Abstract
Association of nuclear lamins with the inner nuclear membrane (INM) is mediated by lipid modifications: either by C-terminal isoprenylation or N-terminal myristoylation. Overexpression of lamins or other lipidated nuclear proteins induces the formation of intranuclear membrane-like arrays. Lamin-induced intranuclear array formation has been observed in Xenopus oocytes as well as in mammalian tissue culture cells. With the use of a membrane-specific fluorescence dye we show here that these arrays are made up of typical lipid membranes. While continuity between these intranuclear membranes and the INM has not been observed so far the presence of integral as well as luminal marker proteins of the endoplasmic reticulum (ER) indicates that these membranes are derived from the nuclear membrane/ER compartment. Earlier studies demonstrated that overexpression of integral membrane proteins of the INM can induce formation of intranuclear membranes, which bud from the INM. Integral membrane proteins reach the INM via the pore membranes while lipidated proteins are imported into the nucleoplasm via the classical NLS pathway where they interact with the INM via their lipid moieties. Together with the previously published data our results show that the formation of intranuclear membranes follows similar routes irrespective of whether the proteins triggering membrane formation are integral membrane or lipidated proteins.
Key words: endoplasmic reticulum, intranuclear membranes, isoprenylation, myristoylation, nuclear envelope, nuclear lamins, Xenopus
Introduction
In eukaryotic cells the nucleoplasmic and cytoplasmic compartments are separated by the nuclear envelope (NE). The NE is an extension of the rough endoplasmic reticulum. It consists of two lipid bilayers, the inner (INM) and outer (ONM) nuclear membranes. The two nuclear membranes are fused at sites where nuclear pore complexes (NPCs) are embedded and the ONM is contiguous with the endoplasmic reticulum. NPCs are the sole gateways for trafficking between nucleus and cytoplasm. The nucleoplasmic side of the INM is associated with the nuclear lamina, a filamentous meshwork that provides mechanical strength to nuclei and serves as a binding platform for the interaction with chromatin and a multitude of inner nuclear membrane proteins.1,2 The nucleoplasm is organized into a number of morphologically and functionally distinct domains.3,4 Unlike cytoplasmic organelles these domains are not bounded by membranes. However, several cases have been reported where intranuclear membranes occur under physiological conditions. The best-analyzed ones are those of the nucleolar channel system (NCS) that appears transiently in the epithelial cell nuclei of post-ovulatory human endometrium.5–8 The NCS consists of layers of tubular membrane cisternae and is often associated with nucleoli and the NE. Overexpression of the nucleolar chaperone Nopp140 in tissue culture cells induces the formation of R-rings, which show remarkable ultrastructural resemblance to the NCS.7,9 R-ring formation initiates at the NE. The presence of endoplasmic reticulum (ER) proteins in R-rings and in the NCS indicates that their membranes are derived from the NE/ER.7,9 Early electron microscopic reports describe membrane-limited tubules and vesicles also in spider crab oocytes,10 in nuclei of sertoli cells of ruminants,11 and in human spermatogenic cells.12 These membrane structures are either associated with nucleoli or are associated with the NE but information about their biogenesis is lacking.
Intranuclear membrane structures have also been described as signs of pathological alterations in a large number of different cell types and tissues.13 Intranuclear membrane structures have been observed after administration of toxic agents.14 In many instances it has been demonstrated that these membranes originate from the inner membrane of the NE and that continuity exists between the perinuclear space and the interior of the membrane bounded cisternae.13
Infection with particular viruses, which replicate and undergo morphogenesis within nuclei of infected cells, is accompanied by overexpression of virus-encoded INM proteins. This leads to hyperproliferation of the INM and eventually to the formation of intranuclear membranes.15–17 The intranuclear membrane vesicles induced by the nucleopolyhedrovirus Autographa californica (baculovirus) ultimately become the envelope of the occlusion-derived virus (ODV).17 Proliferation of the INM can be induced in non-infected cells by expression of particular viral proteins.18–20 Studies with the baculovirus have shed light on how virus-encoded integral membrane proteins are sorted to the INM on their way to the ODV envelopes.21 INM proteins carry an INM-sorting sequence that is recognized by importin-α-16. Importin-α-16 captures the INM protein cotranslationally at the translocon and facilitates its nuclear uptake via the nuclear pore membrane. Membrane budding from the INM then gives rise to the ODV envelopes.17
Intranuclear membranes have also been induced experimentally by overexpression of integral membrane proteins like the lamin B receptor (LBR), an INM protein,22 or a mutant fibroblast growth factor receptor 4 that is targeted to the INM.23 Moreover, formation of intranuclear membrane cisternae after overexpression of particular NPC proteins has been described for nucleoporin Nup153 in mammalian cells24 and for Nup53p in yeast.25 In all the cases either a direct continuity between INM and the intranuclear membranes has been observed or their origin from the NE/ER has been demonstrated by the presence of bona fide ER proteins in these membranes.
Intranuclear membrane-like arrays are also induced by overexpressing intranuclear proteins with a lipidation motif, as seen for lamins overexpressed in amphibian oocytes26 or other cell types.27,28 Induction of the arrays strictly depends on the presence of either an isoprenylation or a myristoylation motif. Nuclear translocation of lamins is mediated by binding to importin α/β via a classical NLS29 followed by uptake through the central pore of the NPC. Within the nucleus the isoprenylated lamins associate with the INM where they form IF-type filaments.30,31
Induction of intranuclear membranes is not restricted to lamins. Expression of chimeric green fluorescent protein (GFP) fused to an NLS and the lipidation motif of N-Ras does also induce intranuclear arrays.26 Similar to the membrane structures induced by expression of integral INM proteins, the membrane-like structures induced by expression of lipidated nuclear proteins form extensive arrays of stacked cisternae. The outer surface of these cisternae is covered by the overexpressed proteins.32 The arrays are bordering the INM at certain points. At the bordering sites the arrays are separated from the INM by an electron dense layer that is of similar thickness as the layers that separate individual stacks of the multilayered arrays (e.g., Fig. 3 in ref. 26). In contrast to the intranuclear membranes described above continuity between the membrane-like sheets and the INM has not been observed.26
Figure 3.
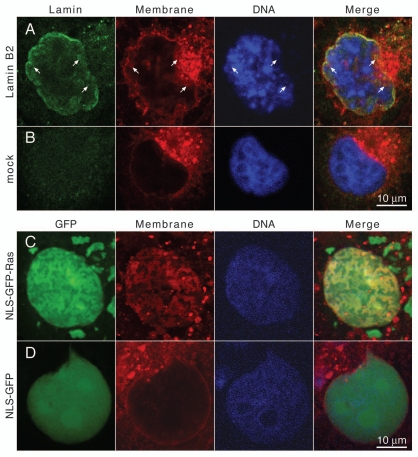
Intranuclear arrays induced by expression of isoprenylated nuclear proteins in Cos-7 cells contain membrane lipids. (A) Cos-7 cells transiently transfected with FLAG-tagged lamin B2 or (B) mock transfected cells were stained for membrane lipids with CM-DiI (red) 24 hours after transfection, then fixed and permeabilized and stained for lamin B2 with mAb M2 and an FITC-conjugated secondary antibody (green) and for DNA with DAPI (blue). (C) Cos-7 cells transiently transfected with NLS-GFP-Ras or (D) NLS-GFP were stained with CM-DiI (red) 24 hours after transfection, they were then fixed and stained for DNA with DAPI (blue). FITC and GFP fluorescence was detected in the green channel. Samples were analyzed with a confocal fluorescence microscope. Mid-nuclear optical sections are shown. Arrows point to intranuclear membranes. The right parts show merged images of all three channels. Size bar for all parts 10 µm.
Intranuclear membranes induced by lipidated proteins could principally arise by two mechanisms: de novo or by budding from pre-existing membranes. There is evidence that nuclei contain large amounts of phospholipid and inositides.33 Analysis of intranuclear lipid distribution revealed that the majority of these lipids is not present in the form of membranes.34 Intranuclear lipid metabolism has been shown to be associated with signal transduction and regulation of enzyme activity.35–37 Since nuclear lamins are lipidated when they enter the nucleoplasm it is possible that they could trigger de novo membrane formation using lipids present in the nucleoplasm. Alternatively, intranuclear membrane formation could be triggered by insertion of the lipid moieties of the proteins into the INM followed by proliferation of the INM.
Here, we report on the characterization of intranuclear membranes that are induced by overexpression of nuclear lamins and other CaaX-isoprenylated proteins. The study was carried out with mammalian tissue culture cells as well as with amphibian oocytes. Amphibian oocytes are particularly suited for this type of analysis. Their nuclear envelopes can easily be isolated and spread, nucleoplasmic side up, onto a microscopic slide. In this way the intranuclear membranes, which adhere to the envelope, are readily accessible to microscopic analysis. With the help of a membrane tracer we show that the intranuclear arrays are indeed made of lipid membranes. Furthermore, we demonstrate that the membrane arrays harbor integral as well as luminal ER marker proteins, which indicates that they are derived from the NE/ER membrane system.
Results
Lamin-induced intranuclear arrays contain membrane lipids.
Exogenous expression of lipidated nuclear proteins induces the formation of intranuclear membrane-like arrays.26–28 To characterize the nature of these arrays FLAG-tagged lamin B2 was expressed in Xenopus oocytes by nuclear injection of plasmid DNA. NE spreads were analyzed by immunofluorescence microscopy (Fig. 1A) and by field-emission scanning electron microscopy (feSEM) (Fig. 1B–D). The intranuclear arrays are attached to the nuclear envelope at certain points (see for example Fig. 3 in ref. 26). At high levels of expression they cover large areas of the INM (Fig. 1A and B). They can be visualized by indirect immunofluorescence microscopy after staining with FLAG-specific antibodies, which specifically decorate the lamin-induced arrays (Fig. 1A). Accessibility of antibodies to the inner layers of the sheets is restricted,26 which results in higher fluorescence signals at the rim of the arrays (Fig. 1A). It does not indicate that lamins accumulate along the edges of the arrays. Immuno-feSEM analysis reveals that the overexpressed lamin B2 is located on the surface of the arrays (Fig. 1C), where it forms a dense layer of IF-type filaments (Fig. 1D).31,32 Transmission electron microscopic (TEM) images of sectioned material show that the arrays form extensive stacks of membrane-like sheets (Fig. 1E).26,32 In TEM images of sectioned material the arrays show a similar appearance as the NE or stacks of ER cisternae (Fig. 1E). Furthermore, the individual layers show a tri-lamellar structure typical for unit membranes. However, a tri-lamellar dark-light-dark pattern is not an unambiguous evidence for the presence of a lipid bilayer. It is known that lipid-free isolated proteins may form ‘membrane-like’ multilayered lamellae. These lamellae show a dark-light-dark pattern in TEM sections, which is indistinguishable from that of lipid bilayers.38
Figure 1.
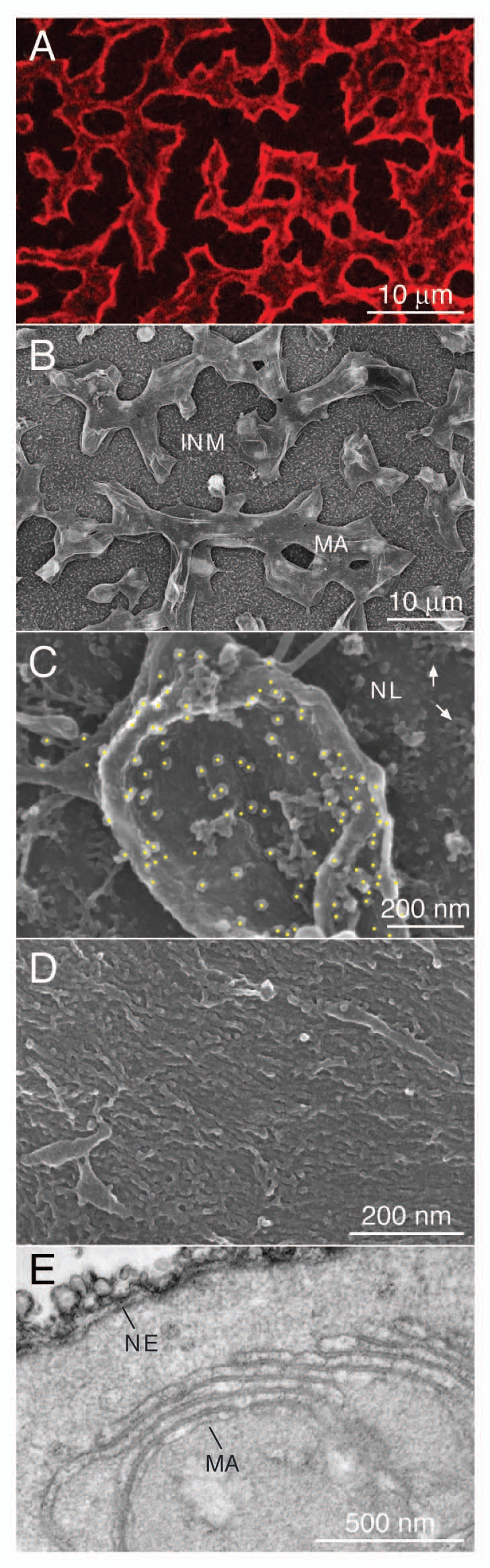
Expression of lamin B2 induces intranuclear membrane-like arrays. FLAG-tagged Xenopus lamin B2 was expressed in Xenopus oocytes by nuclear injection of plasmid DNA. (A) Immunofluorescence microscopy of a nuclear envelope spread of a FLAG-lamin B2 expressing oocyte (top view). FLAG-tagged lamin B2 (red) was detected with mAb M2 and a Cy3-conjugated secondary antibody. (B) feSEM image of a nuclear envelope spread of a lamin B2 expressing oocyte taken at low magnification. Lamin-induced intranuclear arrays (MA) are attached to the inner nuclear membrane (INM). The inner membrane is decorated by numerous pore baskets. (C) Immuno-feSEM detection of FLAG-tagged lamin B2 on the surface of an intranuclear array using mAb M2. Immuno-gold particles were identified in a backscatter image and marked by yellow spots. Gold particles are restricted to the surface of the intranuclear array while the nuclear lamina (NL) is not labeled. Arrows in the upper right point to nuclear pore baskets. (D) feSEM image of the surface of an intranuclear array taken at higher magnification. Individual lamin filaments cover the entire surface of the array. (E) TEM section of an isolated oocyte nucleus expressing lamin B2. Note the similarity between the nuclear envelope cisternae (NE) and the stacked arrays (MA).
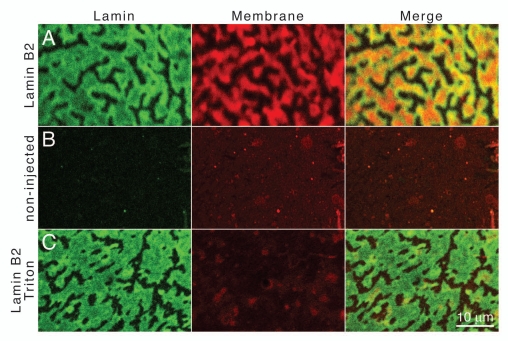
We used the lipophilic dye CM-DiI to probe the intranuclear membrane-like arrays for the presence of lipids. Nuclear envelope spreads of FLAG-tagged lamin B2 expressing oocytes were stained with CM-Dil. FLAG-epitope tagged lamin B2 was detected by indirect immunofluorescence with mAb M2. While the merged image (Fig. 2A, Merge) gives the impression that the lamin signal encloses the membrane signal comparison of the lamin B2 staining (green, Fig. 2A, Lamin) with the CM-DiI membrane staining (red, Fig. 2A, Membrane) shows that the same structures are labeled. However, lamin staining is more intense at the edges of the arrays while membrane staining is stronger in the central parts. This can be explained by the differences in accessibility of the two stains. While the lipophilic membrane dye can easily access all layers of the stack the anti lamin antibodies have limited access to the inner layers of the arrays. After removal of membrane lipids (see below) this rim effect is less pronounced (compare Fig. 2A with C). Areas of the nuclear envelope that are not covered by intranuclear membrane arrays appear relatively dark since exposure times were optimized for the strong signals of the multilayered membrane arrays. Envelope spreads of non-injected control oocytes were negative for lamin B2 staining (Fig. 2B, Lamin) and showed only weak membrane lipid staining (Fig. 2B, Membrane). To demonstrate the specificity of the lipid detection envelope spreads of lamin B2 expressing oocytes were treated with Triton X-100 to remove membrane lipids prior to CM-DiI staining and immunodetection. Lamin filaments are resistant to detergent extraction. Anti-lamin staining was unaffected by the Triton X-100 pre-treatment (Fig. 2C, Lamin), while CM-DiI fluorescence was at background levels after lipid extraction (compare Fig. 2C and A, Membrane). It should be noted that the size and shape of membrane arrays depends on the level of lamin expression. It might vary not only between different NE spreads but also between different areas of the same spread (see also Fig. 5A and C). These results show that the lamin-induced arrays attached to the oocyte nuclear envelopes consist of stacks of lipid membranes that are covered by lamin filaments.
Figure 2.
Detection of lipids in lamin-induced intranuclear arrays. Nuclear envelope spreads of Xenopus oocytes were stained for membrane lipids with CM-DiI (Membrane). FLAG-tagged lamin B2 was detected by indirect immunofluorescence with mAb L7-8C6 and a FITC-conjugated secondary antibody (Lamin). Spreads were analyzed by confocal fluorescence microscopy. (A) Spreads of lamin B2 expressing oocytes, (B) spreads of non-injected control oocytes, (C) spreads of lamin B2 expressing oocytes that were treated with Triton X-100 prior to staining for lipids. The right parts show merged images. Red intensively fluorescing dots in (B and C) (Membrane) are due to small insoluble CM-DiI particles that stick to the samples. Size bar for all parts 10 µm.
Figure 5.
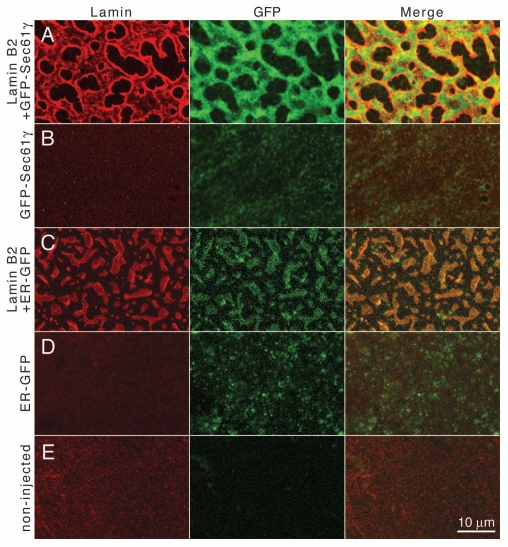
Intranuclear membranes of oocyte nuclei contain luminal and integral membrane ER marker proteins. Nuclear envelope spreads of Xenopus oocytes were stained for FLAG-tagged lamin B2 and detected by indirect immunofluorescence with mAb M2 and a Cy3-conjugated secondary antibody (Lamin) and analyzed by confocal fluorescence microscopy. GFP was detected in the green channel. (A) Spreads of oocytes co-expressing lamin B2 and GFP-Sec61γ, (B) GFP-Sec61γ alone, (C) co-expressing lamin B2 and the luminal ER-GFP, (D) ER-GFP alone, (E) spreads of non-injected control oocytes. The right parts show merged images of the two channels. Size bar for all parts 10 µm.
Lamin-induced intranuclear membrane-like array formation has also been observed in tissue culture cells.27,28 To induce intranuclear array formation Cos-7 cells were transfected with a lamin B2 encoding plasmid vector. Immunodetection of lamins made it necessary to permeabilize the cells after fixation. Permeabilization was done with a detergent concentration lower than normal to minimize membrane lipid extraction, which resulted in weaker lamin immunofluorescence signals compared to signals obtained with the normal staining protocol. Nuclei of lamin B2 expressing cells are slightly lobulated (Fig. 3A, Lamin) and show intranuclear membranes that are stained by CM-DiI (Fig. 3A, Membrane, arrows). Intranuclear CM-DiI positive structures are absent from non-transfected cells (compare Fig. 3B and A, Membrane) while the ER and the NE of both, transfected and non-transfected cells are decorated by the membrane dye. Lipid extraction as a control for the specificity of the membrane lipid staining was not possible with the tissue culture cells since Triton X-100 treatment prior to fixation resulted in detachment of cells from the coverslips.
Lamin-induced intranuclear membrane formation is dependent on lipid modification, either by farnesylation26,28 or by myristoylation.27 We have previously shown that an NLS in conjunction with a CaaX motif can act as minimal targeting motif for the association of proteins with the INM39 and that expression of these proteins induces intranuclear vesicular and tubular structures.26 A GFP protein carrying at its N-terminus the NLS of the large T antigen and at its C-terminus the last 11 amino acid residues of human N-Ras that function as a dual lipidation motif (farnesylation plus palmitoylation) (NLS-GFP-Ras) was expressed in Cos-7 cells. A GFP with an N-terminal NLS but lacking the N-Ras sequences served as control (NLS-GFP). Both chimeric proteins are targeted into the nucleus. Expression of NLS-GFP-Ras induces extensive intranuclear structures (Fig. 3C and GFP) that stain with CM-DiI (Fig. 3C, Membrane). The GFP and CM-DiI signals match each other (Fig. 3C, Merge) but differ from that of chromatin (Fig. 3C and DNA). Since detection of GFP fluorescence could be done without a permeabilization step the fluorescence signals are stronger in this type of experiment (compare intranuclear membrane staining in Fig. 3A and C). Cytoplasmic NLS-GFP-Ras positive structures are not stained by the membrane dye indicating that these structures do not contain membranes. This is in agreement with previous TEM analyses, which showed that membrane-like arrays in NLS-GFP-Ras expressing cells are restricted to the nuclear compartment.26 Together these results show that the intranuclear arrays induced by overexpression of lipidated nuclear proteins consist of lipid membranes.
Intranuclear membranes contain ER marker proteins.
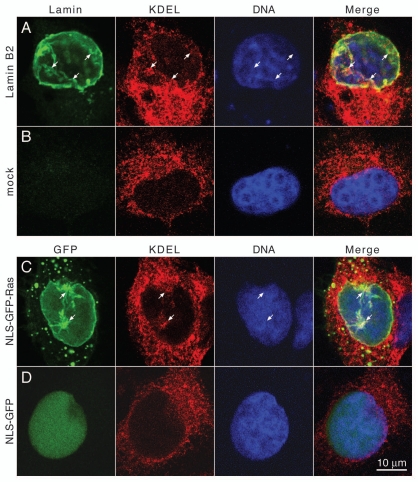
The intranuclear membrane arrays described here are attached to the INM but continuity between these membranes and the INM has never been observed.26,28 We therefore analyzed whether the intranuclear membrane arrays are part of the endomembrane system or might be formed de novo by a hitherto unknown mechanism. If derived from the NE/ER membranes it is reasonable to assume that the intranuclear membranes will contain typical ER proteins, be it luminal or integral membrane proteins. Lamin B2 or the NLS-GFP-Ras chimeras were expressed in Cos-7 cells and cells were immunostained for luminal ER proteins that harbor the ER retrieval sequence KDEL and were analyzed by confocal microscopy. As expected the KDEL antibody intensively stained the ER in transfected and non-transfected cells (Fig. 4A–D, KDEL). While the nucleoplasm of non-transfected and control cells is negative for KDEL-staining (Fig. 4B and D, KDEL) the intranuclear membranes induced by expression lamin B2 or by the NLS-GFP-Ras chimera are stained with anti-KDEL antibodies (Fig. 4A and C, KDEL, Merge, arrows).
Figure 4.
Intranuclear membrane cisternae contain luminal ER proteins. (A) Cos-7 cells transiently transfected with FLAG-tagged lamin B2 or (B) mock transfected cells were fixed and stained for lamin B2 with polyclonal rabbit antibodies directed against the FLAG-epitope and an FITC-conjugated secondary antibody (green), for KDEL-containing ER proteins with mAb 10C3 and a Cy3-conjugated secondary antibody (red) and for DNA with DAPI (blue). (C) Cos-7 cells transiently transfected with NLS-GFP-Ras or (D) transfected with NLS-GFP were stained for KDEL proteins and for DNA as described above. FITC and GFP fluorescence was detected in the green channel. Cells were analyzed by confocal fluorescence microscopy. Mid-nuclear optical sections are shown. Arrows point to intranuclear membranes. The right parts show merged images of all three channels. Size bar for all parts 10 µm.
In addition to the induction of intranuclear membranes overexpression of lipidated nuclear proteins may result in lobulation and/or invagination of the NE. To localize the intranuclear membranes more precisely serial optical sections were obtained by confocal fluorescence microscopy. Z-stacks of the cells shown in Figure 4A and C are presented in the Supplementary Figures S1 and S2, respectively. These images show that membranes are found throughout the interior of the nuclei. The intranuclear membranes are irregularly shaped while the contour of the nuclei is not heavily deformed. The resolution of the confocal microscope does not allow to distinguish between a close association of intranuclear membranes, as it is seen frequently in TEM sections of mammalian cells that overexpress lipidated nuclear proteins (see for example Fig. 5 in ref. 26) and continuity of membranes at putative sites of NE invaginations. However, if all intranuclear membranes would stem from invaginations of the NE one would expect the nuclei to be much more deformed than it is observed in Figure S1 and S2. Taken together, these results indicate that the intranuclear membranes are derived from the NE/ER membranes.
To test for ER markers in Xenopus oocyte nuclei, we expressed two previously described chimeras, ER-GFP40 and Sec61γ-GFP.41 ER-GFP has the leader sequence of protein disulfide isomerase fused to the N-terminus of GFP and a C-terminal ER retention sequence (KDEL) and is cotranslationally translocated into the ER lumen.40 The other marker has GFP fused to the N-terminus of Sec61γ, a subunit of the translocon. Sec61γ is a small tail-anchored protein that is postranslationally inserted into the ER membrane.41 The GFP marker proteins were expressed in oocytes either alone or together with lamin B2. Nuclear envelope spreads were analyzed by confocal microscopy. The nuclear envelope membranes of oocytes that express the ER marker alone show weak but significant GFP fluorescence (compare Fig. 5B and D with the non-injected control E, GFP). ER marker proteins did not induce intranuclear membranes on their own when expressed in oocytes (Fig. 5B and D) nor did they influence the shape of lamin-induced intranuclear membrane arrays (compare Fig. 5A with 1A). On envelope spreads of co-expressing oocytes strong GFP fluorescence was detected for both ER-GFP marker proteins (Fig. 5A and C, GFP). The GFP fluorescence co-localizes with the lamin staining on the intranuclear membranes (Fig. 5A and C, Merge). As also seen in Figures 1A and 2A lamin staining is more intense at the edges of the membrane arrays. Similar results were obtained in transfection experiments with Cos-7 cells (results not shown). Taken together, these results show that intranuclear membranes that are induced upon overexpression of isoprenylated proteins are derived from the NE/ER membrane compartment.
Discussion
Overexpression of lipidated nuclear proteins induces the formation of intranuclear membrane arrays.26–28 In this report we have shown that these arrays are made up of typical lipid membranes and that the membranes are derived from the NE/ER membrane system. Membrane staining was done with CM-DiI, a derivate of the indocarbocyanine dye DiI. CM-DiI is a lipophilic dye with two 18-alkyl chains. The dye is weakly fluorescent in water but highly fluorescent when its alkyl chains are incorporated into membranes. It has been found to stain only membranes.42–44 Lamin-induced arrays in both cellular systems, i.e., Xenopus oocytes and tissue culture cells, as well as arrays induced by expression of a chimeric isoprenylated GFP were specifically stained with CM-DiI. Results with isolated NEs of Xenopus oocytes were particularly conclusive (see Fig. 2) since (1) in spread preparations the membrane arrays are readily accessible to the dye and (2) the multiple layers of the membrane arrays give rise to high signal intensities compared to the relatively weak staining of the underlying NE membranes. Moreover, extraction of the membrane lipids prior to CM-DiI staining demonstrated the specificity of the staining and the fluorescence detection method. Lamin-induced arrays were completely negative for CM-DiI fluorescence after detergent treatment of oocyte nuclear envelope spreads. Since detergent treatment interfered with adhesion of cells to the microscopic slides this type of control was not possible with tissue culture cells. Together with the previously obtained TEM data the detection of membrane lipids unequivocally demonstrates the membranous nature of the intranuclear arrays.
Intranuclear membranes are derived from the NE/ER membranes.
All intranuclear membranes described so far are derived from the NE/ER membrane compartment. This holds true for the NCS of epithelial cells of the human endometrium and NCS-related structures, the R-rings, induced by overexpression of the nucleolar chaperon Nopp140,6 for ODVs produced in insect cells during baculovirus infection,17 for membranes formed after overexpression of particular nuclear proteins22–25 as well as for those membranes formed in nuclei of several pathological cells.13 In the above-mentioned cases the membrane budding from the INM has been either followed directly by live cell imaging6,15,22 or membrane continuity between the intranuclear membranes and the INM has been demonstrated by TEM analysis.6,10,13 In addition, the presence of ER marker proteins within the intranuclear membrane structures has been taken as conclusive evidence for their NE/ER origin.23,24
Overexpression of lamins leads to formation of multilayered stacked membrane cisternae. The surface of these membranes is covered by the overexpressed proteins, which in case of lamins form IF-type filaments. Snapp et al. have proposed a general model for the stacking of membranes.45 Through interaction between proteins on the vesicle surfaces membranes are thought to zipper up into highly compacted, stacked structures.45 Formation of membrane arrays of various shapes has previously been observed in the cytoplasm upon overexpression of ER proteins (reviewed in ref. 45). The affinities of the interacting molecules as well as their shape might determine the diversity of structures observed.45
The lamin-induced membrane stacks are always found in the vicinity of the NE. The NE of the giant nuclei of Xenopus oocytes can easily be separated from the nuclear content that contains chromosomes and nucleoplasmic components.46 The intranuclear membranes co-fractionate quantitatively with the NE fraction indicating that they are in contact with the NE.26 Contact sites have been analyzed in serial sections by TEM. At the contact sites intranuclear membranes and INM run in parallel to each other for a short range leaving a small gap between the aligned membranes.26 Attachment is probably due to interaction between filaments of the endogenous lamina and the filaments on the surface of the intranuclear arrays. Continuity between the INM and the lamin-induced intranuclear membranes has never been observed.26,28 However, our finding that ER marker proteins are present in these membrane arrays demonstrates that intranuclear membranes induced by lamins and other lipidated proteins are derived from NE/ER membranes. The lamin-induced membranes are distinctly different from the tubular channel system present in interphase nuclei of many mammalian cells.47 While two membranes that are continuous with the NE and contain NPCs bound the tubular channels, a single membrane lines the intranuclear membrane cisternae, described here. The fact that a single membrane lines the cisternae indicates that the intranuclear membranes are derived from the INM and excludes the possibility that they form by budding of both, the INM and the ONM. It should be mentioned that the presence of ER/NE marker proteins in intranuclear membranes could be explained by an alternative scenario: After spontaneous formation of membranes within the nucleoplasm ER/NE proteins could have been acquired by a transient fusion/budding with the NE. As membrane fusion does not occur spontaneously and has not been observed in our system we believe that this scenario is rather unlikely.
Overexpression of INM proteins triggers membrane proliferation.
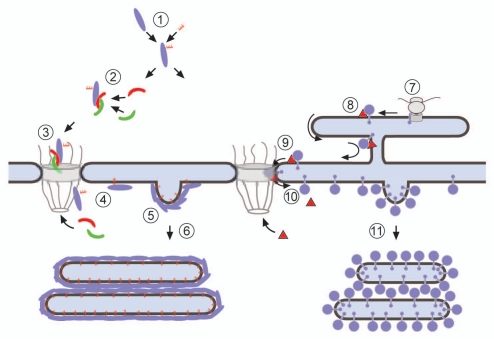
Nuclear lamins and the GFP chimera used in this study carry a classical NLS and are transported via the central NPC channel into the nucleoplasm.29,48 The most probable scenario how isoprenylated proteins induce membrane proliferation is shown in Figure 6. After synthesis and isoprenylation or myristoylation, respectively, in the cytoplasm (Fig. 6-1) the lipidated proteins are imported into the nucleus via the importin-α/β pathway (Fig. 6-2 and 3). Within the nucleus they associate with the INM (Fig. 6-4). Massive insertion of the lipid moieties into the lipid bilayer of the INM then might trigger proliferation of the INM which leads to expansion of the INM (Fig. 6-5) and finally to membrane vesicle budding into the nucleoplasm (Fig. 6-6). Through interaction between proteins on the budded vesicle surfaces vesicles zipper up into flattened and stacked arrays.
Figure 6.
A model for the formation of intranuclear membrane arrays by expression of lipidated nuclear proteins (left) or by expression of integral INM proteins (right). (1) Newly synthesized proteins are lipidated in the cytosol, (2) they bind to importin α/β (red and green, respectively), (3) the cargo/importin α/β complex passes the central channel of the NPC. (4) At the nucleoplasmic side of the NPC the complex is disassembled by the interaction with RanGTP (RanGTP not shown). (5) Binding of the lipidated proteins to the INM triggers membrane proliferation and vesicle budding of the INM. (6) Through interaction between proteins on the budded vesicle surfaces vesicles zipper up into flattened and stacked arrays. (7) Integral INM proteins that are co- or post-translationally inserted into the ER membrane, (8) are recognized by importin-α-16. The complex reaches the NPC membrane by lateral diffusion. (9) Passage via the membrane of the NPC is mediated by importin-α-16, (10) the import complex disassembles by the interaction with Nup50 (Nup50 not shown). (11) Massive accumulation of the integral membrane protein at the INM will induce membrane proliferation and vesicle budding in a similar way as shown for the lipidated proteins. The right part of the scheme has been modified after Rexach.57
Intranuclear membrane proliferation has been previously described after infection with certain viruses. Studies with envelope proteins of nuclear polyhedrosis virus have shed light on how overexpression of integral membrane proteins can lead to formation of intranuclear membranes.18,19,21,49 Viral envelope proteins that are inserted into ER membranes via transmembrane domains (Fig. 6-7) contain INM sorting motifs (INM-SMs) that are recognized by importin-α-16 either cotranslationally at the translocon or posttranslationally21,49 (Fig. 6-8). INM-SMs are not restricted to viral proteins; they are present in a number of bona fide INM proteins.19 Importin-α-16 serves to direct INM-SM-containing proteins to the INM via the pore membrane (Fig. 6-9 and 10). Intranuclear membranes or, in case of baculovirus infection, ODVs are then produced by budding from the INM49 (Fig. 6-11).
Together with the previously published data our observations show that the formation of intranuclear membranes in the course of quite different processes occurs by similar mechanisms. (Over)expression of peripheral or integral INM proteins results in membrane proliferation of the INM irrespective of the way these proteins are delivered to the nucleus and irrespective of how they interact with membranes. Vesicle budding from the INM follows membrane proliferation. Budding might be either into the perinuclear space, as in the case of herpesvirus,20 baculovirus17 and plant nucleorhabdoviruses50 or into the nucleoplasm. The later holds for the formation of the NCS and the R-rings,6 the production of ODVs in the course of baculovirus infection,17 and for membrane formation after experimental overexpression of nuclear proteins. Depending on the frequency of budding continuities between INM and the membranes of the arrays might be observed regularly or, as in case of lamin-induced arrays, not at all.
Whether lamin-induced intranuclear membrane formation occurs in laminopathies as for example in leukodystrophy that is caused by a duplication of the lamin B1 gene and that is accompanied by an increased synthesis of lamin B1 protein is not known.51
Several INM proteins are involved in the regulation of nuclear size and/or nuclear shape.52 The multipass integral membrane protein LBR, for example, influences nuclear lobulation in a dosage dependent manner, i.e., nuclear segmentation is positively correlated with LBR protein levels.53 Other examples are progerin, a permanently isoprenylated mutant version of lamin A and Kugelkern (Kuk/Char), a Drosophila protein. Knockdown of the kuk/char gene prevents nuclear elongation during cellularization in Drosophila while overexpression of the Kuk/Char protein leads to nuclear lobulation and intranuclear membrane growth.53,54 Nuclear growth and intranuclear membrane formation might therefore be two sides of the same coin.
Material and Methods
Animals.
Frogs (Xenopus laevis) were obtained from the African Xenopus facility Knysna, South Africa. Oocyte techniques were as described.55
Cell culture and transfection.
Cos-7 cells (ATCC CRL-1651) were cultured in DMEM with 10% FCS at 37°C, 5% CO2.
Transfections were performed using FuGeneHD (Roche, Mannheim, Germany). 1.5 × 105 cells were seeded in a six-well-plate, grown overnight and transfected according to the manufacturers instruction using 1 µg plasmid DNA per well.
Plasmid.
Plasmids encoding FLAG-epitope tagged Xenopus Lamin B2, chimeric GFP NLS-MT-GFP-N-Ras and NLS-MT-GFP have been described.26,39 Plasmids encoding mGFP Sec61γ and ER-GFP were kindly provided by Dr. Erik Snapp (Albert Einstein College, NY).45
TEM and fe-SEM techniques.
Staining of NE spread preparations.
Membranes of unfixed NE spread preparations were stained with Vybrant CM-Dil (Molecular Probes, Invitrogen, Karlsruhe, Germany). The membrane stain was diluted 1:100 in 5:1 buffer (83 mM NaCl, 17 mM KCl, 10 mM Hepes-KOH, pH 7.2) and added to the NE spreads for 20 minutes. Subsequently NE spreads were washed twice in 5:1 buffer for 10 minutes. For controls membrane extraction was performed prior to membrane staining by incubation of NE spreads in 1% Triton X-100 diluted in 5:1 buffer for 10 minutes. After washing the NE spreads twice in 5:1 buffer for 10 minutes, membrane stain was performed. Immunostaining of NE spreads was as described.26
Staining of tissue culture cells.
For microscopic analysis tissue culture cells were grown on cover slips. For immunostaining cells were washed twice with PBS (140 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.2) followed by fixation for 15 minutes in 3.9% formaldehyde in PBS. Cells were permeabilized by treatment with 5% Triton X-100 in 3.7% formaldehyde in PBS for 10 minutes. Subsequently, cells were incubated in 150 mM glycine in PBS for 10 minutes followed by blocking of unspecific binding sites in 3% BSA in PBS for 15 minutes. Primary antibodies were diluted in 3% BSA in PBS. Incubation of cells with primary antibodies was for 45 minutes followed by three washes in PBS for 10 minutes each. Incubation with secondary antibody was performed for 45 minutes followed by three washes in PBS. Nuclei were stained with 0.1 µg/ml DAPI (Sigma-Aldrich) diluted in PBS for 8 minutes. After washing coverslips were mounted in Mowiol mounting medium 17% Mowiol (Hoechst, Frankfurt, Germany), 33% glycerol, 2.5% DABCO [1,4-diaza-bicyclo(2,2,2,)octan] (Merck, Darmstadt, Germany).
Membranes of tissue culture cells were stained prior to fixation by incubation in CM-Dil diluted 1:100 in DMEM for 20 minutes at 37°C, 5% CO2. Subsequently, cells were washed three times with DMEM for 5 minutes each. Immunostaining was performed as described above. However, permeabilization was done with 0.5% instead of 5% Triton X-100.
Antibodies.
Primary antibodies used were mouse mAb L7-8C6 directed against Xenopus lamin B256 (1:500), mouse mAb M2 against the FLAG epitope (1:500) (cat. no. F-3165, Sigma-Aldrich), mouse mAb 9E10 against the Myc epitope (1:3,000) (cat. no. M-4439, Sigma-Aldrich), rabbit pAb against the FLAG epitope (1:500) (cat. no. F-7452, Sigma-Aldrich), mouse mAb 10C3 recognizing proteins containing the KDEL sequence (1:500) (cat. no. SPA-827D, Stressgen, Ann Arbor, MI). Secondary antibodies were goat anti-rabbit IgG-FITC (1:500) (cat. no. 111-096-003), goat anti-mouse IgG-FITC (1:500) (cat. no. 115-096-003) and goat anti-mouse IgG-Cy3 (1:500) (cat. no. 115-166-003) (Dianova, Hamburg, Germany).
Fluorescence microscopy.
Images were taken with a Zeiss LSM510 (Zeiss, Jena, Germany). DAPI was exited at 364 nm with an UV laser (351, 364 nm). The beam path configuration was: main dichroic beam splitter (HTF) 488/543/633 nm, dichroic beam splitter (NFT) 545 nm, secondary NFT2 490 nm, emission filter BP 385–470 nm. Cy3 was exited at 543 nm with a helium/neon laser. The beam path configuration was: HTF 488/543/633 nm, NFT 545 nm, emission filter LP 560 nm. GFP and FITC were exited with an argon laser at 488 nm. The beam path configuration was: HFT 488/543/633 nm, NFT 545 nm, NFT2 490 nm, emission filter BP 560–615 nm.
Acknowledgements
We would like to thank Erik L. Snapp, Albert Einstein College, NY, for providing GFP plasmids, Rudolf Amann for the access to and Andreas Elrott for help with the LSM at the MPI for Marine Microbiology, Bremen. We are grateful to Martin W. Goldberg, University of Durham, UK and Irm Huttenlauch, Bremen together with whom the electron microscopic images were obtained. We would like to thank the members of the Cell Biology lab in Bremen for support and stimulating discussion and Irm Huttenlauch and Annette Peter, Bremen for critically reading the manuscript.
Abbreviations
- ER
endoplasmic reticulum
- feSEM
field-emission scanning electron microscopy
- GFP
green fluorescent protein
- IF
intermediate filament
- INM
inner nuclear membrane
- INM-SM
inner nuclear membrane sorting motif
- LBR
lamin B receptor
- mAb
monoclonal antibody
- NCS
nucleolar channel system
- NE
nuclear envelope
- NLS
nuclear localization signal
- NPC
nuclear pore complex
- ODV
occlusion-derived virus
- ONM
outer nuclear membrane
- pAb
polyclonal antibody
- TEM
transmission electron microscopy
Footnotes
Previously published online: www.landesbioscience.com/journals/nucleus/article/12352
Supplementary Material
References
- 1.Prokocimer M, Davidovich M, Nissim-Rafinia M, Wiesel-Motiuk N, Bar D, Barkan R, et al. Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med. 2009;13:1059–1085. doi: 10.1111/j.1582-4934.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann H, Strelkov SV, Burkhard P, Aebi U. Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest. 2009;119:1772–1783. doi: 10.1172/JCI38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Misteli T. Concepts in nuclear architecture. Bioessays. 2005;27:477–487. doi: 10.1002/bies.20226. [DOI] [PubMed] [Google Scholar]
- 5.Terzakis JA. The nucleolar channel system of human endometrium. J Cell Biol. 1965;27:293–304. doi: 10.1083/jcb.27.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kittur N, Zapantis G, Aubuchon M, Santoro N, Bazett-Jones DP, Meier UT. The nucleolar channel system of human endometrium is related to endoplasmic reticulum and R-rings. Mol Biol Cell. 2007;18:2296–2304. doi: 10.1091/mbc.E07-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaac C, Pollard JW, Meier UT. Intranuclear endoplasmic reticulum induced by Nopp140 mimics the nucleolar channel system of human endometrium. J Cell Sci. 2001;114:4253–4264. doi: 10.1242/jcs.114.23.4253. [DOI] [PubMed] [Google Scholar]
- 8.Dubrauszky V, Pohlmann G. Strukturveränderungen am Nukleolus vom Korpusendometriumzellen während der Sekretionsphase. Naturwissenschaften. 1960;47:523–524. (Ger). [Google Scholar]
- 9.Isaac C, Yang Y, Meier UT. Nopp140 functions as a molecular link between the nucleolus and the coiled bodies. J Cell Biol. 1998;142:319–329. doi: 10.1083/jcb.142.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinsch GW. Possible Role of Intranuclear Membranes in Nuclear-Cytoplasmic Exchange in Spider Crab Oocytes. J Cell Biol. 1970;47:531–535. doi: 10.1083/jcb.47.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fawcett DW. Ultrastructure of the sertoli cell. In: Hamilton DW, Greep RO, editors. Handbook of Physiology Section 7, Vol V Male Reproductive System. Washington: American Physiological Society; 1975. pp. 21–55. [Google Scholar]
- 12.Chemes HE, Fawcett DW, Dym M. Unusual features of the nuclear envelope in human spermatogenic cells. Anat Rec. 1978;192:493–512. doi: 10.1002/ar.1091920404. [DOI] [PubMed] [Google Scholar]
- 13.Ghadially FN. Ultrastructural pathology of the cell and matrix. London: Butterworths; 1988. [Google Scholar]
- 14.Berciano MT, Fernandez R, Pena E, Calle E, Villagra NT, Rodriguez-Rey JC, et al. Formation of intranuclear crystalloids and proliferation of the smooth endoplasmic reticulum in schwann cells induced by tellurium treatment: association with overexpression of HMG CoA reductase and HMG CoA synthase mRNA. Glia. 2000;29:246–259. doi: 10.1002/(sici)1098-1136(20000201)29:3<246::aid-glia6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Goodin MM, Chakrabarty R, Yelton S, Martin K, Clark A, Brooks R. Membrane and protein dynamics in live plant nuclei infected with Sonchus yellow net virus, a plant-adapted rhabdovirus. J Gen Virol. 2007;88:1810–1820. doi: 10.1099/vir.0.82698-0. [DOI] [PubMed] [Google Scholar]
- 16.Mettenleiter TC. Intriguing interplay between viral proteins during herpesvirus assembly or: the herpesvirus assembly puzzle. Vet Microbiol. 2006;113:163–169. doi: 10.1016/j.vetmic.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 17.Braunagel SC, Summers MD. Molecular biology of the baculovirus occlusion-derived virus envelope. Curr Drug Targets. 2007;8:1084–1095. doi: 10.2174/138945007782151315. [DOI] [PubMed] [Google Scholar]
- 18.Hong T, Summers MD, Braunagel SC. N-terminal sequences from Autographa californica nuclear polyhedrosis virus envelope proteins ODV-E66 and ODV-E25 are sufficient to direct reporter proteins to the nuclear envelope, intranuclear microvesicles and the envelope of occlusion derived virus. Proc Natl Acad Sci USA. 1997;94:4050–4055. doi: 10.1073/pnas.94.8.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braunagel SC, Williamson ST, Saksena S, Zhong Z, Russell WK, Russell DH, et al. Trafficking of ODV-E66 is mediated via a sorting motif and other viral proteins: facilitated trafficking to the inner nuclear membrane. Proc Natl Acad Sci USA. 2004;101:8372–8377. doi: 10.1073/pnas.0402727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klupp BG, Granzow H, Fuchs W, Keil GM, Finke S, Mettenleiter TC. Vesicle formation from the nuclear membrane is induced by coexpression of two conserved herpesvirus proteins. Proc Natl Acad Sci USA. 2007;104:7241–7246. doi: 10.1073/pnas.0701757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saksena S, Summers MD, Burks JK, Johnson AE, Braunagel SC. Importin-alpha-16 is a translocon-associated protein involved in sorting membrane proteins to the nuclear envelope. Nat Struct Mol Biol. 2006;13:500–508. doi: 10.1038/nsmb1098. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Cai S, Lv Q, Jiang Q, Zhang Q, Sodmergen, et al. Lamin B receptor plays a role in stimulating nuclear envelope production and targeting membrane vesicles to chromatin during nuclear envelope assembly through direct interaction with importin beta. J Cell Sci. 2007;120:520–530. doi: 10.1242/jcs.03355. [DOI] [PubMed] [Google Scholar]
- 23.Sørensen V, Brech A, Khnykin D, Kolpakova E, Citores L, Olsnes S. Deletion mutant of FGFR4 induces onion-like membrane structures in the nucleus. J Cell Sci. 2004;117:1807–1819. doi: 10.1242/jcs.01047. [DOI] [PubMed] [Google Scholar]
- 24.Bastos R, Lin A, Enarson M, Burke B. Targeting and function in mRNA export of nuclear pore complex protein Nup153. J Cell Biol. 1996;134:1141–1156. doi: 10.1083/jcb.134.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marelli M, Lusk CP, Chan H, Aitchison JD, Wozniak RW. A link between the synthesis of nucleoporins and the biogenesis of the nuclear envelope. J Cell Biol. 2001;153:709–724. doi: 10.1083/jcb.153.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ralle T, Grund C, Franke WW, Stick R. Intranuclear membrane structure formations by CaaX-containing nuclear proteins. J Cell Sci. 2004;117:6095–6104. doi: 10.1242/jcs.01528. [DOI] [PubMed] [Google Scholar]
- 27.Prüfert K, Alsheimer M, Benavente R, Krohne G. The myristoylation site of meiotic lamin C2 promotes local nuclear membrane growth and the formation of intranuclear membranes in somatic cultured cells. Eur J Cell Biol. 2005;84:637–646. doi: 10.1016/j.ejcb.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Prüfert K, Vogel A, Krohne G. The lamin CxxM motif promotes nuclear membrane growth. J Cell Sci. 2004;117:6105–6116. doi: 10.1242/jcs.01532. [DOI] [PubMed] [Google Scholar]
- 29.Loewinger L, McKeon F. Mutations in the nuclear lamin proteins resulting in their aberrant assembly in the cytoplasm. EMBO J. 1988;7:2301–2309. doi: 10.1002/j.1460-2075.1988.tb03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg MW, Fiserova J, Huttenlauch I, Stick R. A new model for nuclear lamina organization. Biochem Soc Trans. 2008;36:1339–1343. doi: 10.1042/BST0361339. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg MW, Huttenlauch I, Hutchison CJ, Stick R. Filaments made from A- and B-type lamins differ in structure and organization. J Cell Sci. 2008;121:215–225. doi: 10.1242/jcs.022020. [DOI] [PubMed] [Google Scholar]
- 33.Maraldi NM, Santi S, Zini N, Ognibene A, Rizzoli R, Mazzotti G, et al. Decrease in nuclear phospholipids associated with DNA replication. J Cell Sci. 1993;104:853–859. doi: 10.1242/jcs.104.3.853. [DOI] [PubMed] [Google Scholar]
- 34.Mazzotti G, Zini N, Rizzi E, Rizzoli R, Galanzi A, Ognibene A, et al. Immunocytochemical detection of phosphatidylinositol 4,5-bisphosphate localization sites within the nucleus. J Histochem Cytochem. 1995;43:181–191. doi: 10.1177/43.2.7822774. [DOI] [PubMed] [Google Scholar]
- 35.Irvine RF. Nuclear lipid signalling. Nat Rev Mol Cell Biol. 2003;4:349–360. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 36.Albi E, Viola Magni MP. The role of intranuclear lipids. Biol Cell. 2004;96:657–667. doi: 10.1016/j.biolcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Ledeen RW, Wu G. Nuclear sphingolipids: metabolism and signaling. J Lipid Res. 2008;49:1176–1186. doi: 10.1194/jlr.R800009-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber C, Franke WW, Kartenbeck J. Structure and biochemistry of phloem-proteins isolated from Cucurbita maxima. Exp Cell Res. 1974;87:79–106. doi: 10.1016/0014-4827(74)90529-1. [DOI] [PubMed] [Google Scholar]
- 39.Hofemeister H, Weber K, Stick R. Association of prenylated proteins with the plasma membrane and the inner nuclear membrane is mediated by the same membrane-targeting motifs. Mol Biol Cell. 2000;11:3233–3246. doi: 10.1091/mbc.11.9.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nehls S, Snapp EL, Cole NB, Zaal KJ, Kenworthy AK, Roberts TH, et al. Dynamics and retention of misfolded proteins in native ER membranes. Nat Cell Biol. 2000;2:288–295. doi: 10.1038/35010558. [DOI] [PubMed] [Google Scholar]
- 41.Wattenberg B, Lithgow T. Targeting of C-terminal (tail)-anchored proteins: understanding how cytoplasmic activities are anchored to intracellular membranes. Traffic. 2001;2:66–71. doi: 10.1034/j.1600-0854.2001.20108.x. [DOI] [PubMed] [Google Scholar]
- 42.Haugland RP, Spence MTZ, Johnson ID. Handbook of fluorescent probes and research chemicals. Eugene OR, USA (4849 Pitchford Ave., Eugene 97402): Molecular Probes; 1996. [Google Scholar]
- 43.Terasaki M, Jaffe LA. Organization of the sea urchin egg endoplasmic reticulum and its reorganization at fertilization. J Cell Biol. 1991;114:929–940. doi: 10.1083/jcb.114.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honig MG, Hume RI. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986;103:171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snapp EL, Hegde RS, Francolini M, Lombardo F, Colombo S, Pedrazzini E, et al. Formation of stacked ER cisternae by low affinity protein interactions. J Cell Biol. 2003;163:257–269. doi: 10.1083/jcb.200306020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gall JG, Wu Z, Murphy C, Gao H. Structure in the amphibian germinal vesicle. Exp Cell Res. 2004;296:28–34. doi: 10.1016/j.yexcr.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol. 1997;136:531–544. doi: 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalderon D, Richardson WD, Markham AF, Smith AE. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984;311:33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- 49.Braunagel SC, Cox V, Summers MD. Baculovirus data suggest a common but multifaceted pathway for sorting proteins to the inner nuclear membrane. J Virol. 2009;83:1280–1288. doi: 10.1128/JVI.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson AO, Dietzgen RG, Goodin MM, Bragg JN, Deng M. Biology of plant rhabdoviruses. Annu Rev Phytopathol. 2005;43:623–660. doi: 10.1146/annurev.phyto.43.011205.141136. [DOI] [PubMed] [Google Scholar]
- 51.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, et al. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 52.Melcer S, Gruenbaum Y. Nuclear morphology: when round kernels do the Charleston. Curr Biol. 2006;16:195–197. doi: 10.1016/j.cub.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 53.Gravemann S, Schnipper N, Meyer H, Vaya A, Nowaczyk MJM, Rajab A, et al. Dosage effect of zero to three functional LBR-genes in vivo and in vitro. Nucleus. 2010;1:1–12. doi: 10.4161/nucl.1.2.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pilot F, Philippe JM, Lemmers C, Chauvin JP, Lecuit T. Developmental control of nuclear morphogenesis and anchoring by charleston, identified in a functional genomic screen of Drosophila cellularisation. Development. 2006;133:711–723. doi: 10.1242/dev.02251. [DOI] [PubMed] [Google Scholar]
- 55.Stick R, Goldberg MW. Oocytes as an experimental system to analyze the ultrastructure of endogenous and ectopically expressed nuclear envelope components by field-emission scanning electron microscopy. Methods. 2010 doi: 10.1016/j.ymeth.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Stick R. cDNA cloning of the developmentally regulated lamin LIII of Xenopus laevis. EMBO J. 1988;7:3189–3197. doi: 10.1002/j.1460-2075.1988.tb03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rexach MF. A sorting importin on Sec61. Nat Struct Mol Biol. 2006;13:476–478. doi: 10.1038/nsmb0606-476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.