Abstract
The proper communication between organelles is essential for many aspects of eukaryotic life. The coordination of nuclear and cytoplasmic activities in particular is of pivotal importance and depends on transport in and out of the nucleus. The material which translocates through nuclear pores is diverse; it includes numerous proteins, RNAs and large ribonucleoprotein complexes like ribosomal subunits. To ensure the correct nucleocytoplasmic distribution of these components, appropriate mechanisms have to be in place which control traffic across the nuclear envelope. A growing number of studies support the notion that transport through nuclear pore complexes is intimately linked to cell physiology. As such, it has become evident that changes in the cellular environment, either by externally applied stress, aging or disease, alter nuclear traffic. Due to the progress made in the past few years, we are now beginning to understand these processes at the molecular level. Thus, the concept emerges that stress or disease conditions correlate with signaling events which aim at the nuclear transport apparatus. Here, we summarize results from recent publications that provide evidence for the hypothesis that changes in cell physiology modulate nuclear traffic by targeting multiple transport factors. We propose that this traffic control is at least in part mediated by specific signaling events.
Key words: nuclear transport, nucleoporins, stress, oxidants, signaling
Nuclear transport of macromolecules which exceed the diffusion limit of the nuclear pore complex (NPC) depends on specific signals that promote targeting in or out of the nucleus.1–3 Frequently, but not always, the translocation of such macromolecules requires a specialized transport apparatus which moves cargo to its destination. This nuclear transport apparatus is composed of nucleoporins and soluble components which are crucial for the recognition of transport signals. Among these soluble factors are receptors for nuclear localization and nuclear export signals (NLS and NES). In most cases, signals for nuclear import or export are recognized by members of the importin-β family (also known as karyopherin-β) or adaptor proteins, which connect the cargo to its carrier.1–3 The driving force for importin-β dependent transport is the nucleocytoplasmic Ran gradient, with RanGTP in the nucleus and RanGDP in the cytoplasm. Several factors regulate the RanGTPase cycle,1–3 and the correct nucleocytoplasmic distribution and activity of Ran regulators is crucial to maintain a proper Ran gradient. During the passage through the nuclear pore, it is essential for nuclear carriers to transiently interact with nucleoporins. Of particular importance for these interactions are nucleoporins with FG repeats, as they present “stepping stones” during the translocation process. In order to direct carriers through the nuclear pore, repeat-containing nucleoporins are positioned in different parts of the NPC.
Like many biological processes, nucleocytoplasmic transport of proteins and RNAs is dynamic and can be adjusted to the specific requirements of a cell. Notably, early experiments have shown that cancer cells exhibit enhanced nuclear protein transport and that protein kinases and phosphatases play a role in nuclear trafficking in vitro.4,5 Furthermore, in growing cells protein phosphatases are also implicated in nuclear import under stress conditions.6
More recent publications describe significant advances in the field of nuclear transport regulation. As a result, we are now beginning to unravel at the molecular level how changes in the environment and signaling events control movement in and out of the nucleus. In the following, we focus on components of the transport apparatus and their possible role in traffic control. We excluded from our discussion transport regulation that is effected by modification of the cargo and/or changes in the recognition of nuclear transport signals, which have been discussed previously.7
Diverse Mechanisms for Traffic Control
The past few years provided new insights into the mechanisms that participate in the regulation of nuclear traffic, and multiple components were identified that are involved in different aspects of transport control. Among these components are factors with diverse roles in transport, such as Ran, RanBP3, importin-α, importin-β family members and several nucleoporins (summarized in Table 1). Thus, it is likely that many steps of the transport process play a part in the overall control of trafficking.
Table 1.
Targets and mechanisms of nuclear transport control
| Event | Target | Possible consequence | References |
| Apoptosis | Multiple nucleoporins | Degradation of nucleoporins, loss of transport competence | 8, 9 |
| Stress | |||
| Heat shock, severe oxidative stress, hyperosmotic stress | Ran gradient | Collapse of gradient, leading to inhibition of importin-dependent nuclear transport | 12–16 |
| Severe oxidative stress | Importin-β1 | Changes in distribution, degradation by caspases and proteasome | 13 |
| Severe oxidative stress | Nup153 | Changes in distribution, degradation | 13 |
| Heat shock, oxidative stress | Importin-α | Mislocalization, nuclear retention, phosphorylation, changes in nuclear export and import of importin-α | 10, 18–21 |
| Ceramide treatment | CAS | Mislocalization | 28 |
| Ceramide treatment | Importin-α | Mislocalization | 28 |
| Heat and oxidative stress | CAS | Mislocalization, changes in nuclear export of CAS; increased phosphorylation | 10, 18, 19 |
| Oxidative stress | Crm1 | NE association increased, enhanced interaction with Nup62, Nup153 and Ran, reduced association with Nup88; reduced exit from nucleus | 17 |
| Oxidative stress | Nup358 | Reduced concentration at the NE | 17 |
| Oxidative stress | Nup214 | Increase in phosphorylation and GlcNAc modification; reduced interaction with Nup62 and Nup88, enhanced association with NE | 17 |
| Oxidative stress | Nup153 | Mislocalization, increase in phosphorylation and GlcNAc modification, changes in the interaction with other FG nucleoporins; enhanced association with NE | 19 |
| Oxidative stress | Nup62 | Increase in phosphorylation and GlcNAc modification; reduced interaction with Nup153 and Nup214; increased interaction with Crm1; enhanced association with NE | 17 |
| Oxidative stress | Nup98 | Increase in phosphorylation, changes in distribution | 17 |
| Oxidative stress | Nup50 | Increase in nuclear concentration, nuclear retention | 10 |
| Signaling | |||
| RSK and PI3 kinase | RanBP3 | RanBP3 phosphorylation controls Ran gradient | 25 |
| ERK1/2 | Nup50 | Nup50 phosphorylation by ERK decreases binding to importin-β and transportin | 26 |
| PI3 kinase | Importin-α | Changes in post-translational modification and/or localization under normal and oxidative stress conditions | 19 and this contribution (Figs. 1, 2 and Table 2) |
| CAS | |||
| Nup88 | |||
| Nup153 | |||
| Nup62 | |||
| Nup214 | |||
| Nup50 | |||
| Heregulin-dependent growth factor activation | Ran | RanGTP levels increased; may lead to cellular transformation | 32 |
| AMP kinase | Importin-α1 | Acetylation and phosphorylation of importin-α1, control of HuR nuclear import | 29 |
| Aging | |||
| Aging, oxidative stress | Nup153, Nup93 | Increased carbonylation; leaky nuclear pores | 31 |
| Aging | Importin-α; CAS RanBP1 | Reduced levels of transport factors, reduced nuclear import | 30 |
Nuclear transport components that are affected by apoptosis, stress or aging are listed. We have focused on some of the more recent publications. NE, nuclear envelope.
Not only are multiple components contributing to transport regulation, the mechanisms of control can be diverse as well. For instance, changes in cell physiology may alter the stability, concentration, localization or post-translational modification of a specific transport factor. This is exemplified by the degradation of several nucleoporins during apoptosis, some of which are targeted by caspases.8,9 On the other hand, some forms of stress may increase the abundance of nucleoporins.10,11
The nucleocytoplasmic Ran gradient is an essential component of many nuclear transport pathways and its contribution to traffic control has been studied extensively. For example, severe stress conditions can cause a partial or complete collapse of the Ran concentration gradient, thereby inhibiting transport that is mediated by members of the importin-β family.12–16 Such changes to the Ran gradient are conserved among eukaryotes, as the stress-induced collapse of Ran gradients has been described from yeast to man.12–16
Not all forms of stress dissipate the nucleocytoplasmic Ran concentration gradient; nevertheless, such stressors may still interfere with nuclear trafficking. This has been demonstrated for mild stress conditions which have little effect on the Ran concentration gradient, yet inhibit nuclear import and export.10,17 These observations supported the idea that components other than Ran might be targeted by stress and thereby play a role in nuclear transport inhibition. Indeed, several stress-sensitive candidates have been identified by different groups; they include importin-α, multiple members of the importin-β family (importin-β1, CAS—the nuclear exporter for importin-α, the nuclear exporter Crm1) and a growing number of nucleoporins. Interestingly, several of these nucleoporins contain FxFG (Nup358, Nup214, Nup153, Nup62) or GLFG repeats (Nup98), which are crucial for the movement of protein or RNA across the NPC.10,13,17 In addition to repeat containing nucleoporins, Nup88, a protein associated with cytoplasmic NPC filaments, redistributes in response to oxidant exposure and becomes more abundant upon hypertonic stress.10,11
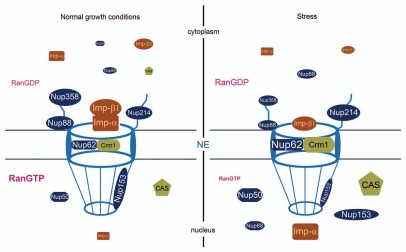
Aside from stress-induced changes in concentration, several transport factors redistribute when cells are exposed to conditions that alter their physiology (summarized in Fig. 1). For example, importin-α and CAS accumulate in nuclei of stressed cells; this relocation is a consequence of different stress treatments, such as heat shock or oxidants.10,18–21 In several cases, the mechanisms that underlie the relocation of transport factors have been identified and it became obvious that these mechanisms are diverse. Thus, changes in the distribution may be caused by nuclear retention, increase in nuclear import, reduction in nuclear export or a combination of these events.10,18–21 Moreover, Crampton et al.17 showed recently that the interactions between multiple components of the transport apparatus are modulated under oxidative stress conditions, which is likely to affect the translocation of many cargos across the nuclear pore. For instance, the oxidant diethyl maleate (DEM) increases the association Crm1/Ran, Crm1/Nup62 as well as Crm1/Nup153, whereas the Crm1/Nup88 interaction is reduced. However, not only does oxidative stress affect the binding of Crm1 to nucleoporins, the associations between nucleoporins can be sensitive to oxidant as well.17 Table 1 and Figure 1 depict examples of such stress-induced changes; they show that nucleoporins which are sensitive to stress are located in the nuclear, central and cytoplasmic portions of the NPC. Thus, it appears that different NPC modules are targeted by oxidants.22 Interestingly, the targets include mobile as well as nucleoporins which are stably associated with the NPC,23 indicating that multiple facets of pore function will be affected.
Figure 1.
Stress alters the localization of nuclear transport components. Several transport components redistribute in cells that have been exposed to stress. This includes repeat-containing nucleoporins, importin-α (Imp-α) and members of the importin-β family, such as importin-β1 (Imp-β1), CAS and Crm1. Furthermore, under severe stress conditions, the nucleocytoplasmic Ran gradient collapses. It should be emphasized that different stressors may have distinct effects on individual transport components. Moreover, not all of the changes depicted here will necessarily occur at the same time. See text and Table 1 for details; NE, nuclear envelope.
Signaling Controls Trafficking
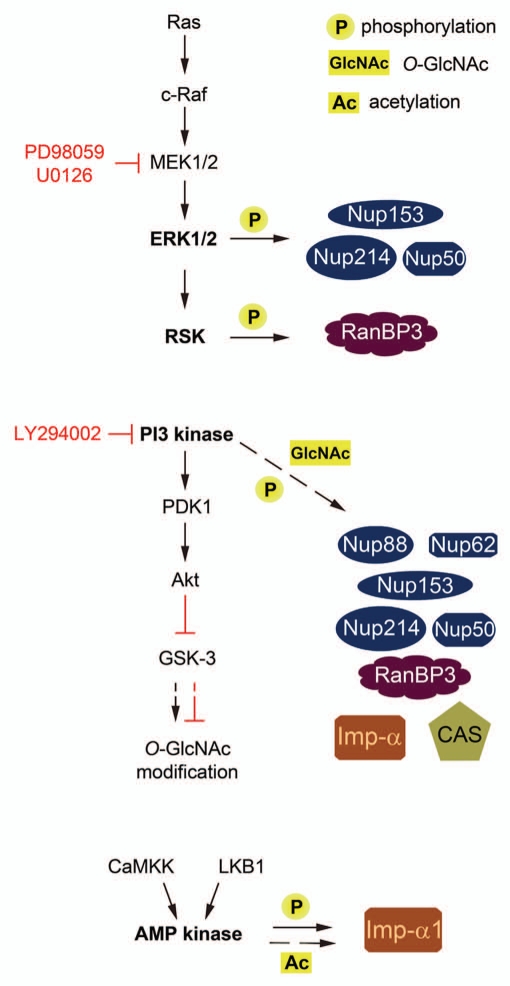
Many aspects of cell physiology are regulated by a large number of signaling events. Among the various signaling routes, members of the MAP kinase family as well as PI3 and AMP kinases are crucial, as they control cell growth, proliferation, apoptosis and the response to stress. Figure 2 depicts an abbreviated version of these signaling pathways; for simplicity we have included only those elements of the signaling routes that are relevant to our discussion.
Figure 2.
Multiple signaling pathways regulate the post-translational modification of nuclear transport components. Kinases which are implicated directly (arrows) or indirectly (dashed arrows) in the post-translational modification of soluble transport factors or nucleoporins are depicted. These modifications include phosphorylation (P), O-GlcNAcylation (GlcNAc) and acetylation (Ac). Depending on the protein to be modified glycogen synthase kinase-3 (GSK-3) activity may increase or decrease O-GlcNAcylation. Kinase inhibitors PD98059, U0126 and LY294002 were used in different studies to evaluate whether specific signaling pathways are implicated in the control of nuclear transport components.
One of the common features of the various stressors that impact nuclear transport is that they induce signaling through multiple kinase cascades. For example, oxidative and heat stress activate both MEK → ERK1/2 and PI3 kinase → Akt pathways24 (Banski et al. unpublished). Based on these observations and the fact that many of the transport components are modified post-translationally, it was reasonable to examine whether these modifications are regulated by stress. Indeed, this seems to be the case, as oxidant treatment correlates with changes in phosphorylation and/or GlcNAc modification, both of soluble transport factors and nucleoporins (Table 1). Interestingly, changes in transport factor modifications are not limited to stress conditions, as modifying ERK or PI3 kinase activities in unstressed cells also impinges on transport factors (Fig. 2). This is exemplified by the regulation of RanBP3 through ERK1/2 → RSK signaling, a regulatory link which ultimately controls the Ran concentration gradient.25 Moreover, ERK-dependent phosphorylation of Nup50 reduces its association with importin-β1 and transportin in vitro, and ERK2 plays a role in the oxidant-induced collapse of the Ran gradient.14,26
Whether or how much modulating individual transport factors contributes to the overall regulation of nuclear trafficking is currently difficult to assess. However, it is noteworthy that the kinase inhibitor PD98059, which interferes with ERK1/2 and ERK5 activation, significantly increases classical nuclear import, both under normal and stress conditions.19 Collectively, these results point to a critical function of ERK activity in nuclear transport, with ERK kinases targeting both soluble factors and nucleoporins.
Despite the importance of ERK pathways, however, it is likely that other signaling cascades play a role in transport control as well; these signaling events may regulate nuclear traffic directly or by crosstalk with ERK pathways. Specifically, the PI3 kinase module has emerged as an excellent candidate for traffic control, as it plays an important role in modulating the localization and post-translational modification of multiple transport components. For example, the nucleocytoplasmic distribution of importin-α, CAS, Nup153 and Nup88 is sensitive to changes in PI3 kinase signaling, and RanBP3 phosphorylation is regulated by the PI3 → Akt pathway.19,25 Furthermore, the distribution of Nup50 which is modulated by oxidative stress,10 depends on PI3 kinase activation (Fig. 3). Interestingly, the effect of PI3 kinase activity is different under normal and oxidative stress conditions. When unstressed cells are treated with LY294002 (an inhibitor of PI3 kinase activation, Fig. 3), Nup50 concentrations increase in the cytoplasm and decrease in the nucleus. By contrast, when cells are exposed to the oxidant diethyl maleate (DEM), LY294002 elevates Nup50 levels in the nucleus (Fig. 3). Consistent with previous observations, these results emphasize that for individual transport factors the consequences of signaling events will be dictated by the physiological state of the cell.19
Figure 3.
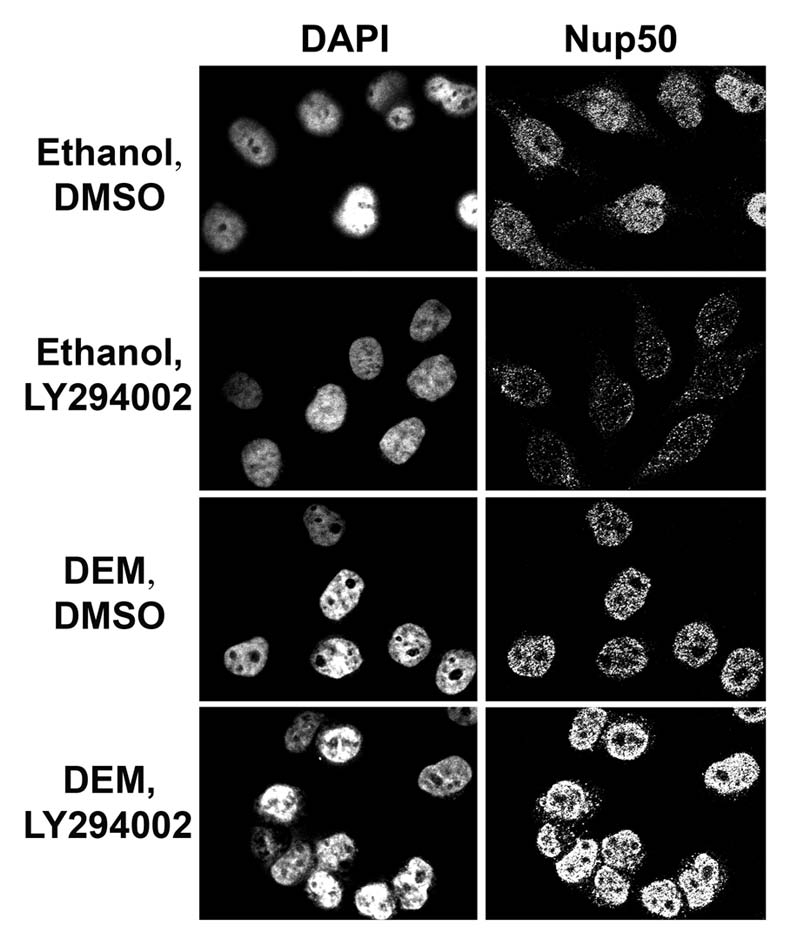
Nup50 distribution is modulated by signaling through PI3 kinase and stress. HeLa cells were treated with the oxidant diethyl maleate (DEM) as previously described.17,19 Cells were incubated with the solvent DMSO or LY294002 as indicated in the figure19 and Nup50 was detected by indirect immunofluorescence following published procedures.10
Given the effect that PI3 kinase inhibitors have on the localization of transport components, we tested whether changing PI3 kinase activity alters the electrophoretic mobility of importin-α, CAS, Nup88, Nup153, Nup62 and Nup214 under normal and oxidative stress conditions (Table 2). As described in previous studies, DEM increases the apparent molecular mass for all of these proteins, which can be attributed at least in part to stress-induced phosphorylation (importin-α, CAS, Nup88) or a combination of phosphorylation and O-GlcNAc modification (Nup153, Nup62, Nup214).17,19 Our unpublished results have shown that the electrophoretic mobility of these proteins is not only modulated by oxidative stress, but also by PI3 kinase activity. In these studies, we observed a complex pattern of changes in mobility, which are summarized in Table 2. In parallel experiments, we quantified how PI3 kinase inhibition affects the concentration of transport components in the nucleus or at the nuclear envelope.19
Table 2.
Oxidative stress and PI3 kinase signaling impact multiple transport components
| Factor | Electrophoretic mobility | Distribution | ||||
| DEM | LY | LY, DEM | DEM | LY | LY, DEM | |
| Importin-α | ↑ | ↑↑ | ↑↑ | Nuc↑, NE↓ | Nuc↑ | Nuc↑ |
| CAS | ↑ | ↑↑ | ↑↑ | Nuc↑ | Nuc↓ | Nuc↑↑ |
| Nup88 | ↑ | ↑↑ | ↑↑(↑) | NE↓ | Nuc↓↓, NE↓↓ | Nuc↓, NE↓↓ |
| Nup153 | ↑↑↑ | ↓↓ | ↓ | Nuc↑↑, NE↑↑ | Nuc↓, NE↓ | Nuc↑, NE↑ |
| Nup62 | ↑↑ | ↑ | ↑↑↑ | nd | nd | nd |
| Nup214 | ↑↑ | ↑ (?) | ↑ (?) | nd | nd | nd |
| Nup50 | nd | nd | nd | Nuc↑ | Nuc↓ | Nuc↑↑ |
Results are compared to what is known about the distribution of transport factors under the same conditions. All changes are relative to unstressed cells that were incubated with the solvent DMSO. LY, LY 294002; Nuc, nucleus; NE, nuclear envelope. ↑, increase; ↓, decrease in electrophoretic mobility or localization. The number of arrows correlates with the level of change; nd, not determined.
At this point, without further studies the interpretation of drug-dependent changes in electrophoretic mobility is speculation. However, we have previously demonstrated that stress-induced shifts in electrophoretic mobility reflect altered phosphorylation and/or O-GlcNAc modification.17,19 Thus, it is possible that mobility shifts induced by LY294002 reflect at least in part differences in post-translational modification. Based on this reasoning, we propose that in response to PI3 kinase inhibition O-GlcNAcylation of both Nup62 and Nup153 might be affected, with Nup153 showing the most pronounced change. Previous results by others may pinpoint the link between these changes and the PI3 → Akt pathway.27 Specifically, O-GlcNAc modification can be sensitive to the inhibition of glycogen synthase kinase-3, an enzyme which is negatively regulated by the Akt (Fig. 2).27 As a consequence of glycogen synthase kinase-3 inhibition, O-GlcNAcylation is upregulated for some, but not affected or downregulated for other proteins. Interestingly, like changes in phosphorylation, changes in O-GlcNAc modification can correlate with a redistribution of the modified protein.27 The same scenario might apply to the nuclear transport apparatus, and it will now be important to determine how O-GlcNAcylation and other post-translational modifications regulate the localization and activity of individual nuclear transport components. On the basis of our previous studies, Nup62 is one of the potential candidates for this type of control, as it is feasible that the stress-induced increase in Nup62 modification stabilizes its association with Crm1.17
Aside from ERK and PI3 kinase signaling, other signaling pathways come into sight which contribute to the control of nuclear transport. For example, the lipid ceramide was shown to regulate nuclear import via activation of the MAPK p38, and p38 activation correlated with the redistribution of importin-α and CAS.28 On the other hand, importin-α1 dependent transport is also affected by AMP kinase, which regulates both the phosphorylation and acetylation of the adaptor (Fig. 2).29
A consensus arising from multiple publications is that changes in signaling may have intricate effects on individual transport factors. For example, preventing the activation of PI3 kinase signaling may alter the concentration in the nucleus, cytoplasm or nuclear envelope, and these changes may be different under normal and stress conditions (Table 2).19 Collectively, these studies indicate that there are complex patterns for the subcellular distribution of transport components, which are influenced by a complicated network of signaling events and possibly linked to post-translational modifications.
Given the modulation of nuclear transport by stress or signaling and the importance of these events for aging, it was crucial to test whether aging impacts nuclear transport or diffusion through NPCs.30,31 Indeed, these studies demonstrated that the concentration of several transport factors is reduced in old cells, and that NPCs which are leaky in old cells display elevated carbonylation of Nup153 and Nup93.
How do Cells Achieve Proper Control of Nuclear Transport?
Work from many studies supports the idea that transport in and out of the nucleus is regulated on several levels. The first layer regulates individual cargos, for example by changing the accessibility or efficiency of transport signals. On a higher level, a group of cargos can be controlled by altering the abundance, distribution or activity of specific factors like Ran, NTF2, Crm1 or CAS.32–35 Finally, many cargos will be affected by altering NPC function, which can change active transport as well as diffusion across nuclear pores. Signaling events can be expected to impact all of these processes. Adding further complexity to traffic control is the fact that many kinases and phosphatases are localized in different cellular compartments and that their distribution is dynamic and in some cases dependent on nuclear transport.36 For MEK → ERK and PI3 → Akt modules in particular, it is noteworthy, that the interplay between these two pathways in nuclei is different from the cytoplasm.24,37 With respect to nuclear transport, it is likely that the nucleocytoplasmic distribution of activated ERK1/2, Akt or AMP-activated kinase and the crosstalk between signaling modules will affect nuclear transport. Not only do the compartment-specific activities of signaling pathways have consequences for individual cargos, they will also impact nuclear and cytoplasmic pools of soluble transport factors and nucleoporins. Eventually, the final destination of a specific cargo will be a culmination of these regulatory events. FoxO3a is one of the examples, where nuclear transport, stress and signaling contribute to the final distribution and ultimately function of the transcription factor.24
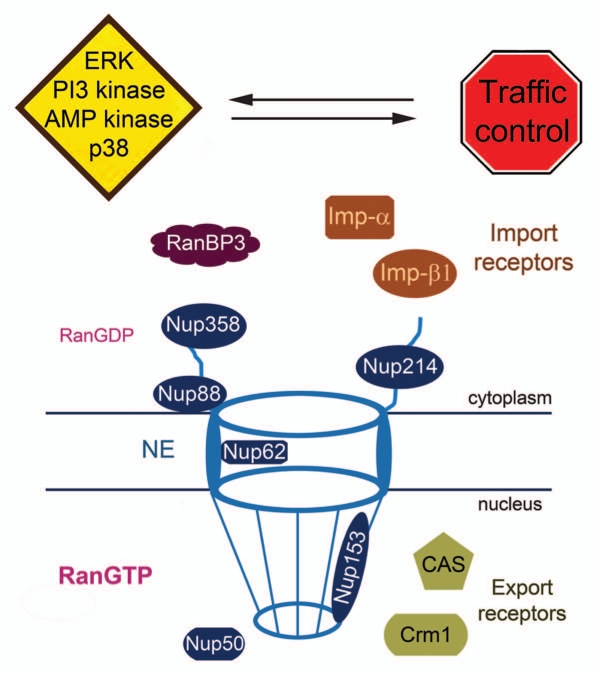
Although there are many open questions, we have combined results from recent publications to propose a simplified model for nuclear traffic control. Figure 4 depicts targets which are currently known to participate in transport regulation. Furthermore, our model emphasizes the mutual relationship between signal transduction and nuclear transport, as signaling controls nuclear transport and vice versa.
Figure 4.
Simplified model for nuclear transport control. Soluble components of the nuclear transport apparatus and nucleoporins (Nups) collectively regulate transport across the NPC. Signaling through PI3 kinase, MEK → ERK, p38 and AMP kinase contributes to different aspects of the transport process. Notably, signaling not only modulates nuclear traffic, nuclear transport also influences the nucleocytoplasmic distribution of signaling events. For simplicity, we have shown signaling events and traffic control only in the cytoplasm, but we expect these processes to take place in the nucleus as well.
What's Next?
Results obtained recently for nuclear transport control provide us with a better understanding of how individual steps required for protein import or export can be modulated at the molecular level. We are also beginning to appreciate how changes in signaling affect specific transport factors or overall movement across NPCs. Our current list of factors which are sensitive to stress or changes in signaling demonstrates that traffic control is exerted by targeting multiple components of the transport apparatus. Key players include repeat containing nucleoporins, importin-α, CAS, Crm1, Ran and RanBP3. Interactions at the nuclear pore, either among nucleoporins or with carriers in transit, are of primary interest, because they impact many different cargos. Future studies will now have to define the possible role of other components in traffic control. This research will likely reveal novel links between nuclear transport and signaling not only in higher but also in lower eukaryotes. As such, it is noteworthy that in S. cerevisiae Snf1 kinase, the ortholog of AMP kinase, controls the localization of nuclear exporters which belong to the importin-β family.38
In the years to come, quantitative analyses of individual factors and transport steps will enable us to identify the bottlenecks for nuclear transport and thereby prime targets for regulation. One of the challenges ahead is to link changes of the transport apparatus to the spatiotemporal organization of signaling events. Ultimately, we will have to integrate these processes to understand how traffic control is achieved.
Acknowledgements
Financial disclosure: N.C., M.K. and S.S. were supported by studentships from NSERC and Heart and Stroke Foundation of Canada. U.S. is supported by grants from CIHR, NSERC and HSFC.
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/11444
References
- 1.Terry LJ, Shows EB, Wente SR. Crossing the Nuclear Envelope: Hierarchical Regulation of Nucleocytoplasmic Transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 2.Poon K, Jans DA. Regulation of nuclear transport: central role in development and transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 3.Weis K. Regulating access to the genome: Nucleocytoplasmic transport throughout the cell cycle. Cell. 2003;112:441–451. doi: 10.1016/s0092-8674(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 4.Feldherr C, Akin D. Stimulation of nuclear import by Simian Virus 40-transformed cell extracts is dependent on protein kinase activity. Mol Biol Cell. 1995;15:7043–7049. doi: 10.1128/mcb.15.12.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlenbach RH, Gerace L. Phosphorylation of the nuclear transport machinery downregulates nuclear protein import in vitro. J Biol Chem. 2000;275:17848–17856. doi: 10.1074/jbc.M001455200. [DOI] [PubMed] [Google Scholar]
- 6.Chu A, Matusiewicz N, Stochaj U. Heat-induced nuclear accumulation of hsc70 proteins is regulated by phosphorylation and inhibited in confluent cells. FASEB J. 2001;15:78–80. doi: 10.1096/fj.00-0680fje. [DOI] [PubMed] [Google Scholar]
- 7.Jans DA, Xiao C, Lam MHC. Nuclear targeting signal recognition: a key control point in nuclear transport? BioEssays. 2000;;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Patre M, Tabbert A, Hermann S, Walczak H, Rackwitz H, Cordes VC, Ferrando-May E. Caspases target only two architectural components within the core structure of the nuclear pore complex. J Biol Chem. 2006;281:1296–1304. doi: 10.1074/jbc.M511717200. [DOI] [PubMed] [Google Scholar]
- 9.Grote P, Schaeuble K, Ferrando-May E. Commuting (to) suicide: an update on nucleocytoplasmic transport in apoptosis. Arch Biochem Biophys. 2007;462:156–161. doi: 10.1016/j.abb.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Kodiha M, Tran D, Qian C, Morogan A, Presley JF, Brown CM, Stochaj U. Oxidative stress mislocalizes and retains transport factor importin-alpha and nucleoporins Nup153 and Nup88 in nuclei where they generate high molecular mass complexes. Biochim Biophys Acta. 2008;1783:405–418. doi: 10.1016/j.bbamcr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Andres-Hernando A, Lanaspa MA, Rivard CJ, Berl T. Nucleoporin 88 (Nup88) is regulated by hypertonic stress in kidney cells to retain the transcription factor tonicity enhancer-binding protein (TonEBP) in the nucleus. J Biol Chem. 2008;283:25082–25090. doi: 10.1074/jbc.M802381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stochaj U, Rassadi R, Chiu J. Stress-mediated inhibition of the classical nuclear protein import pathway and nuclear accumulation of the small GTPase Gsp1p. FASEB J. 2000;14:2130–2132. doi: 10.1096/fj.99-0751fje. [DOI] [PubMed] [Google Scholar]
- 13.Kodiha M, Chu A, Matusiewicz N, Stochaj U. Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death Differ. 2004;11:862–874. doi: 10.1038/sj.cdd.4401432. [DOI] [PubMed] [Google Scholar]
- 14.Czubryt MP, Austria JA, Pierce GN. Hydrogen peroxide inhibition of nuclear protein import is mediated by the mitogen-activated protein kinase, ERK2. J Cell Biol. 2000;148:7–16. doi: 10.1083/jcb.148.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwoebel ED, Ho TH, Moore MS. The mechanism of Ran-dependent nuclear transport by cellular ATP depletion. J Cell Biol. 2002;157:963–974. doi: 10.1083/jcb.200111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley JB, Paschal BM. Hyperosmotic stress signaling to the nucleus disrupts the Ran gradient and the production of RanGTP. Mol Biol Cell. 2007;18:4365–4376. doi: 10.1091/mbc.E07-01-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crampton N, Kodiha M, Shrivastava S, Umar R, Stochaj U. Oxidative stress inhibits nuclear protein export by multiple mechanisms that target FG nucleoporins and Crm1. Mol Biol Cell. 2009;20:5106–5116. doi: 10.1091/mbc.E09-05-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodiha M, Banski P, Ho-Wo-Cheong D, Stochaj U. Dissection of the molecular mechanisms that control the nuclear accumulation of transport factors importin-α and CAS in stressed cells. Cell Mol Life Sci. 2008;65:1756–1767. doi: 10.1007/s00018-008-7588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodiha M, Tran D, Morogan A, Qian C, Stochaj U. Dissecting the signaling events that impact classical nuclear import and target nuclear transport factors. PLoS One. 2009;4:8420. doi: 10.1371/journal.pone.0008420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuta M, Kose S, Koike M, Shimi T, Hiraoka Y, Yoneda Y, et al. Heat-shock induced nuclear retention and recycling inhibition of importin α. Genes Cells. 2004:429–441. doi: 10.1111/j.1356-9597.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto Y, Saiwaki T, Yamashita J, Yasuda Y, Kotera I, Shibata S, et al. Cellular stresses induce the nuclear accumulation of importin α and cause a conventional nuclear import block. J Cell Biol. 2004;165:617–623. doi: 10.1083/jcb.200312008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brohawn SG, Partridge JR, Whittle JRR, Schwartz TU. The nuclear pore complex has entered the atomic age. Structure. 2009;17:1156–1168. doi: 10.1016/j.str.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran EJ, Wente SR. Dynamic nuclear pore complexes, life on the edge. Cel. 2006;125:1041–1053. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Kodiha M, Banski P, Stochaj U. Interplay between MEK and PI3 kinase signaling regulates the subcellular localization of protein kinases ERK1/2 and Akt upon oxidative stress. FEBS Lett. 2009;583:1987–1993. doi: 10.1016/j.febslet.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Yoon SO, Shin S, Liu Y, Ballif BA, Woo MS, Gygi SP, Blenis J. Ran-binding protein 3 phosphorylation links the Ras and PI3-kinase pathways to nucleocytoplasmic transport. Mol Cell. 2008;29:362–375. doi: 10.1016/j.molcel.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, et al. Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat Struct Mol Biol. 2009;16:1026–1035. doi: 10.1038/nsmb.1656. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Pandey A, Hart GW. Dynamic interplay between O-linked N-acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol Cell Proteomics. 2007:1365–1379. doi: 10.1074/mcp.M600453-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Faustino RS, Cheung P, Richard MN, Dibrov E, Kneesch AL, Deniset JF, et al. Ceramide regulation of nuclear protein import. J Lipid Res. 2008;49:654–662. doi: 10.1194/jlr.M700464-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Yang X, Kawai T, López de Silanes I, Mazan-Mamczarz K, Chen P, et al. AMP-activated protein kinase-regulated phosphorylation and acetylation of importin α1. J Biol Chem. 2004;279:48376–48388. doi: 10.1074/jbc.M409014200. [DOI] [PubMed] [Google Scholar]
- 30.Pujol G, Söderqvist H, Radu A. Age-associated reduction of nuclear protein import in human fibroblasts. Biochem Biophys Res Comm. 2002;274:354–358. doi: 10.1016/S0006-291X(02)00492-8. [DOI] [PubMed] [Google Scholar]
- 31.D'Angelo M, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ly TK, Wang J, Pereira R, Rojas KS, Peng X, Feng Q, et al. Activation of the Ran GTPase is subject to growth factor regulation and can give rise to cellular transformation. J Biol Chem. 2010;285:5815–5826. doi: 10.1074/jbc.M109.071886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldherr C, Akin D, Moore MS. The nuclear import factor p10 regulates the functional size of the nuclear pore complex during oogenesis. J Cell Sci. 1998;111:1889–1896. doi: 10.1242/jcs.111.13.1889. [DOI] [PubMed] [Google Scholar]
- 34.Callanan M, Kudo N, Gout S, Brocard M, Yoshida M, Dimitrov S, Khochbin S. Developmentally regulated activity of CRM1/XPO1 during early Xenopus embryogenesis. J Cell Sci. 2000;113:451–459. doi: 10.1242/jcs.113.3.451. [DOI] [PubMed] [Google Scholar]
- 35.Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nature Rev Cancer. 2004:106–117. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- 36.Kodiha M, Rassi JG, Brown CM, Stochaj U. Localization of AMP kinase is regulated by stress, cell density and signaling through the MEK → ERK1/2 pathway. Am J Physiol Cell Physiol. 2007;293:1427–1436. doi: 10.1152/ajpcell.00176.2007. [DOI] [PubMed] [Google Scholar]
- 37.Vereshchagina N, Ramel M, Bitoun E, Wilson C. The protein phosphatases PP2A-B′ subunit Widerborst is a negative regulator of cytoplasmic activated Akt and lipid metabolism in Drosophila. J Cell Sci. 2008;121:3383–3392. doi: 10.1242/jcs.035220. [DOI] [PubMed] [Google Scholar]
- 38.Quan X, Yu J, Bussey H, Stochaj U. The localization of nuclear exporters of the importin-β family is regulated by Snf1 kinase, nutrient supply and stress. Biochim Biophys Acta. 2007;1773:1052–1061. doi: 10.1016/j.bbamcr.2007.04.014. [DOI] [PubMed] [Google Scholar]