Abstract
A pH- and temperature-responsive, injectable hydrogel has been designed to take advantage of the acidic microenvironment of ischemic myocardium. This system can improve therapeutic angiogenesis methods by providing spatio-temporal control of angiogenic growth factor delivery. The pH- and temperature-responsive random copolymer, poly(N-isopropylacrylamide-co-propylacrylic acid-co-butyl acrylate) (p[NIPAAm-co-PAA-co-BA]), was synthesized by reversible addition fragmentation chain transfer polymerization. This polymer was a liquid at pH 7.4 and 37 °C but formed a physical gel at pH 6.8 and 37 °C. Retention of biotinylated basic fibroblast growth factor (bFGF) between 0-7 days after injection into infarcted rat myocardium was 10-fold higher with hydrogel delivery versus saline. Following 28 days of treatment in vivo, capillary and arteriolar densities were increased 30-40% by polymer+bFGF treatment versus saline+bFGF or polymer-only controls. Treatment with polymer+bFGF for 28 days resulted in a 2-fold improvement in relative blood flow to the infarct region versus day 0, whereas saline+bFGF or polymer-only had no effect. Fractional shortening determined by echocardiography was significantly higher following treatment with polymer+bFGF (30±1.4%) versus saline (25±1.2%) and polymer alone (25±1.8%). By responding to local changes in pH and temperature in an animal model of ischemia, this hydrogel system provided sustained, local delivery of bFGF, improved angiogenesis, and achieved therapeutic effects in regional blood flow and cardiac function.
Keywords: angiogenesis, hydrogel, thermally-responsive material, fibroblast growth factor, controlled drug release, copolymer
INTRODUCTION
Ischemic heart disease is a leading cause of death worldwide and presents significant morbidity. Therapeutic angiogenesis, or the delivery of angiogenic growth factors to promote revascularization of ischemic tissue, is a promising approach to treat ischemic heart disease [1]. However, this approach has been met with limited clinical success because of several challenges that must be overcome from a delivery standpoint [2, 3]. In particular, angiogenic growth factors must be retained locally at the ischemic site to prevent systemic side effects and be gradually released to allow adequate time for growth of new blood vessels.
Biomaterials that can provide spatial and temporal control of angiogenic growth factor delivery can improve the clinical viability of therapeutic angiogenesis approaches [4-6]. Prior approaches that have shown evidence of increased blood vessel growth in infarcted myocardial tissue include delivery of basic fibroblast growth factor (bFGF) with sustained release gelatin [4, 7] or chitosan [8, 9], or sequential administration of vascular endothelial growth factor (VEGF) followed by platelet-derived growth factor (PDGF) via an alginate hydrogel [10]. There are still significant limitations, however, that must be overcome before clinical implementation. First, the ability to maintain functional improvement at later time points remains a challenge. Shao et al. did not find sustained functional improvement at 4 weeks despite encouraging echocardiography results at 2 weeks following bFGF delivery with a gelatin-based system to infarcted rat hearts [7]. Also, delivery systems that require external stimuli such as an ultraviolet light source for gel formation [8] could be challenging to implement clinically. Finally, while these systems were capable of providing sustained release in vitro, the kinetics of growth factor release in vivo remained unclear in many of these studies. Release kinetics could be quite different in vivo due to animal movement, dilution by bodily fluids, or changes in the surrounding environment following acute injury (e.g. changes in pH due to ischemia, changes in extracellular milieu due to the inflammatory response). Thus, there remains a need for new biomaterials that offer logistically feasible delivery methods, display sustained delivery in vivo, and demonstrate functional improvement in therapeutic angiogenesis applications.
Polymers responsive to clinically-relevant stimuli such as pH or temperature have significant potential for use as biomaterials [11, 12]. In particular, changes in pH can be used as stimuli for drug delivery to regions of local acidosis as found in ischemic myocardium (pH 6-7) [13, 14]. For example, incorporation of carboxylic acid-derived monomers such as acrylic acid [15], methacrylic acid [16], ethylacrylic acid [17], or propylacrylic acid [18] has been used to create copolymers that respond to acidic pH values. In addition, increasing temperature from room temperature to body temperature (37 °C) can facilitate phase change in stimuli-responsive polymers such as in Pluronics® [19] or poly(N-isopropylacrylamide) [20].
We have developed a sharply pH- and temperature-responsive injectable hydrogel system composed of a random terpolymer of N-isopropylacrylamide (NIPAAm), propylacrylic acid (PAA), and butyl acrylate (BA) (p[NIPAAm-co-PAA-co-BA]) [21]. This polymer exists as a liquid at room temperature and pH 7.4 but becomes a gel at 37 °C and pH 6.8. We hypothesized that the ability of this polymer to form a reversible gel under conditions of intermediate acidity allows it to first act as a depot system for the release of angiogenic growth factors to ischemic tissue, and second, to promote polymer dissolution and elimination once the tissue has returned to physiological pH.
In this study, the time course of exogenous bFGF release from the hydrogel in vivo between 0 and 7 days was quantified using biotinylated bFGF. Furthermore, the ability of our hydrogel drug delivery system to achieve measurable therapeutic benefits in angiogenesis, regional blood flow, and cardiac function in a rat model of myocardial ischemia was evaluated.
MATERIALS AND METHODS
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used as received unless otherwise described. N-isopropylacrylamide (NIPAAm) was recrystallized from hexane. 2,2′-Azobisisobutyronitrile (AIBN) was recrystallized from methanol. Propylacrylic acid (PAA) was synthesized as shown previously [22]. Butyl acrylate (BA) was purified by distillation. 2-dodecylsulfanylthiocarbonylsulfanyl-2-methyl propionic acid (DMP) was obtained as a gift from Drs. Charles L. McCormick (University of Southern Mississippi) and John Lai (Noveon Company). Basic fibroblast growth factor (bFGF) was generously donated by Scios (Mountain View, CA). PD-10 desalting columns were purchased from GE Healthcare (Piscataway, NJ). FluoSpheres microspheres were purchased from Molecular Probes (Eugene, OR). Polycarbonate filters (10 μm) were purchased from GE Healthcare (Waukesha, WI). Primary antibodies for immunohistochemistry and western blot were purchased from Abcam (Cambridge, MA) (rat endothelial cell antigen, RECA-1; smooth muscle α-actin, α-SMA; and α-tubulin) or BD Pharmingen (BD Biosciences, San Jose, CA) (CD45). Biotin-SP-AffiniPure Goat Anti-Mouse IgG secondary antibody was purchased from Jackson ImmunoResearch (West Grove, PA). ProtOn biotin labeling kit, Quant*Tag biotin quantification kit, peroxidase anti-mouse IgG (H+L) secondary antibody, goat serum, Vectastain Elite ABC reagent, and 3,3′-diaminobenzidine (DAB) substrate were purchased from Vector Labs (Burlingame, CA). Tissue protein extraction reagent (T-PER), Halt protease inhibitor cocktail, Precise protein polyacrylamide gels (8-16%), and streptavidin-horseradish peroxidase were purchased from Pierce (Rockford, IL). 3,3′,5,5′-Tetramethylbenzidine 1-component Membrane Peroxidase Substrate was purchased from KPL (Gaithersburg, MD).
Animals
A total of 77 male Fischer 344 rats (Charles River, Wilmington, MA) were used in this study. Twenty-two rats were used to quantify retention of biotinylated bFGF (bFGF-biotin) by western blot. Rats were sacrificed at 0, 1, 2, or 7 days post-surgery. Fifty-seven male Fischer rats, aged 8-10 weeks, were used for efficacy studies involving survival surgery with a planned endpoint of 28 days post-surgery. These rats underwent echocardiography measurements, injection of fluorescent microspheres for blood flow analysis, and preparation of tissues for histology. All animals were treated according to the guidelines and policies set forth by the Institutional Animal Care and Use Committee at the University of Washington.
Polymer synthesis and characterization
Poly(NIPAAm-co-PAA-co-BA) was prepared by reversible addition fragmentation chain transfer (RAFT) polymerization as previously described [21] with DMP as the chain transfer agent (CTA), AIBN as the initiator, and 50 w/w % dimethylformamide (DMF) as the solvent. Solutions were purged with nitrogen for 30 min and then allowed to polymerize at 60 °C for 18 h. The polymers were then diluted in acetone and precipitated twice into ethyl ether. After drying the product under high vacuum for 24 h, the polymer yields were determined gravimetrically. Molecular weight of p(NIPAAm-co-PAA-co-BA) was quantified by gel permeation chromatography (GPCmax VE2001, Viscotek, Houston, TX) by comparison to a series of poly(methyl methacrylate) standards. High-performance liquid chromatography (HPLC)-grade DMF containing 0.01 mol/L LiBr was used as the eluent. Copolymer compositions were determined by proton nuclear magnetic resonance spectroscopy (1H-NMR) by comparing the peak area of the NIPAAm amide N-H signal at 7.2 ppm with the total peak area between 3.7 and 4.1 ppm (corresponding to the sum of the NIPAAm isopropyl C-H and the butyl acrylate ester – O-CH2 proton peaks) and the total peak area between 0.7 and 2.2 ppm (encompassing the remainder of C-H protons).
Biotinylation of bFGF
To quantify bFGF retention following hydrogel delivery into infarcted myocardium, bFGF was biotinylated (bFGF-biotin) using a ProtOn biotin labeling kit according to the manufacturer’s instructions (Vector Laboratories, Burlingame, CA).
Polymer sample preparation
To prepare P(NIPAAm-co-PAA-co-BA) random copolymers for injection, polymer samples were dissolved in DPBS (with a small amount of 1 N NaOH added to promote solubility), purified by PD-10 columns then lyophilized. Lyophilized polymer was re-dissolved in Dulbecco’s phosphate buffered saline (DPBS), and the pH of the polymer samples was adjusted with cold 1 N HCl until the polymer samples were capable of gel formation at 37 °C (pH 6.8 at 37°C). Chilled samples were filtered through 0.2-μm filters. For western blot time course studies, bFGF-biotin was diluted in sterile DPBS then incorporated into the polymer solution by gentle mixing such that each 40 μL injection contained 2.5 μg of bFGF-biotin with a final polymer concentration of 50 mg/mL (5 w/v %). For efficacy studies, bFGF and heparin (to stabilize bFGF) were diluted in sterile DPBS then incorporated into the polymer solution by gentle mixing to achieve a final concentration of 5 μg of bFGF and 50 μg heparin per 40 μL (injection volume) and 80 mg/mL (8 w/v %) of polymer (polymer+bFGF). Three controls were prepared in a similar manner: saline, polymer, and saline+bFGF (all contained equal heparin concentrations).
Rat myocardial infarct model
A permanent occlusion rat myocardial infarct model was used as described previously [23, 24]. Briefly, rats were anesthetized with isoflurane (5%), intubated, and supported with a mechanical ventilator. The heart was accessed through a left thoracotomy, and a retractor was used to keep the chest wall open during the procedure. A sterile suture was used to permanently occlude the left anterior descending coronary artery (LAD) approximately 1 mm distal to the distal edge of the left atrium to create a medium-sized infarct (typically involving ~30% of the left ventricle (LV)). Ten minutes after the onset of ischemia, two 20 μL injections (40 μL total) of polymer solution or saline were placed directly into the central region of the infarct zone of the myocardium using a 29 gauge needle. The chest was then closed with sutures and the animal was allowed to recover.
Tissue homogenization and western blotting
Rats injected with bFGF-biotin were sacrificed at 0, 1, 2, or 7 days post-surgery. Hearts were harvested and then cut in half perpendicular to the long axis with a scalpel to separate the heart into the apical and basal halves. Tissue samples were homogenized in T-PER then centrifuged for 5 minutes at 16,000 x g. The supernatant was transferred to clean microcentrifuge tubes and frozen at −80 °C until further use. To detect bFGF-biotin, samples were evaluated with western blotting using a streptavidin-horseradish peroxidase detection system. The loading control, α-tubulin (50 kDa), was detected using a peroxidase-conjugated secondary antibody. For quantification, blots were digitally scanned and the density of the bands was quantified with ImageJ software (NIH, Bethesda, MD). Values are reported as percent relative density (%) = (band density of sample) / (band density of the saline control at 0 h) x 100. A saline control at 0 h sample was included on every blot for normalization.
Echocardiography
For efficacy studies, rats were assigned randomly into 4 groups: saline, polymer, saline+bFGF, and polymer+bFGF. Echocardiography measurements were taken prior to surgery in 10 rats, and at 2, 14, and 28 days after surgery in all surviving rats to quantify left chamber dimensions. Rats were lightly anesthetized with isoflurane (2% isoflurane in room air supplemented with 100% O2) and chest walls were shaved for echocardiography measurements as previously described [24]. Parameters examined include the left-ventricular end diastolic dimension (LVEDD), left-ventricular end systolic dimension (LVESD), and heart rate (beats per minute). The percent fractional shortening (FS%) was calculated by the following equation: fractional shortening = (LVEDD – LVESD) / LVEDD x 100. All measurements were performed by a blinded observer.
Microsphere injection for regional myocardial blood flow
FluoSpheres polystyrene microspheres (15 μm diameter) (Molecular Probes, Eugene, OR) were injected into the chamber of the left ventricle of the heart via the apex to evaluate blood flow [25, 26]. Sixteen rats received injection of 200,000 yellow-green (excitation/emission: 505/515 nm) microspheres immediately prior to infarct and 200,000 red-orange (565/580 nm) microspheres immediately after infarct and just prior to closure of the animal. The remaining 41 rats did not receive microsphere injections on day 0. The differences in the fluorescent spectra of the microspheres injected on day 0 allowed us to compare the differences in myocardial blood flow pre- and post-infarction on day 0. At 28 days post-surgery, all surviving rats were intubated, their chests reopened and their left ventricular cavities injected with 200,000 green (450/480 nm) microspheres in a 200 μL volume in a similar manner as before. The microspheres were allowed to circulate for 5 minutes, and the rats were then euthanized with an overdose of sodium pentobarbital.
Tissue harvest and sampling
Immediately after sacrifice, hearts were harvested then rinsed in PBS. Hearts were sliced into ~1 mm thick short-axis sections using a rat heart slicer matrix (Zivic Instruments, Pittsburgh, PA). At least five sections per heart were collected starting at the apex; remaining heart tissue (closer to the base) was discarded (Figure 1). At least two sections per heart were fixed in methyl Carnoy’s fixative and stored at 4 °C until further processing for histological analysis. The remaining three sections were used for microsphere analysis, and the infarct and uninjured regions were isolated with the aid of a dissecting microscope using a scalpel. The peri-infarct regions were discarded. The non-infarct regions from all three slices were combined into a single 15 mL polypropylene tube, weighed, and stored at −80 °C until further processing. Similarly, the infarct regions from all three slices were combined, weighed, and frozen.
Figure 1.

Preparation of heart sections for histology and microsphere regional blood flow analysis. Infarcted and uninjured sections were individually isolated for microsphere (flow) analysis. RV, right ventricle; LV, left ventricle.
Microsphere analysis
Heart samples were prepared for microsphere analysis as previously described [26, 27]. Briefly, tissue samples were digested in KOH with 2% Tween 80, and microspheres were separated from the digested tissue by negative pressure filtration using polycarbonate filters (10 μm) and a suction filter apparatus. Fluorescent dye was extracted from the microspheres using 2-(2-ethoxyethoxy)ethyl acetate. After centrifugation, supernatant samples were read in a fluorescence spectrometer (Perkin Elmer LS-50, Waltham, MA). Relative fluorescence intensity was determined in the supernatant at each of the three wavelengths used in this study, with correction for spectral overlap. Raw data were reported as relative fluorescence intensity (FI) units. To calculate relative regional myocardial blood flow, relative FI units of the pooled infarct samples and uninjured samples were normalized to tissue weight. Blood flow to the infarct region was expressed as a percentage of that in the non-infarcted region using the following formula: (Relative fluorescence intensity in infarct region / tissue weight) / (Relative fluorescence intensity in uninjured region / tissue weight) x 100.
Morphometric analysis
Hematoxylin and eosin (H&E)-stained sections located approximately 2 mm proximal to the apex were used to measure the thickness of the infarcted free wall, thickness of the uninjured septal wall, LV endocardial circumference, and LV epicardial circumference. Images were digitally scanned and distances were measured using ImageJ software (NIH, Bethesda, MD). Five measurements of the infarcted free wall (LV scar) and three measurements of the uninjured septal wall were taken per sample and values were averaged for each heart. Expansion index was calculated according to the following equation: (LV cavity/total LV area) x (septal thickness/LV scar thickness).
Immunohistochemistry and vessel density quantification
Immunohistochemical staining was used to identify and quantify cells positive for rat endothelial cell antigen-1 (RECA-1), alpha-smooth muscle actin (a-SMA), or CD45. Slides were deparaffinized, rehydrated, then blocked in 1.5% goat serum in PBS for 60 min at room temperature. Excess serum was blotted away and slides were incubated with the primary antibody (RECA-1 (mouse anti-rat): 1:100 dilution; α-SMA (mouse): 1:100; CD45 (mouse anti-rat): 1:30) prepared in 1.5% goat serum in PBS for 60 min at room temperature or overnight at 4 °C. Slides were rinsed in PBS then incubated with goat anti-mouse IgG secondary antibody in PBS containing 1.5% goat serum (1:400) for 30 min at room temperature. Slides were rinsed in PBS, incubated with Vectastain Elite ABC reagent, then detected with DAB according to the manufacturer’s instructions. Slides were then rinsed in tap water and counterstained with hematoxylin as described previously. Images were taken using a 20x objective (Nikon E800, Melville, NY) in a section of each heart approximately 2 mm proximal to the apex. Capillary density was determined by manual counting in a blinded manner as described previously in slides stained with RECA-1 antibody [28]. Capillaries were identified by positive RECA-1 antibody staining of the endothelial cell(s), and any distinct endothelial cell or group of endothelial cells was counted as a vessel. Vessel lumens did not have to be present in the microscopic image to be counted as a capillary [28]. Arteriole density was quantified in a similar manner, with arterioles being identified by positive α-SMA antibody staining of the vascular smooth muscle cell and a visible lumen with an inner diameter > 10 μm [29].
Statistics
Statistical significance between groups was evaluated using an unpaired t-test, with significance achieved when p<0.05. Values are reported as mean±SEM unless noted otherwise.
RESULTS
Polymer characterization
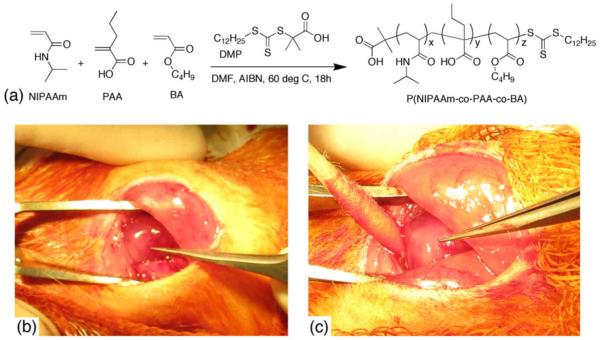
P(NIPAAm-co-PAA-co-BA) was synthesized by RAFT polymerization (Figure 2a) with a feed composition of 80 mol % NIPAAm, 10 mol % PAA, and 10 mol % BA and a calculated polymer composition of 67 mol % NIPAAm, 18 mol % PAA, and 15 mol % BA by 1H-NMR. The number-average molecular weight (Mn) and polydispersity index (PDI) of the polymer as determined by gel permeation chromatography were 28 kDa and 1.13, respectively. This polymer was selected because of its ability to undergo a sol-to-gel phase transition in the expected pH range of ischemic myocardium (pH 6–7 [13, 14]), with a gel pH of 6.8 at 37 °C. Images of acutely ischemic rat hearts injected with saline or hydrogel are shown in Figures 2b and 2c, respectively. A persistent white, raised mass was observed following polymer injection, whereas saline-only injections typically caused transient blanching (temporary change of color from dark pink to light pink) of the tissue. Twenty-two rats were used to quantify retention of bFGF-biotin by western blot analysis. Four rats were sacrificed at 0 h post-surgery, and the remaining rats were sacrificed at 1, 2, or 7 days post-surgery (100% survival rate). Forty-nine out of 57 rats used for efficacy studies remained alive at 28 days post-surgery (86% survival rate). Eight rats died less than 24 h post-surgery due to complications of myocardial infarction.
Figure 2.
(a) Synthesis of p(N-isopropylacrylamide-co-propylacrylic acid-co-butyl acrylate) by reversible addition fragmentation chain transfer (RAFT) polymerization. (b) and (c) Photos of ischemic rat hearts immediately after injection of 40 μL of either (b) saline or (c) p(NIPAAm-co-PAA-co-BA). Forceps are pointing to injection site. Note the greater extent of blanched tissue in the hydrogel-injected heart.
Quantification of bFGF retention following hydrogel delivery to infarct model
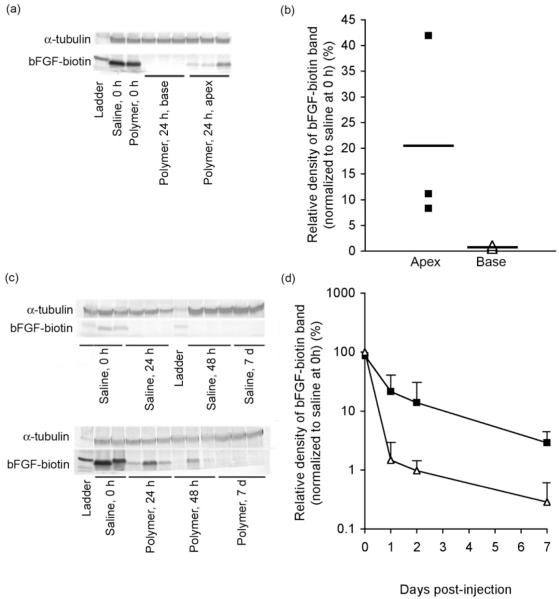
The hydrogel provided local retention of bFGF-biotin in the apex with minimal diffusion to the basal half of the heart (Figure 3a-b). Basic FGF could be detected in the apex half of the heart at 24 h post-injection following delivery with polymer, with a relative band density of 20% compared to saline control at 0 h. In contrast, little to no bFGF was detectable in the basal half of the heart at 24 h post-injection following delivery with polymer (relative density < 1%), confirming that the polymer system was able to retain bFGF at the site of injection with minimal diffusion to distant regions of the heart.
Figure 3.
Quantification of bFGF-biotin retention by western blot analysis following injection into infarcted rat myocardium. Localization of bFGF to apex but not base half of heart at 24 h demonstrated by (a) western blot and (b) densitometry results. Quantification of bFGF release kinetics demonstrated by (c) western blot and (d) densitometry results. Heart samples were harvested at 0 h, 24 h, 48 h, or 7 days post-injection then homogenized to facilitate detection of bFGF-biotin by western blot. Samples injected with either phosphate buffered saline (PBS) (△in panels b and d) or polymer (■in panels b and d) (28 kDa, 5 w/v % in PBS) as the delivery vehicle. Each 40 μL injection contained 2.5 μg of bFGF-biotin. Values reported as mean±SD are normalized to α-tubulin and to bFGF band density of saline control at 0 h, n=3 for each time point.
Between 1-7 days post-injection, the amount of bFGF-biotin recovered from homogenized apex tissue was consistently higher following delivery with polymer compared to saline vehicle (Figure 3c-d). Immediately after injection, recovery of bFGF-biotin was nearly identical in the saline and polymer animals. However, at 1, 2 and 7 days post-injection, we observed relative band densities of 21%, 13%, and 3% of bFGF-biotin when delivered with polymer, respectively. In contrast, when delivered with saline, band densities of only 2% and 1% of bFGF-biotin were observed at 1 and 2 days, respectively, and virtually no bFGF-biotin could be detected by western blot at 7 days.
Echocardiography
Values for fractional shortening (FS%) as a function of time are shown in Figure 4. In non-infarcted animals, FS% averaged 46±3%. At two days post-infarction, all groups showed significant depression of systolic function, with FS% of 31±0.8% and no differences among groups. Systolic function decreased further by 2 weeks as left ventricular remodeling progressed, with FS% ranging from 25-28%, again with no differences among treatment groups. By 28 days, however, the FS% in the polymer+bFGF group (30±1.4%) was significantly higher than the FS% in the saline (25±1.2%) and polymer control (25±1.8%) groups (p<0.05 vs. either saline or polymer controls).
Figure 4.
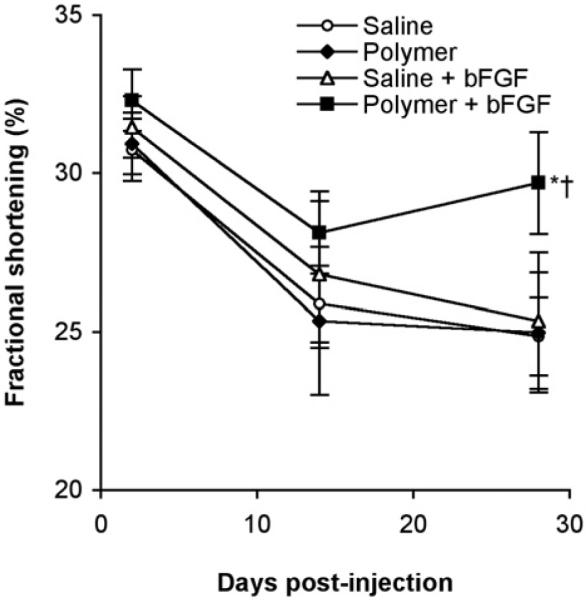
Systolic fractional shortening determined by echocardiography at 2, 14, and 28 days post-surgery in rats injected with polymer+bFGF (■, n=16), saline+bFGF (△, n=8), polymer (◆, n=9), or saline (|○, n=15). All injections contained 50 μg heparin. Values shown are mean±SEM. *p<0.05 versus saline at 28 days; †p<0.05 versus polymer at 28 days.
Regional myocardial blood flow
Blood flow in the infarct zone was significantly increased in the polymer+bFGF group at 28 days (26±5% of blood flow to uninjured myocardium) compared to the post-infarct group on day 0 (10±3%) and the polymer alone group at 28 days (13±3%) (Figure 5). Relative blood flow to the infarct region in the polymer alone and saline+bFGF (16±4%) groups did not show any improvement at 28 days compared to post-infarct on day 0. However, there was no difference in relative blood flow between the saline alone and polymer+bFGF groups at 28 days.
Figure 5.
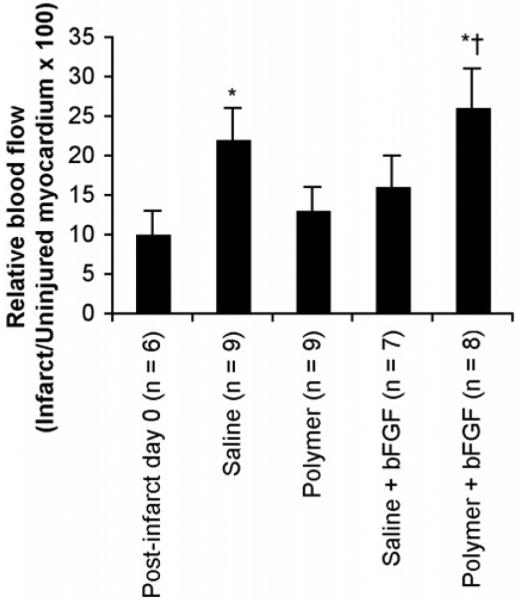
Regional myocardial blood flow to infarct region after 28 days of treatment, expressed as a percentage of the blood flow to the uninjured myocardium. Relative flow quantified using fluorescent polystyrene microspheres. Post-infarct day 0 indicates baseline data from red-orange microspheres injected on day 0, while saline, polymer, saline+bFGF, and polymer+bFGF groups indicate data from green microspheres injected at the time of sacrifice (28 days post-surgery). Infarcted and uninjured tissue samples were isolated at 28 days post-injection at the time of sacrifice, and fluorescence intensity values were normalized to tissue weights. Values shown are mean±SEM. *p<0.05 versus post-infarct day 0, †p<0.05 versus polymer at 28 days.
Histology Studies: Expansion Index, Inflammation and Angiogenesis
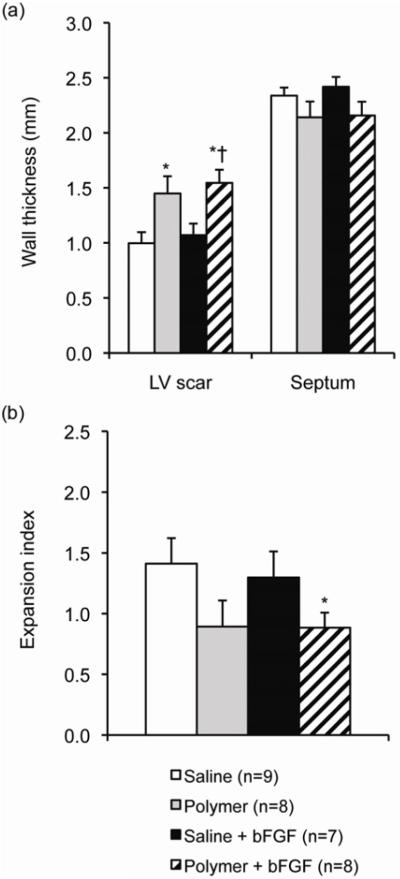
The wall thickness of the LV scar in animals treated with polymer+bFGF (1.5±0.1 mm) was statistically increased compared to animals treated with saline alone (1.0±0.1 mm) or saline+bFGF (1.1±0.1 mm) (Figure 6a). In addition, wall thickness was increased in animals treated with polymer alone (1.4±0.2 mm) compared to saline alone. The expansion index in the polymer+bFGF group (0.9±0.1) was also significantly lower than that of the saline group (1.4±0.2, p<0.05 versus polymer+bFGF) (Figure 6b).
Figure 6.
Morphometry of heart sections at 28 days post-injection. (a) Wall thicknesses of the infarcted free wall (LV scar) and uninjured septal wall (septum) of the left ventricle at 28 days. (b) Expansion index. Sections were taken from approximately 2 mm proximal to the apex and stained with H&E. Values shown are mean±SD. *p<0.05 versus saline. †p<0.05 versus saline+bFGF.
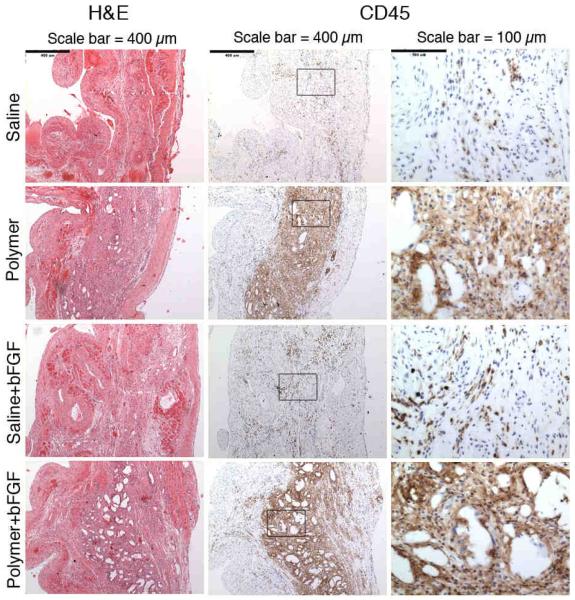
There was a higher inflammatory response as indicated by CD45 staining in animals injected with polymer compared to saline at 28 days (Figure 7). The inflammatory cells were predominantly mononuclear. Neutrophils were not noted, while foreign body giant cells were occasionally seen (not shown). In addition, many of the polymer-treated animals had large void spaces in the tissue that were not seen in saline-treated animals. These void spaces were likely residual polymer that had dissolved in the solvents used during tissue fixation and histological processing.
Figure 7.
Representative images stained with hematoxylin and eosin (H&E) (left column) or CD45 (middle and right columns) at 28 days post-injection with saline, polymer, saline+bFGF, or polymer+bFGF. Scale bar indicates 400 μm in left and middle columns and 100 μm in right column. Note the intense CD45+ inflammatory infiltrate in hearts receiving polymer injections. Void spaces likely reflect residual polymer, dissolved during histological processing.
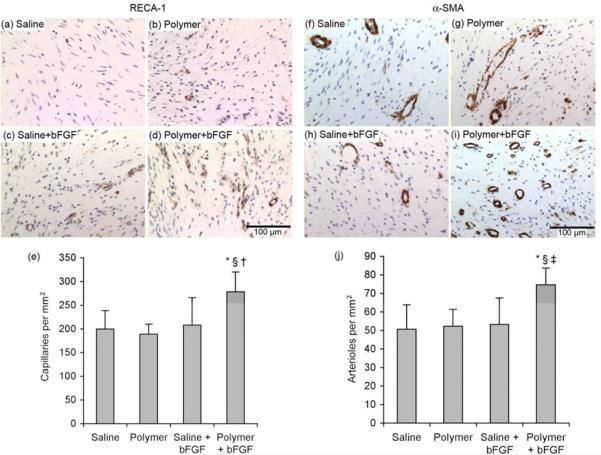
The principal hypothesis of this study was that our pH-responsive hydrogel would enhance angiogenic effects of bFGF therapy. In support of this notion, histological studies showed a significant improvement in the angiogenic response following delivery of bFGF with the hydrogel. There was a significant increase by approximately 30% in both capillary (Figure 8a-e) and arteriolar (Figure 8f-j) densities as quantified by the number of RECA-1-positive or α-SMA-positive microvessels, respectively, per mm2 in the polymer+bFGF group compared to saline, polymer alone, and saline+bFGF.
Figure 8.
(a-e) Capillary density evaluated by rat endothelial cell antigen-1 (RECA-1) staining and (f-j) arteriolar density evaluated by smooth muscle α-actin (α-SMA) staining. (a-e) Representative images of sections with endothelial cell staining (dark brown stain indicates positive antibody staining for RECA-1) after 28 days of treatment with (a) saline (n=9), (b) polymer (n=9), (c) saline+bFGF (n=7), or (d) polymer+bFGF (n=9). (e) Quantification of capillary density following 28 days of treatment. (f-j) Representative images of sections with smooth muscle cell staining (dark brown stain indicates positive antibody staining for α-SMA) after 28 days of treatment with (f) saline (n=9), (g) polymer (n=9), (h) saline+bFGF (n=7), or (i) polymer+bFGF (n=9). (j) Quantification of arteriolar density following 28 days of treatment. Scale bar indicates 100 μm. *p<0.001 versus saline, §p<0.001 versus polymer, †p<0.05 versus saline+bFGF. ‡p<0.001 versus saline+bFGF.
DISCUSSION
The injectable, pH- and temperature-responsive hydrogel system, p(NIPAAm-co-PAA-co-BA), was tested for its efficacy as a delivery system for angiogenic growth factors in vivo. P(NIPAAm-co-PAA-co-BA) was a liquid at room temperature but formed a gel after injection into ischemic myocardium. This hydrogel system was capable of both sustained release and local delivery of bFGF. The efficacy of our p(NIPAAm-co-PAA-co-BA) hydrogel was tested in a rat model of myocardial infarct. We demonstrated that this system is able to provide spatio-temporal control of bFGF delivery, which in turn promoted increased angiogenesis, enhanced blood flow, and improved cardiac function.
The dual ability of this hydrogel to respond to both pH and temperature allowed us to tune the system to provide a rapidly gelling but still reversible hydrogel system that will dissolve away from the site as the tissue returns to physiological pH.Basic FGF-biotin delivered with this system remained localized near the site of injection, as bFGF-biotin could only be detected in the apex region; little to no bFGF-biotin could be detected in the base samples tested. In addition, the amount of bFGF-biotin recovered at 1, 2, and 7 days was enhanced 10-fold with polymer delivery compared to saline delivery. Using this system to deliver bFGF, there was a significant increase in capillary and arteriolar densities in animals treated with polymer+bFGF compared to saline, polymer alone, or saline+bFGF at 28 days post-surgery. These results demonstrate the significant potential for this pH- and temperature-responsive system to provide spatial and temporal control of angiogenic growth factor delivery and be used as an effective therapy following acute myocardial infarction.
We found improved blood flow to the infarct region with our polymer system. In general, regional blood flow measurements at 28 days correlated well with histological analysis of microvascular density. There was a statistically higher relative blood flow in the polymer+bFGF group at 28 days compared to baseline (immediately after infarct on day 0). Also, both regional blood flow measurements and histology showed that polymer+bFGF treatment was superior to polymer-alone at 28 days. There was one notable discrepancy between the results seen with regional blood flow measurements compared to histology: microsphere data indicated no difference between saline-only and polymer+bFGF at 28 days. This is contrast to the histological results showing that saline-only, polymer only, and saline+bFGF had no effect on microvascular density, while polymer+bFGF substantially increased microvascular density. One potential explanation is that rather than achieving venule-to-arteriole anastomosis during capillary formation, we instead had venule-to-venule connections. If this occurred, we could still see increased microvascular density with histology, however, an improvement in blood flow would not be observed as the fluorescent microspheres were only embedded in the arterial side. Note, however, that angiogenesis on the post-capillary side would not explain our observation that blood flow was enhanced in polymer+bFGF compared to saline+bFGF animals. An alternative, and perhaps more likely explanation, is that there may have been inadvertent contamination of some of the infarct samples with non-ischemic tissue in the saline-only group. We were unable to prove this definitively, however, so data from all animals are included in this paper.
The increased angiogenesis combined with the increased thickness of the infarcted wall in the polymer+bFGF group likely facilitated the improvement in cardiac function as measured by echocardiography at 28 days post-injection compared to saline alone and polymer alone. These results are consistent with previous work from our group in mice where bFGF was knocked out or overexpressed [30]. In that study we found that genetic deletion of bFGF inhibited angiogenesis and fibroblast proliferation post-infarction, in association with worsened ventricular remodeling and contractile function. Conversely, overexpression of bFGF enhanced angiogenesis and fibroblast proliferation, in association with reduced ventricular remodeling and better contractile function. The long term benefit observed with bFGF protein therapy in the current study contrasts with results found by Shao et al., who found that there was no difference in FS% four weeks after delivery of bFGF by a gelatin hydrogel compared to saline [7]. However, our results are consistent with those described by Wang et al., who found approximately 9-10% improvement in FS% following delivery of bFGF by a chitosan hydrogel compared to saline [9]. These results demonstrate the significant potential for our pH- and temperature-responsive system to be an effective therapy following acute myocardial infarction.
The polymer alone group also had increased wall thickness of the LV scar but not increased angiogenesis compared to saline alone. Although this did not translate into significant differences in FS% between the saline and polymer groups, these results suggest that injection of the polymer itself may be capable of preventing the wall thinning that occurs during the normal progression of wound healing following myocardial infarction [31]. This is in agreement with others who have suggested that some biomaterials can increase LV thickness and improve cardiac function in the absence of exogenous growth factor delivery [32, 33]. This may be due to increased inflammatory cell migration to the injection site in animals treated with polymer, which can facilitate collagen deposition [34].
There was evidence of a chronic inflammatory response marked by macrophage infiltration and occasional foreign body giant cells observed near the polymer injection site at 28 days post-surgery. Whether this inflammatory response is beneficial to further facilitate an angiogenic effect or detrimental to our therapeutic outcome remains unclear. In the context of myocardial infarct, inflammatory cells such as monocytes and macrophages play an important role in wound healing. It has been shown that certain subsets of monocytes are responsible for clearing away dead cardiomyocytes, stimulating angiogenesis, or depositing collagen [34]. Monocytes and macrophages are capable of secreting angiogenic growth factors to promote angiogenesis [35]. Thus, it is possible that the increased presence of macrophages in animals treated with polymer could provide beneficial effects to improve the angiogenic response of our system. However, the lack of control over the extracellular milieu also has the potential for having a detrimental effect on angiogenesis. In addition, it is unclear how long the inflammatory response will be activated and whether a chronic inflammatory response would be detrimental in this context. Time points later than 28 days will be necessary determine whether the inflammatory response persists after the polymer is completely eliminated from the myocardium.
CONCLUSIONS
We have shown that delivery of bFGF to infarcted rat myocardium with the stimuli-responsive hydrogel, p(NIPAAm-co-PAA-co-BA), can increase microvessel density, improve regional blood flow, and improve cardiovascular function after 28 days of treatment. In addition, we quantified the rate of release of bFGF-biotin from the hydrogel system over a 7-day period following injection into infarcted myocardium and confirmed the ability of this polymer to provide spatio-temporal control over bFGF delivery. Based on the results described, this system has strong potential for further development as a vehicle for therapeutic growth factors. Future work should be aimed at modifying the polymer system to improve drug retention and reduce inflammation.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Jennifer Deem and Sarah Dupras for their technical assistance with animal surgeries and analysis of echocardiography results, Dowon An for processing fluorescent microsphere samples, and Veronica Muskheli for assistance with histology. In addition, the authors thank Charles L. McCormick (University of Southern Mississippi) and John Lai (Noveon) for their generous donation of the DMP CTA and Scios Inc. for their donation of bFGF. This project was funded by NIH grants R01 HL64387, R01 HL084642, R01 EB2991, P01 HL094374 and the University of Washington’s Mouse Metabolic Phenotyping Center U24 DK076126. J.C.G. was supported by NIH T32 EB001650 and the Poncin Scholarship Fund.
LIST OF ABBREVIATIONS
- AIBN
2,2′-azobisisobutyronitrile
- BA
butyl acrylate
- bFGF
basic fibroblast growth factor
- CTA
chain transfer agent
- DAB
3,3′-diaminobenzidine
- DMF
dimethylformamide
- DMP
2-dodecylsulfanylthiocarbonylsulfanyl-2-methyl propionic acid
- DPBS
Dulbecco’s phosphate buffered saline
- FS%
fractional shortening
- GPC
gel permeation chromatography
- H&E
hematoxylin and eosin
- 1H-NMR
proton nuclear magnetic resonance
- LV
left ventricle
- LVEDD
left ventricular end diastolic dimension
- LVESD
left ventricular end systolic dimension
- NIPAAm
N-isopropylacrylamide
- PAA
propylacrylic acid
- RAFT
reversible addition fragmentation chain transfer
- RECA-1
rat endothelial cell antigen-1
- SEM
standard error of the mean
- α-SMA
alpha-smooth muscle actin
- T-PER
tissue-protein extraction reagent
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Freedman SB, Isner JM. Therapeutic angiogenesis for ischemic cardiovascular disease. J Mol Cell Cardiol. 2001;33:379–93. doi: 10.1006/jmcc.2000.1329. [DOI] [PubMed] [Google Scholar]
- 2.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–93. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 3.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 4.Iwakura A, Fujita M, Kataoka K, Tambara K, Sakakibara Y, Komeda M, et al. Intramyocardial sustained delivery of basic fibroblast growth factor improves angiogenesis and ventricular function in a rat infarct model. Heart Vessels. 2003;18:93–9. doi: 10.1007/s10380-002-0686-5. [DOI] [PubMed] [Google Scholar]
- 5.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 6.Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–51. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Shao ZQ, Takaji K, Katayama Y, Kunitomo R, Sakaguchi H, Lai ZF, et al. Effects of intramyocardial administration of slow-release basic fibroblast growth factor on angiogenesis and ventricular remodeling in a rat infarct model. Circ J. 2006;70:471–7. doi: 10.1253/circj.70.471. [DOI] [PubMed] [Google Scholar]
- 8.Fujita M, Ishihara M, Morimoto Y, Simizu M, Saito Y, Yura H, et al. Efficacy of photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 in a rabbit model of chronic myocardial infarction. J Surg Res. 2005;126:27–33. doi: 10.1016/j.jss.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Zhang X, Li Y, Ma Y, Zhang Y, Liu Z, et al. Improved myocardial performance in infarcted rat heart by co-injection of basic fibroblast growth factor with temperature-responsive chitosan hydrogel. J Heart Lung Transplant. 2010;29:881–7. doi: 10.1016/j.healun.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–85. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Matsusaki M, Akashi M. Novel functional biodegradable polymer IV: pH-sensitive controlled release of fibroblast growth factor-2 from a poly(gamma-glutamic acid)-sulfonate matrix for tissue engineering. Biomacromolecules. 2005;6:3351–6. doi: 10.1021/bm050369m. [DOI] [PubMed] [Google Scholar]
- 12.Shim WS, Kim J-H, Park H, Kim W, Kwon IC, Lee DS. Biodegradability and biocompatibility of a pH- and thermo-sensitive hydrogel formed from a sulfonamide-modified poly(e-caprolactone-co-oactinde)-poly(ethylene glycol)-poly(e-caprolactone-co-lactide) block copolymer. Biomaterials. 2006;27:5178–85. doi: 10.1016/j.biomaterials.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Khabbaz KR, Zankoul F, Warner KG. Intraoperative metabolic monitoring of the heart: II. Online measurement of myocardial tissue pH. Ann Thorac Surg. 2001;72:S2227–33. doi: 10.1016/s0003-4975(01)03284-2. discussion S33-4, S67-70. [DOI] [PubMed] [Google Scholar]
- 14.Kumbhani DJ, Healey NA, Birjiniuk V, Crittenden MD, Josa M, Treanor PR, et al. Determinants of regional myocardial acidosis during cardiac surgery. Surgery. 2004;136:190–8. doi: 10.1016/j.surg.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Hoffman AS. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature. 1995;373:49–52. doi: 10.1038/373049a0. [DOI] [PubMed] [Google Scholar]
- 16.Brazel CS, Peppas NA. Synthesis and characterization of thermo- and chemomechanically responsive poly(N-isopropylacrylamide-co-methacrylic acid) hydrogels. Macromolecules. 1995;28:8016–8020. [Google Scholar]
- 17.Lackey CA, Murthy N, Press OW, Tirrell DA, Hoffman AS, Stayton PS. Hemolytic activity of pH-responsive polymer-streptavidin bioconjugates. Bioconjug Chem. 1999;10:401–5. doi: 10.1021/bc980109k. [DOI] [PubMed] [Google Scholar]
- 18.Yin X, Hoffman AS, Stayton PS. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules. 2006;7:1381–5. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- 19.Alexandridis P, Hatton TA. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modeling. Colloids Surf A Physicochem Eng Asp. 1995;96:1–46. [Google Scholar]
- 20.Han CK, Bae YH. Inverse thermally-reversible gelation of aqueous N-isopropylacrylamide copolymer solutions. Polymer. 1998;39:2809–12. [Google Scholar]
- 21.Garbern JC, Hoffman AS, Stayton PS. Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules. 2010;11:1833–9. doi: 10.1021/bm100318z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrito M, Tirrell DA. Poly(2-ethylacrylic acid) Macromol Synth. 1992;11:59–62. [Google Scholar]
- 23.Anderl JN, Robey TE, Stayton PS, Murry CE. Retention and biodistribution of microspheres injected into ischemic myocardium. J Biomed Mater Res A. 2009;88:704–10. doi: 10.1002/jbm.a.31917. [DOI] [PubMed] [Google Scholar]
- 24.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 25.Hale SL, Vivaldi MT, Kloner RA. Fluorescent microspheres: a new tool for visualization of ischemic myocardium in rats. Am J Physiol. 1986;251:H863–8. doi: 10.1152/ajpheart.1986.251.4.H863. [DOI] [PubMed] [Google Scholar]
- 26.Van Oosterhout MF, Willigers HM, Reneman RS, Prinzen FW. Fluorescent microspheres to measure organ perfusion: validation of a simplified sample processing technique. Am J Physiol. 1995;269:H725–33. doi: 10.1152/ajpheart.1995.269.2.H725. [DOI] [PubMed] [Google Scholar]
- 27.Glenny RW, Bernard S, Brinkley M. Validation of fluorescent-labeled microspheres for measurement of regional organ perfusion. J Appl Physiol. 1993;74:2585–97. doi: 10.1152/jappl.1993.74.5.2585. [DOI] [PubMed] [Google Scholar]
- 28.Schor AM, Pendleton N, Pazouki S, Smither RL, Morris J, Lessan K, et al. Assessment of vascularity in histological sections: effects of methodology and value as an index of angiogenesis in breast tumours. Histochem J. 1998;30:849–56. doi: 10.1023/a:1003437619956. [DOI] [PubMed] [Google Scholar]
- 29.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–60. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 30.Virag JA, Rolle ML, Reece J, Hardouin S, Feigl EO, Murry CE. Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol. 2007;171:1431–40. doi: 10.2353/ajpath.2007.070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisman HF, Bush DE, Mannisi JA, Weisfeldt ML, Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation. 1988;78:186–201. doi: 10.1161/01.cir.78.1.186. [DOI] [PubMed] [Google Scholar]
- 32.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–68. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polverini PJ, Cotran PS, Gimbrone MA., Jr. Unanue ER. Activated macrophages induce vascular proliferation. Nature. 1977;269:804–6. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]