Abstract
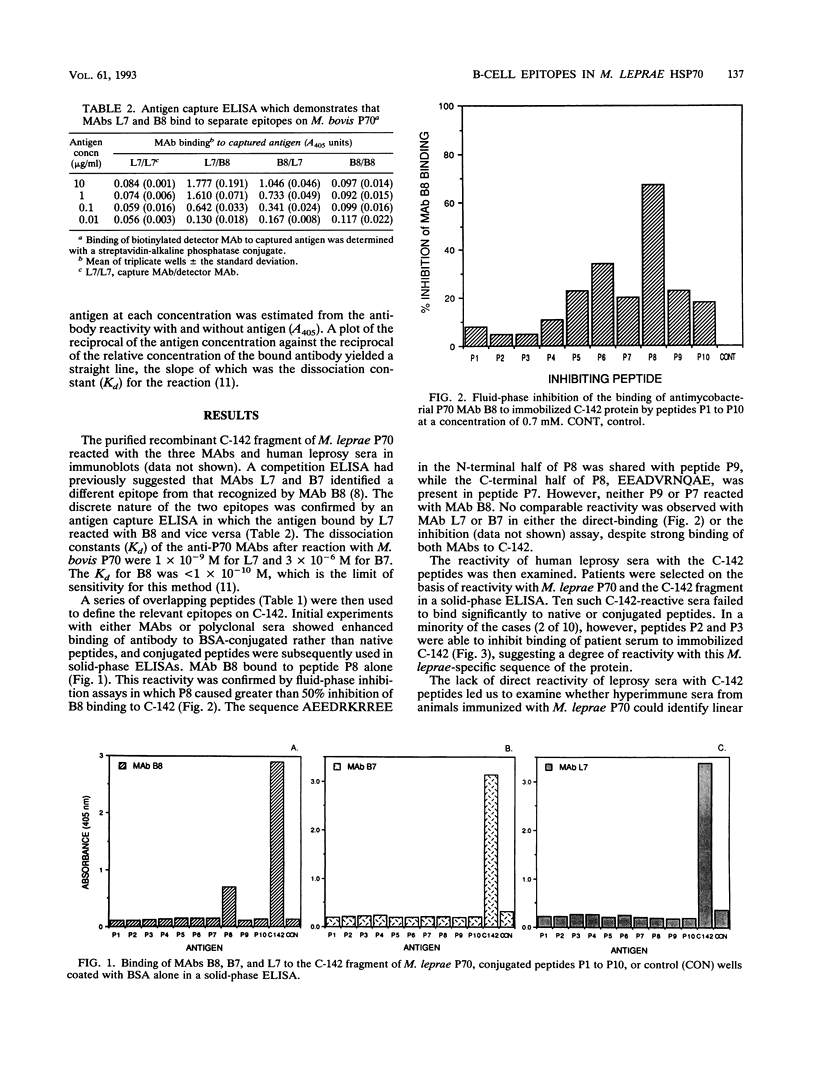
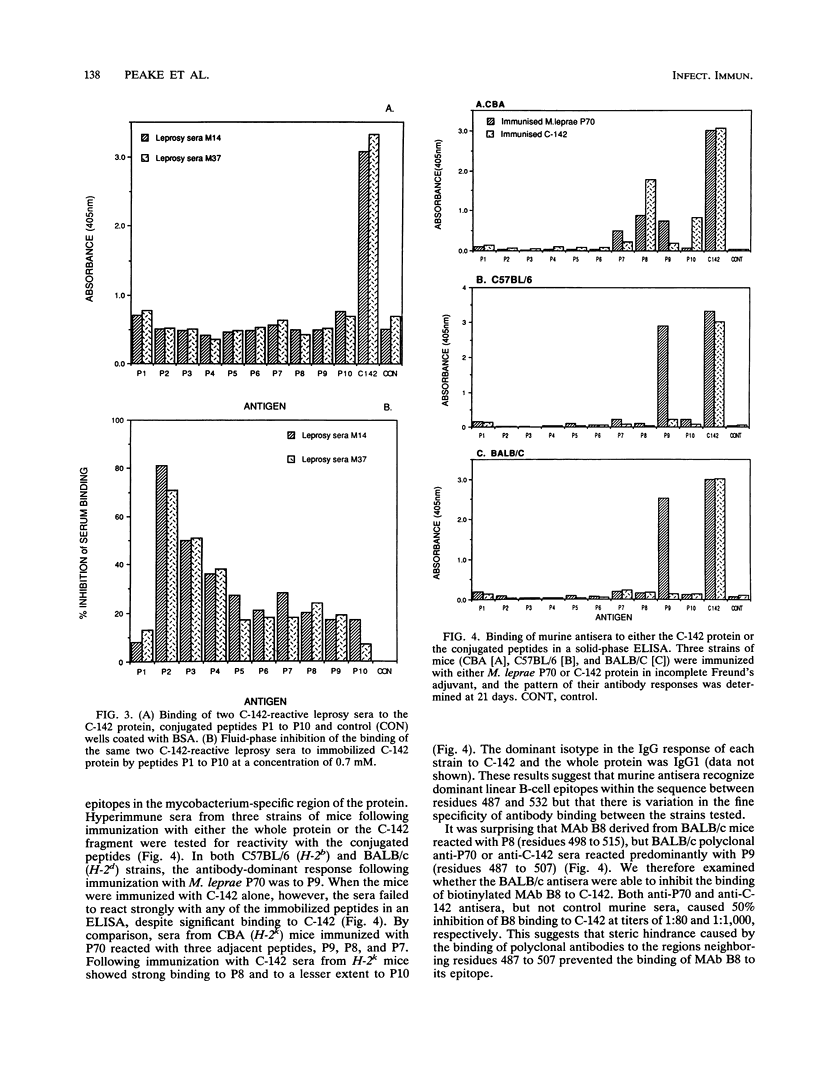
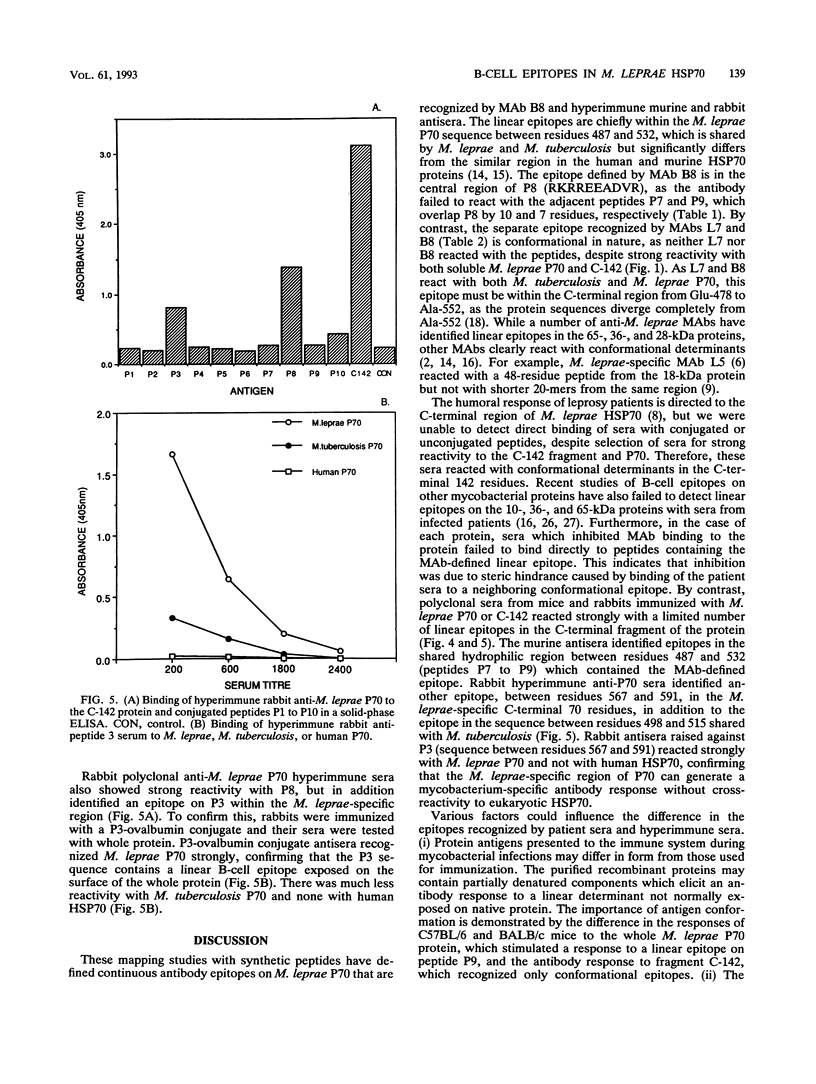
The C-terminal region of the Mycobacterium leprae 70-kDa heat-shock protein is the major target for the humoral immune response to this protein and contains M. leprae-specific sequences. To examine B-cell responses to this region more closely, we constructed and expressed a recombinant fragment of the M. leprae P70 gene that encodes the C-terminal 142 residues (C-142) and synthesized a series of 10 overlapping peptides to encompass this region. The affinities of three monoclonal antibodies (MAbs) reactive with this region of P70 were measured, and the binding site of the highest-affinity MAb was determined to lie between residues 498 and 515. This reactivity was confirmed by a fluid-phase inhibition enzyme-linked immunosorbent assay. By contrast, sera from leprosy patients which were strongly reactive with the C-142 fragment failed to bind directly to the conjugated or unconjugated peptides. To determine whether the M. leprae-specific C-terminal 70 residues could stimulate B-cell responses, the reactivity of hyperimmune anti-M. leprae P70 antisera with the peptides was examined. Rabbit polyclonal anti-M. leprae P70 antisera recognized epitopes between residues 498 and 515 and in the M. leprae-specific region between residues 567 and 591. The latter, in turn, when coupled to ovalbumin, was able to generate a strong anti-P70 response specific for mycobacterial, but not human, HSP70. Three strains of mice immunized with either C-142 or P70 recognized epitopes in the region between residues 487 and 532, but the response varied with the strain and immunogen. These data demonstrate that two regions in the C-terminal portion of M. leprae P70 contain linear B-cell epitopes recognized by MAbs or hyperimmune serum. Sera from leprosy patients, however, react predominantly with conformational determinants in the immunodominant C-terminal part of the protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E., Garsia R. J., Hellqvist L., Holt P., Basten A. T cell reactivity to the purified mycobacterial antigens p65 and p70 in leprosy patients and their household contacts. Clin Exp Immunol. 1990 May;80(2):206–212. doi: 10.1111/j.1365-2249.1990.tb05235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Barry M. E., Buchanan T. M. Exact definition of species-specific and cross-reactive epitopes of the 65-kilodalton protein of Mycobacterium leprae using synthetic peptides. J Immunol. 1988 Jul 15;141(2):607–613. [PubMed] [Google Scholar]
- Booth R. J., Harris D. P., Love J. M., Watson J. D. Antigenic proteins of Mycobacterium leprae. Complete sequence of the gene for the 18-kDa protein. J Immunol. 1988 Jan 15;140(2):597–601. [PubMed] [Google Scholar]
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Inglis A. S. Immunoreactivity of a 70 kD protein purified from Mycobacterium bovis Bacillus Calmette-Guerin by monoclonal antibody affinity chromatography. J Exp Med. 1986 Sep 1;164(3):695–708. doi: 10.1084/jem.164.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Raison R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J Immunol. 1985 Dec;135(6):4171–4177. [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport M. P., McKenzie K. R., Basten A., Britton W. J. The variable C-terminal region of the Mycobacterium leprae 70-kilodalton heat shock protein is the target for humoral immune responses. Infect Immun. 1992 Mar;60(3):1170–1177. doi: 10.1128/iai.60.3.1170-1177.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty T. M., Booth R. J., Love S. G., Gibson J. J., Harding D. R., Watson J. D. Characterization of an antibody-binding epitope from the 18-kDa protein on Mycobacterium leprae. J Immunol. 1989 Mar 1;142(5):1691–1695. [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985 Mar 18;77(2):305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Garsia R. J., Hellqvist L., Booth R. J., Radford A. J., Britton W. J., Astbury L., Trent R. J., Basten A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect Immun. 1989 Jan;57(1):204–212. doi: 10.1128/iai.57.1.204-212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M. Molecular technology: peptide epitope mapping and the pin technology. Southeast Asian J Trop Med Public Health. 1990 Dec;21(4):523–533. [PubMed] [Google Scholar]
- Hunt C., Calderwood S. Characterization and sequence of a mouse hsp70 gene and its expression in mouse cell lines. Gene. 1990 Mar 15;87(2):199–204. doi: 10.1016/0378-1119(90)90302-8. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatser P. R., De Wit M. Y., Kolk A. H., Hartskeerl R. A. Characterization of murine B-cell epitopes on the Mycobacterium leprae proline-rich antigen by use of synthetic peptides. Infect Immun. 1991 Jan;59(1):433–436. doi: 10.1128/iai.59.1.433-436.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- McKenzie K. R., Adams E., Britton W. J., Garsia R. J., Basten A. Sequence and immunogenicity of the 70-kDa heat shock protein of Mycobacterium leprae. J Immunol. 1991 Jul 1;147(1):312–319. [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake P., Basten A., Britton W. J. Characterization of the functional properties of the 70-kDa protein of Mycobacterium bovis. J Biol Chem. 1991 Nov 5;266(31):20828–20832. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Sachs D. H., Schechter A. N., Eastlake A., Anfinsen C. B. An immunologic approach to the conformational equilibria of polypeptides. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3790–3794. doi: 10.1073/pnas.69.12.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Jenner P. J., Jeyakumar L. H., Colston M. J. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect Immun. 1990 Jun;58(6):1937–1942. doi: 10.1128/iai.58.6.1937-1942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Stabel L. F., Suykerbuyk M. E., De Wit M. Y., Klatser P. R., Kolk A. H., Hartskeerl R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an immunoreactive region of proline-rich repeats. Infect Immun. 1990 Jan;58(1):80–87. doi: 10.1128/iai.58.1.80-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadiee A. R., Gillis T. P., Shannon E. J. Confirmation of a false-positive result associated with a competition inhibition assay used for detecting antibodies to a protein epitope of Mycobacterium leprae. Clin Exp Immunol. 1990 Mar;79(3):397–402. doi: 10.1111/j.1365-2249.1990.tb08102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbon A., Hartskeerl R. A., Kolk A. H. Murine and human B cell epitope mapping of the Mycobacterium tuberculosis 10-kD heat shock protein using overlapping peptides. Clin Exp Immunol. 1991 Oct;86(1):6–12. doi: 10.1111/j.1365-2249.1991.tb05765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]


