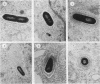
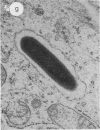
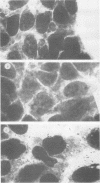
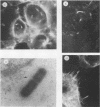
Abstract
Listeria ivanovii has been considered to be pathogenic to animals but has rarely been found associated with human infections. It has been claimed that L. ivanovii lacks the actA gene, which in L. monocytogenes encodes a protein required for interaction with host cell actin. Using fluorescence microscopy and electron microscopy, we demonstrate that L. ivanovii can invade mammalian cells, lyse the phagosomal membrane, polymerize host cell actin, reorganize actin to form tails, and spread from cell to cell. However, no DNA homologous to the actA gene could be detected by polymerase chain reaction. Further, L. ivanovii lacks the 90-kDa surface protein which in L. monocytogenes is encoded by actA. Despite the ability to spread from cell to cell, L. ivanovii differed significantly from L. monocytogenes in being unable to form plaques on monolayers of 3T3 fibroblast cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Goebel W. Recent developments in the study of virulence in Listeria monocytogenes. Curr Top Microbiol Immunol. 1988;138:41–58. [PubMed] [Google Scholar]
- Clerc P. L., Ryter A., Mounier J., Sansonetti P. J. Plasmid-mediated early killing of eucaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect Immun. 1987 Mar;55(3):521–527. doi: 10.1128/iai.55.3.521-527.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. F., Dennis S. M., Harris J. O. Fractionation of Listeria monocytogenes serotype 5. Am J Vet Res. 1973 Aug;34(8):1093–1099. [PubMed] [Google Scholar]
- Cossart P., Mengaud J. Listeria monocytogenes. A model system for the molecular study of intracellular parasitism. Mol Biol Med. 1989 Oct;6(5):463–474. [PubMed] [Google Scholar]
- Dabiri G. A., Sanger J. M., Portnoy D. A., Southwick F. S. Listeria monocytogenes moves rapidly through the host-cell cytoplasm by inducing directional actin assembly. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6068–6072. doi: 10.1073/pnas.87.16.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann E., Wehland J., Rohde M., Pistor S., Hartl M., Goebel W., Leimeister-Wächter M., Wuenscher M., Chakraborty T. A novel bacterial virulence gene in Listeria monocytogenes required for host cell microfilament interaction with homology to the proline-rich region of vinculin. EMBO J. 1992 May;11(5):1981–1990. doi: 10.1002/j.1460-2075.1992.tb05252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Frehel C., Gouin E., Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991 Jun 28;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Kathariou S., Kuhn M., Sokolovic Z., Kreft J., Köhler S., Funke D., Chakraborty T., Leimeister-Wächter M. Hemolysin from Listeria--biochemistry, genetics and function in pathogenesis. Infection. 1988;16 (Suppl 2):S149–S156. doi: 10.1007/BF01639739. [DOI] [PubMed] [Google Scholar]
- Haas A., Dumbsky M., Kreft J. Listeriolysin genes: complete sequence of ilo from Listeria ivanovii and of lso from Listeria seeligeri. Biochim Biophys Acta. 1992 Feb 28;1130(1):81–84. doi: 10.1016/0167-4781(92)90466-d. [DOI] [PubMed] [Google Scholar]
- Kocks C., Gouin E., Tabouret M., Berche P., Ohayon H., Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992 Feb 7;68(3):521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- Kreft J., Funke D., Haas A., Lottspeich F., Goebel W. Production, purification and characterization of hemolysins from Listeria ivanovii and Listeria monocytogenes Sv4b. FEMS Microbiol Lett. 1989 Jan 15;48(2):197–202. doi: 10.1111/j.1574-6968.1989.tb03298.x. [DOI] [PubMed] [Google Scholar]
- Kreft J., Funke D., Schlesinger R., Lottspeich F., Goebel W. Purification and characterization of cytolysins from Listeria monocytogenes serovar 4b and Listeria ivanovii. Acta Microbiol Hung. 1989;36(2-3):189–192. [PubMed] [Google Scholar]
- Kuhn M., Kathariou S., Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 Jan;56(1):79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Prévost M. C., Mounier J., Sansonetti P. J. A nonvirulent mutant of Listeria monocytogenes does not move intracellularly but still induces polymerization of actin. Infect Immun. 1990 Nov;58(11):3477–3486. doi: 10.1128/iai.58.11.3477-3486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Haffner C., Domann E., Goebel W., Chakraborty T. Identification of a gene that positively regulates expression of listeriolysin, the major virulence factor of listeria monocytogenes. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8336–8340. doi: 10.1073/pnas.87.21.8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier J., Ryter A., Coquis-Rondon M., Sansonetti P. J. Intracellular and cell-to-cell spread of Listeria monocytogenes involves interaction with F-actin in the enterocytelike cell line Caco-2. Infect Immun. 1990 Apr;58(4):1048–1058. doi: 10.1128/iai.58.4.1048-1058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notermans S. H., Dufrenne J., Leimeister-Wächter M., Domann E., Chakraborty T. Phosphatidylinositol-specific phospholipase C activity as a marker to distinguish between pathogenic and nonpathogenic Listeria species. Appl Environ Microbiol. 1991 Sep;57(9):2666–2670. doi: 10.1128/aem.57.9.2666-2670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Connelly P. S., Portnoy D. A. Actin filament nucleation by the bacterial pathogen, Listeria monocytogenes. J Cell Biol. 1990 Dec;111(6 Pt 2):2979–2988. doi: 10.1083/jcb.111.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland J. A., Dominguez L., Rodriguez-Ferri E. F., Suarez G. Purification and characterization of two Listeria ivanovii cytolysins, a sphingomyelinase C and a thiol-activated toxin (ivanolysin O). Infect Immun. 1989 Dec;57(12):3928–3935. doi: 10.1128/iai.57.12.3928-3935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland J. A., Kocks C., Dramsi S., Ohayon H., Geoffroy C., Mengaud J., Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992 Jan;60(1):219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. Actin-binding proteins--regulators of cell architecture and motility. Nature. 1982 Apr 29;296(5860):811–816. doi: 10.1038/296811a0. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A., Prevost M. C., Sansonetti P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992 Jul 9;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]