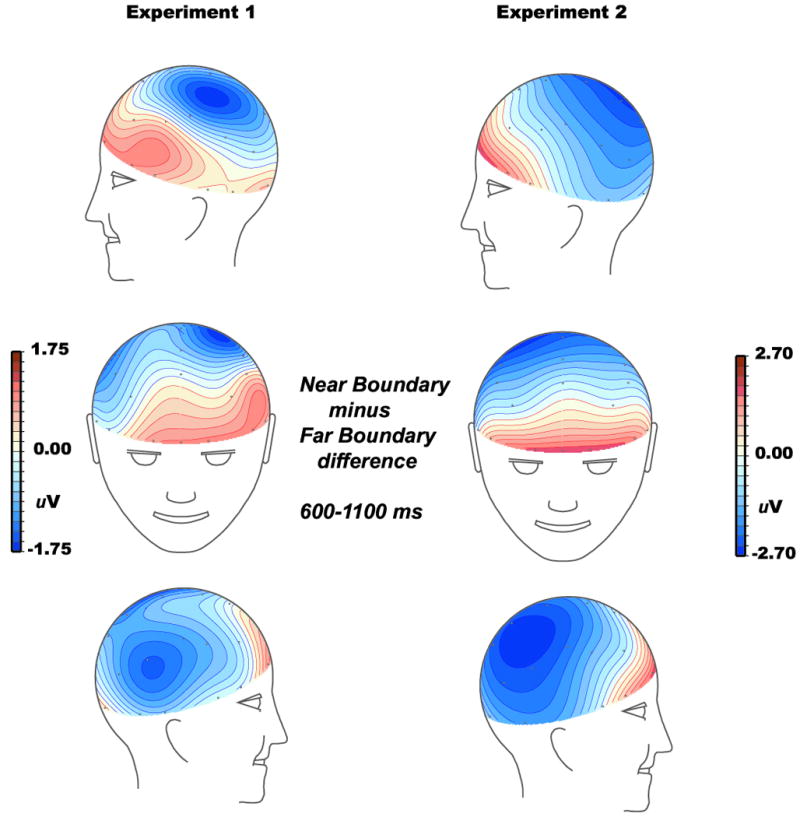
Spline-interpolated topographic maps of the ERP difference between Near Boundary and Old Far Boundary conditions. The mapping algorithm included electrode sites from the scalp and forehead (i.e, Fp sites) only, not those below the eyes (Le sites) because there were too few sites near the Le placements to allow adequate interpolation of voltages.
For the 600-1100 ms latency range that we've previously used to quantify late frontal effects during categorization tasks (Folstein & Van Petten, 2004, Folstein et al., 2008), the impact of distance from the category boundary was evaluated by ANOVAs taking boundary condition (Old Far versus Near) and scalp location as factors. For the midline sites (Fpz to Oz, 7 levels), the enhanced frontal positivity but smaller posterior Slow Wave in the Near Boundary condition led to a significant interaction between boundary condition and anterior-posterior location (F(6,90) = 5.79, p = .002, ε = .46), without a main effect of condition (F < 1). The lateral scalp sites near the midline (Fp1, Fp2, Fc3, Fc4 through O1 and O2, 7 levels of anterior-posterior together with left/right location) similarly yielded a null main effect of condition (F < 1), a condition by anterior-posterior interaction (F(6,90) = 4.67, p < .02, ε = .41), and two interactions reflecting the opposing lateral asymmetries of the anterior and posterior boundary effects (condition by left/right, F(1,15) = 19.5, p < .0005; condition by anterior-posterior by left/right, F(6,90) = 5.58, p < .0002, ε = .79). Finally, the three pairs of electrode sites farther from the midline -- over the temporal lobe (Ft7/8, Tp7/8, T5/6) -- yielded only a condition by left-right interaction (F(1,15) = 17.3, p < .001). Below, the late frontal and Slow Wave effects are analyzed independently in the separate scalp regions where they are maximal.
The impact of perceptual novelty that was irrelevant to the categorization rule – new versus trained body-parts in the Old versus New Far Boundary conditions – was analyzed as above and yielded no significant effects. Similarly, the contrasts between Common and Novel Others yielded no significant effects or interactions for the manipulation of perceptual novelty in rule-relevant dimensions.
Late frontal effect
Figures 4 and 5 indicate that more positive ERPs for Near Boundary stimuli were confined to prefrontal and frontotemporal sites. For the late time window of 600-1100 ms, comparison of the Near and Old Far Boundary conditions at the lateral sites Fp1, Fp2, F7, F8, Ft7 and Ft8 was conducted via an ANOVA with condition, anterior-to-posterior, and left/right as factors, and yielded an interaction between Boundary condition and left/right (F(1,15) = 16.3, p < .001). Followup ANOVAs showed that the larger positive potential in the Near Boundary than Far Boundary condition was significant at the three left frontal/frontotemporal sites(F(1,15) = 4.62, p < .05) but not the corresponding sites over the right hemisphere (F < 1). Figure 4 also shows that the largest difference between the Near and Far Boundary conditions appears at the most anterior site used – the electrode placed below the right eye to detect vertical eye movements and blinks. Because the direction of the effect (Near Boundary more positive) is the same as at sites above and below the eyes, the effect cannot be attributed to EOG contamination from vertical eye movements or blinks. To pursue this finding, Experiment 2 includes electrodes below both the right and left eyes, at locations more systematically determined than in Experiment 1.
Slow Wave
The more posterior boundary effect – smaller positive Slow Wave for the Near than Far Boundary conditions – was largest at central and centroparietal scalp sites (maximum amplitudes of 1.40 and 1.42 uV at C4′ and Cp4, respectively) and thus measured at lateral sites C3′, C4′, Cp3 and Cp4 in the 600-1100 ms latency. Comparison of the Old Far and Near Boundary conditions yielded a Boundary by left/right interaction (F(1,15) = 19.8, p < .0005), confirming the rightward asymmetry apparent in Figure 5. However, followup ANOVAs did not yield a significant effect of boundary distance for either the left or right sites (Fs < 1.58), nor for any individual electrode. The posterior Slow Wave effect of boundary distance was thus small and unreliable in Experiment 1 (although it proved to be of larger amplitude in Experiment 2).
ERPs timelocked to the behavioral response
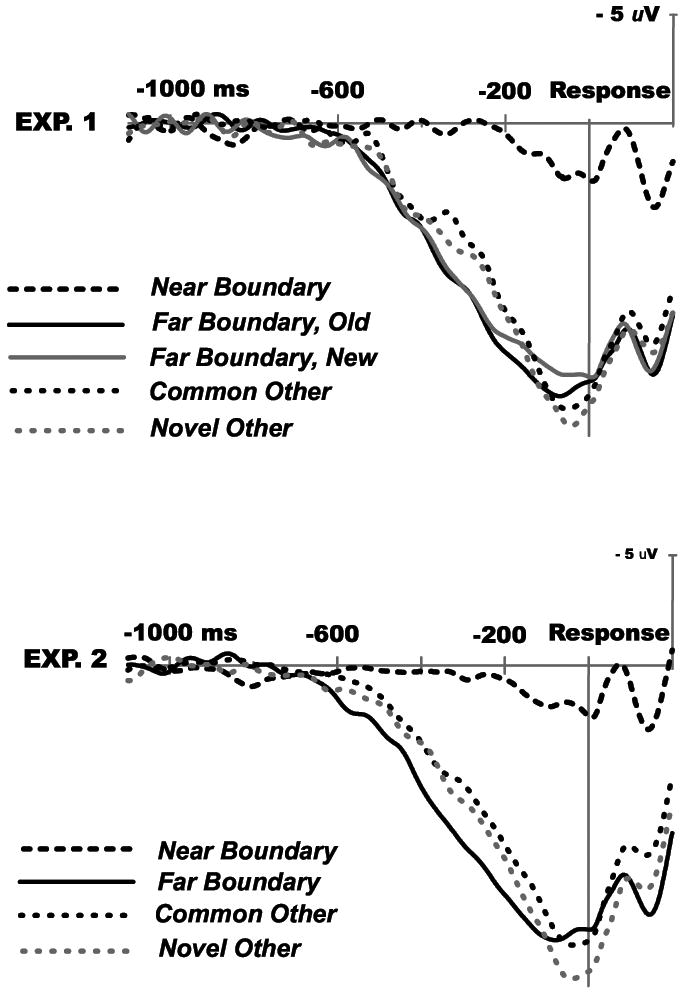
We take the uncoupling of P3 latency from reaction time created by the boundary manipulation to indicate that this brain response reflects stimulus evaluation, but only an initial stage of evaluation. A possible alternative account is that the multifeatural stimuli elicited multiple P3s or related components (such as the Slow Wave), and that a late P3-like component was not captured by the 1100 ms poststimulus recording epoch. For instance, it has been suggested by some authors that there are multiple centroparietal positive components that are more closely related to stimulus evaluation versus response selection, and these blend together when the time between stimulus and response is fairly brief, but are separated when this interval is long (see Christensen, Ivkovich & Drake, 2001; Falkenstein, Hohnsbein & Hermann, 1994; Verleger, 1997; Verleger, Jaœkowski & Wascher, 2005). According to this account, the prolonged RTs in the Near Boundary condition might be accompanied by a late positivity just preceding the behavioral response, a positivity reflecting response selection. If instead the P3 in the current experiment is related only to stimulus evaluation then its amplitude should be larger in ERP averages timelocked to stimulus presentation than in those timelocked to the behavioral response. We thus formed response-locked averages and compared these to the stimulus-locked data.
Figure 6 shows response-locked averages at the parietal midline site Pz. Four of the five conditions showed substantial positive peaks just prior to the response, but there was an almost total lack of a response-locked P3 in the Near Boundary condition, strongly supporting a dissociation between P3 and response. To quantify this impression, we first compared the response-locked P3 across conditions, focusing on the Near Boundary and Old Far Boundary conditions. The response-locked P3 peak was defined as the largest positive amplitude between −400 and 0 milliseconds prior to the response, measured with respect to a −1100 to −900 ms baseline. This measure was performed on 10 hz low-pass filtered ERPs at the six centroparietal and parietal sites used for the standard P3 analyses above. Far Boundary peak amplitude was significantly greater than Near Boundary amplitude (F(1,15) = 25.8, p < .0001).
Figure 6.
Grand average response-locked event-related potentials from midline parietal electrode Pz. Time zero marks response onset.
To quantify the relationship between the stimulus and response-locked P3, peak amplitude measures for the two conditions were entered into a 2 × 2 ANOVA with factors of Condition and Locking (stimulus vs. response). Main effects of Condition (F(1,15) = 26.1, p < .0005) and Locking (F(1,15) = 48.7, p < .0001) were qualified by a significant Condition by Locking interaction (F(1,15) = 39.5, p < .0001). Followup analyses clarified the interaction: although the stimulus-locked P3 was larger than the response-locked P3 in the Far Boundary condition (12.5 versus 11.0 uV, F(1,15) = 13.4, p < .005), it was much larger in the Near Boundary condition (12.0 versus 4.0 uV, F(1,15) = 48.2, p < .0001)1.
Larger amplitudes in the stimulus-locked than response-locked averages suggest that the P3 here was triggered by stimulus evaluation rather than response selection. In the conditions with stimulus-response intervals (RTs) that are both short and have little variability, the P3 visible in the response-locked averages is most simply attributed to the residue of the P3 triggered by the stimulus. For the Near Boundary condition with long and variable RTs, the P3 elicited by the stimulus is poorly synchronized to the response and thus of very small amplitude.
Discussion
As expected, stimuli that belonged to one category according to the experimental rule proved more difficult to categorize when they contained a feature consistent with a different category – the Near Boundary condition. This difficulty was reflected in lower accuracy and greatly prolonged RTs relative to all other conditions. Slow decisions in the Near Boundary condition were not, however, accompanied by delayed P3 components. Instead, the P3 in all conditions reached peak amplitude within 500 ms of stimulus presentation, shortly before the behavioral response in the easier conditions, but some 800 ms before the mean reaction time in the Near Boundary condition. Analyses of the response-locked P3 showed that this dissociation was not due to differences in the degree of overlap between stimulus and response-locked P3 subcomponents in the Far versus Near Boundary conditions. The results are consistent with our proposal that the P3 component indexes an initial stage of assigning stimulus features to categories, but that this process is not sufficient to determine category membership when the mapping between individual features and categories is not one-to-one.
After the peak of the P3, the easier Far Boundary conditions elicited somewhat more positive ERPs than the Near Boundary condition at central scalp sites. That effect – which we identify as the Slow Wave – proved to be statistically unreliable in Experiment 1, so that discussion is reserved for after Experiment 2.
Our second prediction was that stimuli with features of two distinct categories would elicit larger ERPs over prefrontal cortex than stimuli whose features all suggested the same response. This prediction was grounded in the hypothesis that categorization problems that cannot be solved after detection of single features or simple conjunctions trigger a secondary analysis that is more demanding of executive functions dependent on prefrontal cortex. This prediction was also confirmed, although the difference between Near and Far Boundary stimuli was not as large as expected, and significant only at prefrontal and frontotemporal sites on the left side. The lateral asymmetry of this effect was unexpected, as prefrontal effects in previous studies using similar stimuli were not significantly asymmetric (Folstein & Van Petten, 2004; Folstein et al., 2008).
N2 amplitude was also assessed as a possible measure of either response conflict (in the comparison of Near and Far Boundary conditions) or perceptual novelty (in the comparison of Common and Novel Others). While the Near Boundary condition elicited a slightly larger N2 than the Far Boundary condition, the effect did not replicate in Experiment 2 (see also the lateralized readiness potentials for these experiments, reported in Folstein, 2007). We suspect therefore, that, even if the Near Boundary stimuli did elicit some conflict monitoring in Experiment 1, the contribution of this variable to the very large RT delay was minor. Interpretation of this N2 effect was further hindered by the lack of a novelty N2 in either the Novel Part or Novel Other condition. We hypothesized that the novelty manipulation for the Novel Other condition was weakened by the fact that, although the Novel Others were never seen during training, their frequency during the test phase was similar to the Common Others. We therefore strengthened this manipulation in Experiment 2 by increasing the difference in frequency between the Novel and Common Others during the test phase.
Experiment 2
Participants
Sixteen healthy young adults participated (7 women, 11 men); their mean age was 24 with 15 years of formal education; 14 were right handed. All provided informed consent and reported no history of neurological disorder or use medication that could affect the central nervous system. Data from one additional participant were excluded due to excessive blink and movement artifact.
Materials and Procedure
The category structure, relevant stimulus dimensions and instructions to the participants were identical to Experiment 1. The experimental conditions were also the same except that the New Far Boundary condition was removed and a small number of Near Boundary Others (stimuli with one Mog or Nib property and two Other properties) were presented during training to encourage participants to attend to all Other features. Instructions were the same as in Experiment 1. During training, participants categorized 60 Far Boundary stimuli, 60 Near Boundary stimuli, 30 Common Others, and 12 Near Boundary Others over ten blocks of trials. During the test phase, participants categorized 100 Far Boundary stimuli, 60 Near Boundary stimuli, 140 Common Others, and 50 Novel Others. In the test phase, Novel Others thus occurred in only 14.2% of the trials which was expected to increase the likelihood of observing a novelty N2 effect. This change in stimulus probability structure also made the response category of “other” more probable (54% of the total trials). “Mog” and “nib” response categories remained equiprobable (23% each), and the manipulation of distance from the category boundary remained orthogonal to response probability. Table 1, bottom, shows the frequencies of relevant and irrelevant features in each condition.
Electrophysiological Methods
Electrophysiological procedures were identical to Exp. 1 except for minor changes in the electrode montage. Scalp sites C3 and C4 substituted for C3′ and C4′. Lateral electrode pair Tp7/8 was removed in favor of including two anterior sites below each eye. These served to detect blinks and vertical eye movements (via polarity-inverted potentials as compared to Fp sites above the eyes), but also as data channels. These were labeled Le3 and Le4 and placed 15% of the inter-aural distance lateral to Fpz, and 20% of the nasion-to-inion distance below Fpz.
Results
Accuracy
Accuracy was 99% or better in all conditions except for a significantly lower 86% in the Near Boundary condition results (ts(15) > 5.05, ps < .001).
Reaction time
Figure 2 (top right) shows a much slower mean RT for the Near Boundary condition than the other three conditions (main effect of condition in an omnibus ANOVA, F(3,45) > 70.9, p < .001, ε = .389; t-tests for Near Boundary versus other conditions, (ts(15) > 8.37, ps < .0005). Because an additional ANOVA without the Near Boundary condition was still significant (F(2,30) = 12.8, p < .01), follow-up t-tests were performed on the other conditions, revealing that Far Boundary stimuli elicited significantly slower reaction times than the Common Other and Novel Other conditions (ts(15) > 3.52, ps < .005), which did not differ from each other (t(15) = 1.66, p > .1).
N2
The right side of Figure 3 shows that the Novel Other stimuli elicited a larger N2 than the Common Others. This effect was not significant with our standard measure of mean amplitude in the 190-370 ms latency range (F(1,15) = 2.06, p > .1), but was significant for a peak amplitude measure within the same latency range (F(1,15) = 5.14, p < .05.
Figure 7 shows ERPs elicited by the Near Boundary and Far Boundary stimuli. Unlike Experiment 1, the N2 was not larger for the Near than Far Boundary condition. Instead, the Near Boundary condition elicited a slightly more positive ERP in the N2 latency range, leading to a condition by anterior/posterior interaction (F(3,45) = 4.88, p < .05, ε = .462). Followup tests for this interaction yielded a marginal effect of condition at the prefrontal (Fp1, Fpz, Fp2) sites only (F(1,15) = 4.31, p < .06), while there was no effect of condition at the frontal, frontocentral, or central electrode chains. This prefrontal difference in the N2 analysis epoch appears to be the leading edge of the late prefrontal positivity, which was larger in the Near Boundary than Far Boundary condition (see below).
Figure 7.
Grand average event-related potentials in Experiment 2 from all scalp sites. Electrode sites are arranged from anterior (top row) to posterior (bottom row) and from left to right, roughly corresponding to head location. Time zero marks stimulus onset.
Stimulus-locked P3
Amplitude
P3 amplitude was somewhat smaller in the Near than Far Boundary condition (F(1,15) = 11.1, p < .005), and this difference was larger at parietal than centroparietal sites (condition by anterior/posterior interaction, F(1,15) = 7.35, p < .05). The Common and Novel Other conditions did not differ in P3 amplitude (F(1,15) = 1.80).
Latency
As in Experiment 1, P3 latency did not differ between the Near and Far Boundary conditions (F(1,15) = 2.80) or between the Common and Novel Other conditions (F < 1). Like Exp. 1, both of the Other conditions elicited slower P3 latencies than the Near and Far Boundary conditions despite having faster RTs (Common Other vs. Near Boundary, F(15) = 8.93, p < 01; Novel Other vs. Near Boundary, F(15) = 7.12, p < .02; Common Other vs. Far Boundary, F(1,15) = 3.75, p = .07; Novel Other vs. Far Boundary, F(1,15) = 4.22, p = .06).
Late epoch: Late frontal positivity and centroparietal Slow Wave
Figure 7 shows that, after the P3, distance from the category boundary elicited a pattern of effects like that of Exp. 1: more positive ERPs for Near Boundary stimuli at anterior sites, and less positive ERPs over more posterior scalp. The similarity in scalp distribution of the two late effects across experiments is illustrated in Figure 5. These effects were analyzed as in Exp.1: ANOVAs on mean amplitude measures in the 600-1100 ms latency window for the midline, near-lateral, and far-lateral (temporal) scalp sites, followed by analyses of the frontal and centroparietal regions of interest where the late frontal and Slow Wave effects were largest.
For the midline sites, the enhanced frontal positivity but smaller posterior Slow Wave in the Near as compared to Far Boundary condition led to a significant interaction between boundary condition and anterior-posterior location (F(6,90) = 7.48, p = .005, ε = .28). Both sets of lateral scalp sites similarly yielded condition by anterior-posterior interactions (near-lateral: F(6,90) = 5.17, p < .05, ε = .22; far-lateral, F(2,30) = 8.82, p <.01, ε = .52). As the followup analyses below show, the late frontal effect was larger over the left side, whereas the Slow Wave effect was larger over the right. The rightward asymmetry of the Slow Wave dominated the omnibus analysis of near-lateral sites, leading to an interaction of boundary condition by left/right (F(1,15) = 22.0, p < .0005). In contrast, the leftward asymmetry of the late frontal effect dominated the omnibus analysis of sites over the temporal lobe, also leading to a boundary by left/right interaction (F(1,15) = 14.6, p < .0002).
Comparisons between the Common Other and Novel Other conditions were performed as above for the midline, near-lateral and far-lateral electrode chains, and yielded no significant effects or interactions for the manipulation of perceptual novelty in rule-relevant dimensions.
Late prefrontal positivity
Mean amplitudes at sites Fp1, Fp2, F7, F8, Ft7 and Ft8 in the 600-1100 ms latency range showed a significant boundary effect (F(1,15) = 5.78, p < .05), and a boundary by left/right interaction reflecting the leftward asymmetry of the effect (F(1,15) = 15.5, p = .001). Followup analyses showed that the main effect of boundary was significant at the three left sites (F(1,15) = 9.27, p < .01) but not the three right sites F(1,15) = 2.50). Figure 8 shows that the largest boundary effect was, however, evident at the most anterior sites recorded – those below the two eyes (Le3, Le4, near both the inferior surface of the frontal lobes and the temporal poles). The late positive boundary effect was robust at these sites (F(1,15) = 37.5, p < .0001), where it was not significantly asymmetric (condition by left/right, (F(1,15) = 2.37). The bilateral symmetry of the boundary effect at the Le3-Le4 sites indicates that it cannot be attributed to electrooculographic signals generated by horizontal eye movements. There was no difference between the Common and Novel Other conditions in analyses of the six frontal/frontotemporal sites, or the lower eye sites (Fs < 1).
Figure 8.
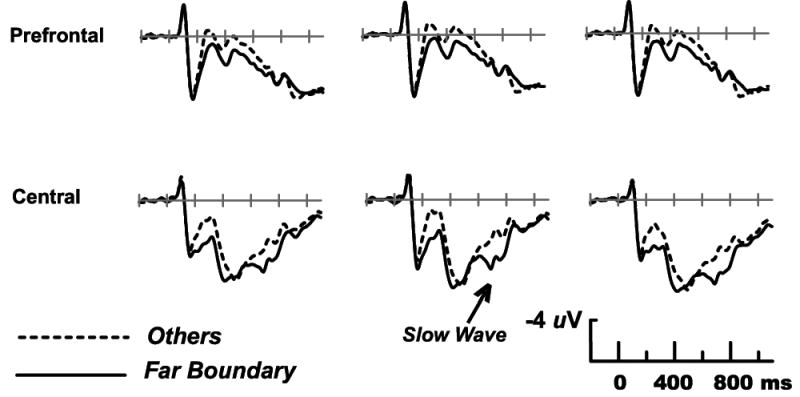
Grand average ERPs in Experiment 2 from prefrontal sites Fp1, Fpz, Fp2 (left to right) and central scalp sites C3, Cz, C4.
Slow Wave
As in Exp. 1, the additional positivity elicited by the Far Boundary condition was largest over central and centroparietal scalp, and measured at those lateral sites for maximal separation from the frontal boundary effect. The larger Slow Wave for the Far Boundary condition yielded a main effect of condition (F(1,15) = 9.94, p < .01), and a boundary by left/right interaction reflecting the rightward asymmetry of the effect (F(1,15) = 16.7, p < .001). The effect was of larger amplitude than in Exp. 1, and was thus independently significant at both the left and right scalp sites (F(1,15) = 4.57 and 15.1, p < .05 and p < .002, respectively).
Dissociation of the late frontal positivity and Slow Wave
The comparison of the Near and Far Boundary conditions led to effects of opposite polarity in different scalp regions. One might wonder if these are a single electrophysiological effect for which the frontal versus central scalp regions lie at opposite ends of an electrical dipole. If this were so, more positive central ERPs (larger Slow Waves) would invariably be accompanied by less positive frontal ERPs. The critical comparison for addressing this possibility is between the Far Boundary and Other conditions, all conditions with relatively fast and accurate categorization decisions hypothesized to require fewer executive resources (and thus less frontal engagement) than the Near Boundary condition. Figure 8 shows that the Far Boundary condition exhibited a larger Slow Wave than the Other conditions (Common and Novel collapsed), evident as a second positive peak after the P3. The Slow Wave difference between the Far Boundary and Other conditions was of shorter duration than the boundary effect above, but still resulted in a main effect of condition in the 600-1100 ms latency window (central and centroparietal sites, F(1,15) = 8.16, p < .02). There was no sign of a reversed-polarity difference at anterior sites, where Far Boundary stimuli instead continued to elicit slightly more positive ERPs, but not significantly so (frontal and frontoemporal sites, main effect of condition, F < 1, interactions between condition and scalp location, Fs < 1.94). These analyses suggest that the anterior and posterior effects evident in the Near/Far Boundary comparison are likely to be independent phenomena: a larger frontal positivity for the Near Boundary condition, and a larger Slow Wave for the Far Boundary condition.
Response-locked ERPs
The response-locked ERPs in Experiment 2 showed the same pattern as in Experiment 1 (Figure 6), with all conditions showing a clear response-locked P3 wave except for the Near Boundary condition, in which ERPs were almost completely flat. Our analyses again focused on the contrast between the Near and Far Boundary conditions, defining peak amplitude as the most positive voltage in a latency window of -400 to 0 ms with respect to a -1100 to -900 baseline1. Far Boundary positive peak amplitude was larger than Near Boundary peak amplitude (F(1,15) = 30.3, p < .0005).
Stimulus- and response-locked amplitudes were compared in an ANOVA with Condition and Locking as factors; this returned a main effect of Condition, a main effect of Locking, and a Condition by Locking interaction (all F(1,15) > 25.8, p < .0001. The source of the interaction was the same as in Exp. 1: the Far Boundary condition had a larger stimulus- than response-locked P3 (2.2 uV larger, F(1,15) = 39.4, p < .0001), but the Near Boundary condition had a much larger stimulus- than response-locked P3 (9.1 uV larger, F(1,15) = 38.3, p < .0001).
Summary
The results of Experiment 2 were broadly similar but stronger than Experiment 1. The Common and Novel Other conditions elicited shorter RTs than either the Near or Far Boundary conditions, but slightly longer P3 latencies. In both experiments, stimuli near the category boundary substantially delayed decisions about category membership but had no impact on the peak latency of the P3. In both experiments, the Near Boundary condition also elicited an enhanced late prefrontal positive component at left frontal electrode sites and at sites just below the eyes. The late frontal effect is one we predicted based on previous results, although its asymmetry was unexpected. The manipulation of distance from the category boundary also elicited a second late effect, after the peak of the parietal P3: larger positive potentials for the easier Far Boundary conditions. This Slow Wave effect was largest over central and centroparietal sites in both experiments, but proved to be larger and more statistically robust in Experiment 2 than Exp. 1.
Unlike Experiment 1, distance from the category boundary did not influence the anterior N2 component, hypothesized to reflect monitoring of response conflict. Novelty, unlike boundary distance, did modulate the N2, with Novel Others eliciting a larger N2 than Common Others, suggesting that we were successful in increasing the strength of the novelty manipulation. Because there was no boundary-related N2, the novelty and conflict N2 could not be directly compared.
General Discussion
Dissociations between reaction time and P3 latency
One goal of this study was to examine whether object categorization according to a complex logical rule engages a qualitatively different system from the one indexed by the P3. One type of evidence that might support the existence of multiple systems is a dissociation between P3 latency and decision time as expressed in behavioral reaction time. In both experiments, Near Boundary stimuli that shared one feature with the opposite category elicited reaction times that were at least twice as long as RTs to Far Boundary stimuli with features from only one category (∼600 milliseconds in absolute terms). Despite the large effect on reaction time, there was no difference between these conditions in P3 latency. The possibility that P3s were elicited by response selection rather than stimulus evaluation was explored via ERPs timelocked to the behavioral response, but these showed little hint of a P3 immediately preceding the response to Near Boundary stimuli.
Another variable that can delay reaction time independently of stimulus evaluation is the triggering of control processes, which include response inhibition and monitoring of response conflict, processes that often elicit frontocentral N2 components (Gehring, Gratton, Coles, & Donchin, 1992; Pfferbaum, Ford, Weller & Kopell, 1985). Although the Near Boundary condition elicited a slightly larger N2 than the Far Boundary condition in Experiment 1, this did not occur in Experiment 2 despite a very large impact of boundary condition on RT. This finding adds to a handful of recent results in which conflicting information has not elicited an enhanced N2 (Fox, Michie, Wynn & Mayberry, 2000; Folstein et al., 2008). These recent studies may be useful in placing limiting conditions on conclusions from simpler speeded flanker and go/no-go tasks. We conclude that even if there was some monitoring of response conflict in Experiment 1, the majority of the RT boundary effect was driven by a different process.
If reaction time was not slowed by processes such as inhibition of incorrect response preparation, the most likely explanation is that late evaluation processes, not reflected in the P3, were responsible for the delay3. We suggest that P3 latency indexes the timing of necessary initial stages of stimulus evaluation, such as the identification of single stimulus features or perhaps two-feature conjunctions. For the binary classification tasks that have been extensively studied with ERPs – those in which a decision can be based on single features or non-overlapping conjunctions of features – these first-pass evaluation processes are typically sufficient to arrive at a final decision. Simple stimulus-response rules thus tend to produce close relationships between RT and P3 latency, and little ERP activity between the P3 and the response (with the possible exception of the motor cortex activity reflected in the Readiness Potential). In the current paradigm, detection and even categorical labeling of the stimulus features would be sufficient to lead to a first-pass division between stimuli that should be labeled “other” and those requiring either a “mog” or “nib” judgment. For both the Near and Far Boundary stimuli, final assignment to the “mog” or “nib” category would require something more: separate counts of Mog and Nib features, and comparison of those counts to the assigned categorization rule. These subsequent processes were associated with late positive potentials over frontal and central scalp that varied in opposite directions for the Near and Far Boundary conditions, discussed below. It is an interesting possibility that, with extended training, the feature conjunctions used here could form larger units, moving control of the final decision away from working memory and to the earlier process indexed by the P3.
After the P3: Late centroparietal ERPs preceding categorization decisions
In both experiments, positive potentials occurred after the apparent peak of the P3, in roughly the same scalp region but with a slightly more anterior topography (centroparietal rather than parietal maximum). These proved to be largest for the Far Boundary stimuli whose three relevant features were all associated with a single category. Similar potentials have been sporadically observed and written about for many years and variously labeled as Slow Wave, a second P3, P4, and P-CR (see Squires, Squires & Hillyard, 1975; Johnson & Donchin, 1985; Christensen, Ivkovich & Drake, 2001; Falkenstein, Hohnsbein & Hoorman, 1994, respectively). These different names accompany different functional interpretations. “P4” and “P-CR” have been suggested to reflect a stage of selecting a specific behavioral response, a process that can be made more difficult by increasing the number of response options or introducing spatial incompatibility between stimuli and correct responses (Christensen et al., 2001; Falkenstein et al., 1994). This interpretation is unlikely to be relevant to the boundary effect observed here, as comparison of stimulus- and response-locked averages did not reveal a clear response-preceding positivity separate from that triggered by stimulus presentation. In contrast, the “Slow Wave” and “multiple P3” labels/interpretations have been offered as neurophysiological correlates of cognitive processing that follows initial stimulus identification, the central topic of the current study. After noting that Slow Waves are particularly prevalent in response to stimuli that convey feedback about task performance, Johnson and Donchin (1985) suggested that these were second P3s that reflected updating of mental models and revisions of plans to handle upcoming trials, processes that followed identification of the “correct” or “wrong” signal value of the stimuli. Although arguing that the Slow Wave should be considered distinct from the P3, García-Larrea and Cézanne-Bert (1998) similarly suggested that “one relevant feature common to paradigms evoking large PSW [positive slow wave] is that, in them, target identification does not close the cognitive epoch, but rather prompts the execution of a second task…” (pg 261).
For the explicitly combinatorial categorization rule assigned in the current experiments, a plausible candidate for the “second task” is feature counting, after identification of one or two rule-relevant features. For the stimuli assigned a response label of “Other”, any single feature or gestalt impression would have been sufficient to drive a correct response. In contrast, the larger Slow Wave for the Far Boundary stimuli may have reflected a more deliberate feature count, a process that could only serve to confirm the category assignment suggested by preliminary identification of one or two categorical features. For the Near Boundary stimuli with a mixture of features suggestive of different categories, the observation of a smaller Slow Wave may reflect continued uncertainty when feature counts are inconsistent with a single category. We suggest that the frontal positivity that was largest in the difficult Near Boundary condition reflected a shift to a qualitatively different strategy when feature counting yielded conflicting evidence for two different categories, a strategy that required greater engagement of prefrontal cortex.
Prefrontal activity in categorization and memory tasks
Several neuropsychological studies have implicated prefrontal cortex in complex problem solving tasks employing multiple stimulus dimensions. In particular, solving problems with high “relational complexity” is impaired by lesions to prefrontal cortex, and activates dorsolateral and inferior prefrontal cortex in hemodynamic imaging studies (Holyoak & Kroger, 1995; Kroger et al., 2002; Christoff et al., 2001). Prefrontal regions were also activated by a recent fMRI categorization study that shared several key elements with the current experiments (Koenig et al., 2005). Participants learned to categorize artificial creatures using either an explicit rule or a similarity strategy. Stimuli were Members if they shared at least three out of four features with a prototype and were non-members if they shared two features (Low Distortion condition) or one to zero features (High Distortion condition). The Low Distortion stimuli were akin to our Near Boundary condition in that they shared features with a different response category. Like our Near Boundary condition, the Low Distortion condition elicited longer reaction times than the other conditions. This condition also elicited greater hemodynamic activation in left dorsolateral prefrontal cortex (BA 46). In participants that used an explicit rule, left inferior frontal gyrus (BA 44/6) was globally more active than in participants using a similarity strategy, and more active in the Low Distortion condition than in the other conditions. This pattern of activation is consistent with the prefrontal distribution of the late prefrontal positive wave observed in the current study and also with the consistent left lateralization of that wave.
The time course and distribution of the frontal boundary effect was similar to that observed in a comparison of different categorization strategies in Folstein and Van Petten (2004). In that study, participants who used many stimulus features in their categorization strategy had much larger frontal positive waves than participants who attended disproportionately to a single feature. The previous study also manipulated distance from category boundary but, unlike the current study, did not lead to a significant enhancement of the frontal positivity for Near Boundary stimuli. We believe that the critical difference between the designs is that even the Far Boundary in the previous study contained one feature consistent with a different category, so that both boundary conditions included evidence consistent with more than one category.
Similar late prefrontal positive components have been observed in source memory tests that require participants to judge conjunctions of stimulus attributes as studied or unstudied, for instance whether a test word occurred in a previously studied list and whether it was spoken by the same voice (Kuo & Van Petten, 2006, 2008; Senkfor & Van Petten, 1998; Trott, Friedman, & Ritter, 1997; Van Petten, Senkfor, & Newberg, 2000; Wilding & Doyle, 1996). These episodic memory tasks share some formal properties of the categorization rule used here: 1) single stimulus attributes do not signal the correct response, which must be based on attribute conjunctions, and 2) single attributes are associated with multiple responses (i.e., studied words may require a response of “old same voice” or “old different voice”, just as the presence of a horse-like body shape here may require a “mog” or a “nib” response, depending on the other stimulus features). In parallel with our earlier categorization study (Folstein & Van Petten, 2004), prefrontal positive ERPs in episodic memory tests have also been shown to vary according to the strategy of an individual participant, being larger in those participants who rely more strongly on attribute conjunctions than on single features (Van Petten, Luka, Rubin, & Ryan, 2002). Finally, similar prefrontal positivities have occasionally been observed in mental arithmetic tasks in association with more difficult problems (Iguchi & Hashimoto, 2000; Ruchkin, Johnson, Canoune & Ritter, 1991; Ruchkin, Johnson, Mahaffey & Sutton, 1988). Because these various prefrontal positivities have appeared in different experiments with different participants, it is as yet unclear whether they are the same or merely similar. Even within the current experiments, the topography of the frontal effects is suggestive of multiple sources, given that the boundary effect was left-lateralized at standard prefrontal and frontotemporal sites, but bilaterally symmetric at anterior sites below the eyes. Although sites below the eyes are rarely utilized as data channels in EEG recordings, these are close to both the inferior surface of the frontal lobes and the temporal poles and frequently show ERP activity similar to that recorded at Fp sites on the forehead (see e.g., Senkfor & Van Petten, 1998).
Broadly, the literature suggests that prefrontal positive waves index recruitment of executive processes and the current experiments indicate that object categorization using complex logical rules recruits these processes as well. The best cognitive description of the particular executive processes invoked by complex categorization rules has not yet been formalized. For instance, although “working memory” is a broad theoretical construct often invoked to explain frontal engagement in a variety of tasks, numerous ERP studies have manipulated working memory load in a variety of paradigms, and these manipulations have generally been associated with slow negative potentials (sometimes continuing for several seconds), rather than the positive potential observed here. A common paradigm used to examine maintenance of information is to assign a matching-to-sample task and record ERPs during the S1-S2 interval. The amplitude of a slow negative potential is larger during the retention interval when more stimulus elements must be retained, and the scalp distribution of this slow negativity depends on the nature of the material retained (Mecklinger & Pfeifer, 1996; Ruchkin, Johnson, Grafman, Canoune, & Ritter, 1992, 1997). A mental rotation task has been used to examine manipulation of information in working memory; the amplitude of a slow negative potential over parietal cortex increases linearly with the angle of rotation (Roeder, Rösler, & Hennighausen, 1997). Finally, comparisons between sentences of varying syntactic complexity have been used to evaluate both retention and manipulation of information during language comprehension. Frontal and prefrontal ERPs show the greatest sensitivity to sentence complexity manipulations, but are again marked by more negative potentials as working memory load increases (King & Kutas, 1995; Mecklinger, Schriefers, Steinhauer, & Friederici, 1995; Münte, Schiltz, & Kutas, 1998). The index of multiple feature evaluation observed here is thus unlike that observed in canonical manipulations of working memory load. One property that differentiates both the categorization task used here and the source memory tests described above from these other paradigms is the need to compare the contents of working memory (features of the current stimulus) with long-term memory for the categorization rule or previously studied items. Coordination between working- and long-term memory may be the core process driving frontal positivities, but this formulation will require further experimentation and theoretical refinement.
Acknowledgments
Funding was provided by the National Institute for Mental Health (MH073703). We are grateful for the extensive and careful work of MungChen Wong in stimulus preparation.
Footnotes
It is important to note, however, that stimuli need not be categorically rare (less than 50%) to elicit a P3. When stimuli consist of two items presented with 50% probabilities each, the one designated as the target elicits a larger P3 than the one designated as the nontarget (see Johnson, 1988 for an integrative review of the factors influencing P3 amplitude).
We also analyzed response-locked ERPs relative to the same pre-stimulus baseline used for the stimulus-locked averages (see Verleger et al., 2005 for a similar procedure), with results much like the analyses reported in the main text. The interested reader can examine these analyses in Folstein (2007).
In both experiments, we also examined the Lateralized Readiness Potential for signs that that participants began to prepare the wrong response hand on Near Boundary trials, given that these stimuli contained one feature suggestive of the wrong response (see e.g., Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988; Osman, Bashore, Coles, Donchin, & Meyer, 1992). No evidence of incorrect response preparation was observed; figures and analyses are available in Folstein (2007).
References
- Allen SW, Brooks LR. Specializing the operation of an explicit rule. Journal of Experimental Psychology: General. 1991;120:3–19. doi: 10.1037//0096-3445.120.3.278. [DOI] [PubMed] [Google Scholar]
- Anllo-Vento L, Hillyard SA. Selective attention to the color and direction of moving stimuli: electrophysiological correlates of hierarchical feature selection. Perception & Psychophysics. 1996;58:191–206. doi: 10.3758/bf03211875. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Alfonso-Reese LA, Turken U, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Azizian A, Freitas AL, Parvaz MA, Squires NK. Beware misleading cues: Perceptual similarity modulates the N2/P3 complex. Psychophysiology. 2006;43:253–260. doi: 10.1111/j.1469-8986.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- Azizian A, Freitas AL, Watson TD, Squires NK. Electrophysiological correlates of categorization: P300 amplitude as index of target similarity. Biological Psychology. 2006;71:278–288. doi: 10.1016/j.biopsycho.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Breton F, Ritter W, Simson R, Vaughan HG., Jr The N2 component elicited by stimulus matches and multiple targets. Biological Psychology. 1988;27:23–44. doi: 10.1016/0301-0511(88)90003-8. [DOI] [PubMed] [Google Scholar]
- Christensen CA, Ivkovich D, Drake KJ. Late positive ERP peaks observed in stimulus-response compatibility tasks tested under speed-accuracy instructions. Psychophysiology. 2001;38:404–416. [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Coles MGH, Gratton G, Bashore TR, Eriksen CW, Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:529–533. doi: 10.1037//0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography & Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41:665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise! Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Kopell BS. The Stroop effect: brain potentials localize the source of interference. Science. 1981;214:938–940. doi: 10.1126/science.7302571. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Kruschke JK. Rules and exemplars in category learning. Journal of Experimental Psychology: General. 1998;127:107–140. doi: 10.1037//0096-3445.127.2.107. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoorman J. Effects of choice complexity on different subcomponents of the late positive complex of the event-related potential. Electroencephalography and Clinical Neurophysiology. 1994;92:148–160. doi: 10.1016/0168-5597(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Folstein JR. Unpublished doctoral dissertation. University of Arizona; 2007. On the category's edge: Event-related potential correlates of novelty and conflicting information in rule-based categorization. [Google Scholar]
- Folstein JR, Van Petten C. Multidimensional rule, unidimensional rule, and similarity strategies in categorization: event-related potential correlates. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:1026–1044. doi: 10.1037/0278-7393.30.5.1026. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N200: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C, Rose SA. Novelty and conflict in the categorization of complex stimuli: N2 and P3 correlates. Psychophysiology. 2008;45:467–479. doi: 10.1111/j.1469-8986.2007.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AM, Michie PT, Wynne CD, Maybery MT. ERP correlates of response inhibition to elemental and configural stimuli in a negative patterning task. Clinical Neurophysiology. 2000;111:1045–1053. doi: 10.1016/s1388-2457(00)00257-1. [DOI] [PubMed] [Google Scholar]
- García-Larrea L, Cézanne-Bert G. P3, Positive slow wave and working memory load: a study on the functional correlates of slow wave activity. Electroencephalography and Clinical Neurophysiology. 1998;108:260–273. doi: 10.1016/s0168-5597(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Gratton G, Coles MGH, Donchin E. Probability effects on stimulus evaluation and response processes. Journal of Experimental Psychology: Human Perception & Performance. 1992;18:198–216. doi: 10.1037/0096-1523.18.1.198. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: a psychophysiological analysis. Journal of Experimental Psychology: Human Perception & Performance. 1988;14:331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Grossman M, Smith EE, Koenig P, Glosser G, DeVita C, Moore P, McMillan C. The neural basis for categorization in semantic memory. Neuroimage. 2002;17:1549–1561. doi: 10.1006/nimg.2002.1273. [DOI] [PubMed] [Google Scholar]
- Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A. Dissociating neural correlates of cognitive components in mental calculation. Cerebral Cortex. 2001;11:350–359. doi: 10.1093/cercor/11.4.350. [DOI] [PubMed] [Google Scholar]
- Hahn U, Prat-Sala M, Pothos EM, Brumby DP. Exemplar similarity and rule application. Cognition. 2010;114:1–18. doi: 10.1016/j.cognition.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Holyoak KJ, Kroger JK. Forms of reasoning: insight into prefrontal functions? Annals of the New York Academy of Sciences. 1995;769:253–263. doi: 10.1111/j.1749-6632.1995.tb38143.x. [DOI] [PubMed] [Google Scholar]
- Ilan AB, Polich J. P300 and response time from a manual Stroop task. Clinical Neurophysiology. 1999;110:367–373. doi: 10.1016/s0168-5597(98)00053-7. [DOI] [PubMed] [Google Scholar]
- Iguchi Y, Hashimoto I. Sequential information processing during a mental arithmetic is reflected in the time course of event-related brain potentials. Clinical Neurophysiology. 2000;111:204–213. doi: 10.1016/s1388-2457(99)00244-8. [DOI] [PubMed] [Google Scholar]
- Johansen MK, Palmeri TJ. Are there representational shifts during category learning? Cognitive Psychology. 2002;45:482–553. doi: 10.1016/s0010-0285(02)00505-4. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr . The amplitude of the P300 component of the event-related potential: review and synthesis. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in Psychophysiology. Vol. 3. Connecticut: JAI Press; 1988. pp. 69–138. [Google Scholar]
- Johnson R, Jr, Donchin E. P300 and stimulus categorization: two plus one is not so different from one plus one. Psychophysiology. 1980;17:167–178. doi: 10.1111/j.1469-8986.1980.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr, Donchin E. Second thoughts: Multiple P300s elicited by a single stimulus. Psychophysiology. 1985;22:182–194. doi: 10.1111/j.1469-8986.1985.tb01584.x. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Kok A, Smulders FTY. Event-related potentials to conjunctions of spatial frequency and orientation as a function of stimulus parameters and response requirements. Electroencephalography & Clinical Neurophysiology. 1993;88:51–63. doi: 10.1016/0168-5597(93)90028-n. [DOI] [PubMed] [Google Scholar]
- King JW, Kutas M. Who did what and when? Using word- and clause-level ERPs to monitor working memory usage in reading. Journal of Cognitive Neuroscience. 1995;7:376–395. doi: 10.1162/jocn.1995.7.3.376. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning & Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, Gee J, Grossman M. The neural basis for novel semantic categorization. Neuroimage. 2005;24:369–383. doi: 10.1016/j.neuroimage.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: A parametric study of relational complexity. Cerebral Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Kuo TY, Van Petten C. Prefrontal engagement during source memory retrieval depends on the prior encoding task. Journal of Cognitive Neuroscience. 2006;18:1133–1146. doi: 10.1162/jocn.2006.18.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TY, Van Petten C. Perceptual difficulty in source memory encoding and retrieval: Prefrontal versus parietal electrical brain activity. Neuropsychologia. 2008;46:2243–2257. doi: 10.1016/j.neuropsychologia.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Masaki H, Takasawa N, Yamazaki K. An electrophysiological study of the locus of the interference effect in a stimulus-response compatibility paradigm. Psychophysiology. 2000;37:464–472. [PubMed] [Google Scholar]
- Mecklinger A, Pfeifer E. Event-related potentials reveal topographical and temporal distinct neuronal activation patterns for spatial and object working memory. Cognitive Brain Research. 1996;4:211–224. doi: 10.1016/s0926-6410(96)00034-1. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Schriefers H, Steinhauer K, Friederici AD. Processing relative clauses varying on syntactic and semantic dimensions: An analysis with event-related potentials. Memory & Cognition. 1995;23:477–494. doi: 10.3758/bf03197249. [DOI] [PubMed] [Google Scholar]
- Münte TF, Schiltz K, Kutas M. When temporal terms belie conceptual order. Nature. 1998;395:71–73. doi: 10.1038/25731. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM, Palmeri TJ. An exemplar-based random walk model of speeded classification. Psychological Review. 1997;104:266–300. doi: 10.1037/0033-295x.104.2.266. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM, Palmeri TJ. A rule-plus-exception model for classifying objects in continuous-dimension spaces. Psychonomic Bulletin & Review. 1998;5:345–369. [Google Scholar]
- Osman A, Bashore TR, Coles MGH, Donchin E, Meyer DE. On the transmission of partial information: Inferences from movement-related brain potentials. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:217–232. doi: 10.1037//0096-1523.18.1.217. [DOI] [PubMed] [Google Scholar]
- Patalano AL, Smith EE, Jonides J, Koeppe RA. PET evidence for multiple strategies of categorization. Cognitive, Affective & Behavioral Neuroscience. 2001;1:360–370. doi: 10.3758/cabn.1.4.360. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalography & Clinical Neurophysiology. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Stark CEL, Squire LR. Contrasting cortical activity associated with category memory and recognition memory. Learning & Memory. 1998;5:420–428. [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, Stark CEL, Squire LR. Cortical areas supporting category learning identified using functional MRI. Proceedings of the National Academy of Sciences, USA. 1998;95:747–750. doi: 10.1073/pnas.95.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr G, Brooks LR. Perceptual manifestations of an analytic structure: The priority of holistic individuation. Journal of Experimental Psychology: General. 1993;122:92–114. doi: 10.1037//0096-3445.122.1.92. [DOI] [PubMed] [Google Scholar]
- Remy F, Mirrashed F, Campbell B, Richter W. Mental calculation impairment in Alzheimer's disease: a functional magnetic resonance imaging study. Neuroscience Letters. 2004;358:25–28. doi: 10.1016/j.neulet.2003.12.122. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R, Jr, Canoune H, Ritter W. Event-related potentials during arithmetic and mental rotation. Electroencephalography & Clinical Neurophysiology. 1991;79:473–487. doi: 10.1016/0013-4694(91)90167-3. erratum appears in Electroencephalography & Clinical Neurophysiology, 1992, 85, 396-8. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R, Grafman J, Canoune H, Ritter W. Distinctions and similarities among working memory processes: An event-related potential study. Cognitive Brain Research. 1992;1:53–66. doi: 10.1016/0926-6410(92)90005-c. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R, Jr, Grafman J, Canoune H, Ritter W. Multiple visuospatial working memory buffers: Evidence from spatiotemporal patterns of brain activity. Neuropsychologia. 1997;35:195–209. doi: 10.1016/s0028-3932(96)00068-1. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Johnson R, Jr, Mahaffey D, Sutton S. Toward a functional categorization of slow waves. Psychophysiology. 1988;25:339–353. doi: 10.1111/j.1469-8986.1988.tb01253.x. [DOI] [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what? An event-related potential investigation of source and item memory. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Herrmann CS. Effects of task difficulty on evoked gamma activity and ERPs in a visual discrimination task. Clinical Neurophysiology. 2002;113:1742–1753. doi: 10.1016/s1388-2457(02)00266-3. [DOI] [PubMed] [Google Scholar]
- Smid HGOM, Jakob A, Heinze HJ. An event related potential study of visual selective attention to conjunctions of color and shape. Psychophysiology. 1999;36:264–279. doi: 10.1017/s0048577299971135. [DOI] [PubMed] [Google Scholar]
- Smith EE, Patalano AL, Jonides J. Alternative strategies of categorization. Cognition. 1998;65:167–196. doi: 10.1016/s0010-0277(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Smulders FTY, Kenemans JL, Schmidt WF, Kok A. Effects of task complexity in young and old adults: Reaction time and P300 latency are not always dissociated. Psychophysiology. 1999;36:118–125. doi: 10.1017/s0048577299961590. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electrophysiology and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W. Item and source memory: Differential age effects revealed by event-related potentials. NeuroReport. 1997;8:3373–3378. doi: 10.1097/00001756-199710200-00036. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ, Rubin SR, Ryan JP. Frontal brain activity predicts individual performance in an associative memory exclusion test. Cerebral Cortex. 2002;12:1180–1192. doi: 10.1093/cercor/12.11.1180. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Senkfor AJ, Newberg WM. Memory for drawings in locations: Spatial source memory and event-related potentials. Psychophysiology. 2000;37:551–564. [PubMed] [Google Scholar]
- Verleger R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology. 1997;34:131–156. doi: 10.1111/j.1469-8986.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Verleger R, Jaœkowski P, Wascher E. Evidence for an integrative role of P3b in linking reaction to perception. Journal of Psychophysiology. 2005;19:165–181. [Google Scholar]
- Wijers AA, Mulder G, Okita T, Mulder LJM, Scheffers MK. Attention to color: an analysis of selection, controlled search, and motor activation, using event-related potentials. Psychophysiology. 1989;26:89–109. doi: 10.1111/j.1469-8986.1989.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]


