Abstract
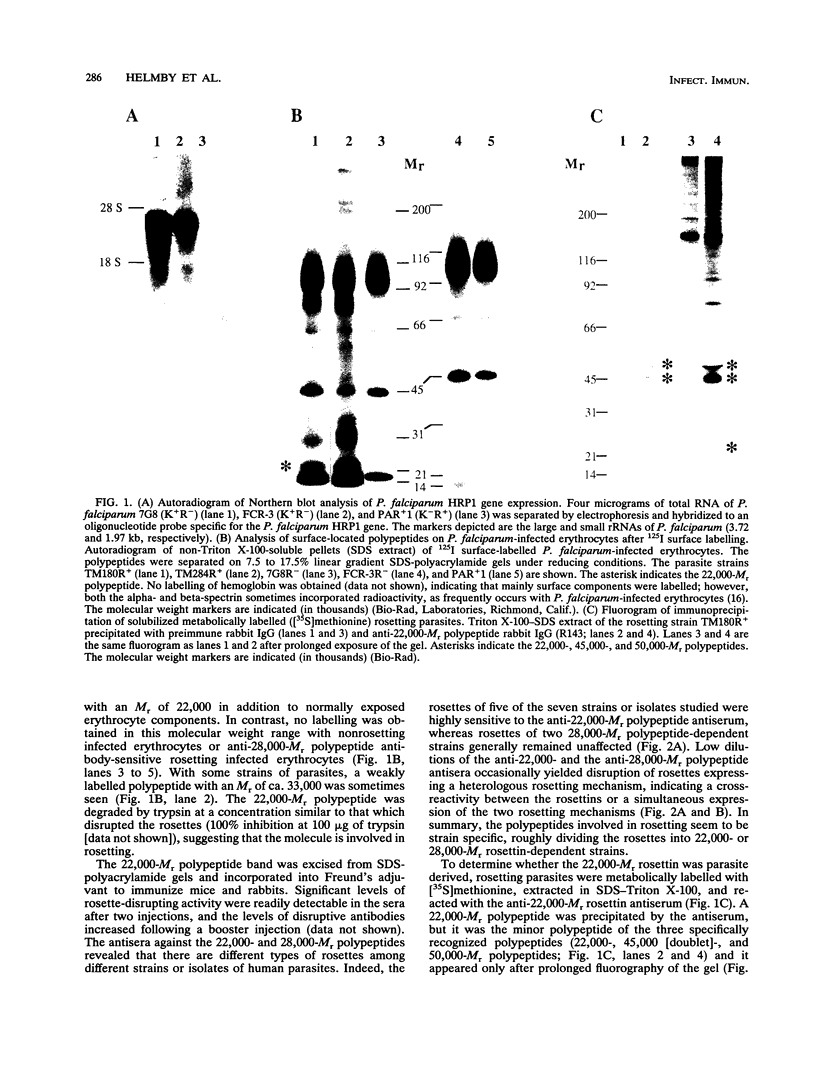
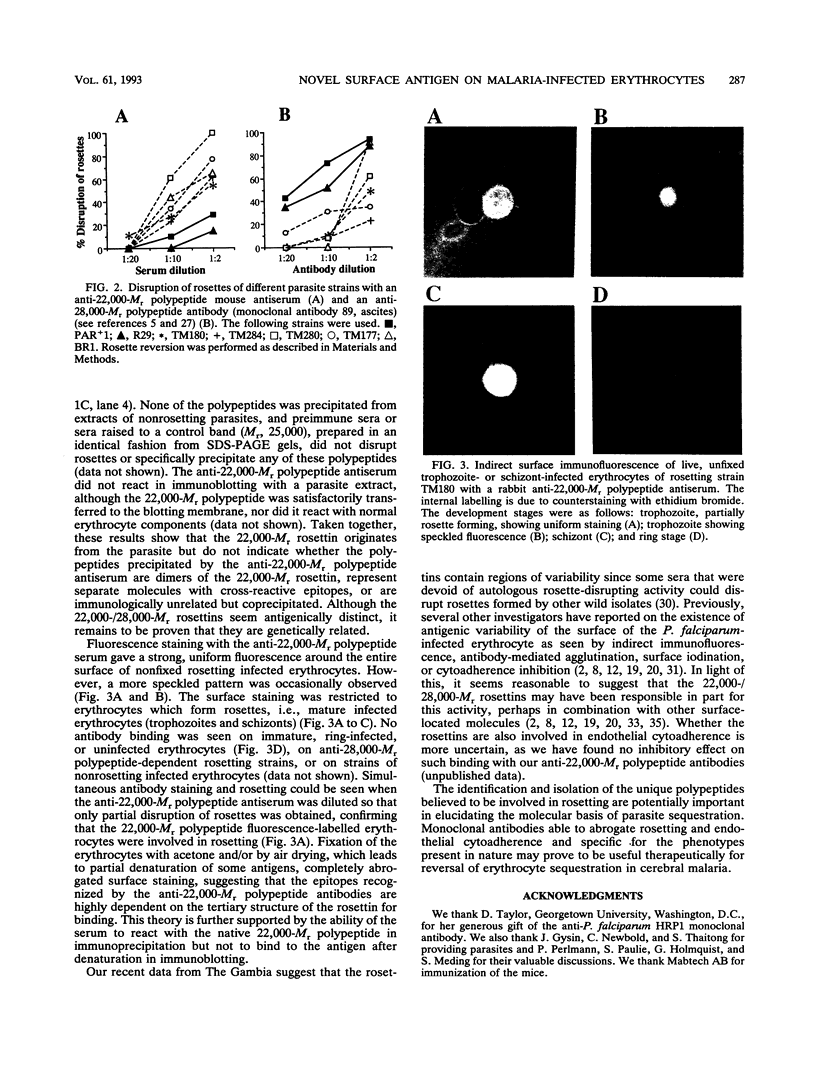
Spontaneous binding of uninfected erythrocytes to Plasmodium falciparum-infected erythrocytes (rosetting) has been suggested to have a critical role in the induction of cerebral malaria. We report here that rosetting can be mediated by several molecular mechanisms involving parasite polypeptides with M(r)s of 22,000 or 28,000, termed rosettins. Antibodies to either polypeptide disrupt rosettes in a strain-specific fashion. Rosettes of five of the seven isolates examined thus far are more easily disrpted by anti-22,000-M(r) rosettin antibodies than by anti-28,000-M(r) rosettin antibodies. Polyclonal anti-22,000-M(r) rosettin antibodies raised in mice or rabbits strongly and strain specifically stain the surface of nonfixed erythrocytes infected with late asexual stages of rosetting P. falciparum. Simultaneous antibody staining and rosetting are seen when the anti-22,000-M(r) rosettin antiserum is diluted so that only partial disruption of rosettes is obtained, confirming that the fluorescence-labelled infected erythrocytes are involved in rosetting. The 22,000-M(r) rosettin is accessible for surface iodination on erythrocytes infected with strains of rosetting parasites sensitive to anti-22,000-M(r) rosettin antibodies, whereas no labelling occurred on either normal erythrocytes or nonrosetting-P. falciparum-infected erythrocytes. Purified anti-22,000-M(r) rosettin serum immunoglobulin G immunoprecipitated three parasite-derived polypeptides with M(r)s of 22,000, 45,000 (doublet), and 50,000 from lysates of [35S]methionine-labelled, parasite-infected erythrocytes. Our results suggest that rosetting is mediated by strain-specific, antigenically distinct, P. falciparum-derived polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Iseki M., Barnwell J. W., Taylor D., Oo M. M., Howard R. J. The pathology of human cerebral malaria. Am J Trop Med Hyg. 1990 Aug;43(2 Pt 2):30–37. doi: 10.4269/ajtmh.1990.43.30. [DOI] [PubMed] [Google Scholar]
- Aley S. B., Sherwood J. A., Marsh K., Eidelman O., Howard R. J. Identification of isolate-specific proteins on sorbitol-enriched Plasmodium falciparum infected erythrocytes from Gambian patients. Parasitology. 1986 Jun;92(Pt 3):511–525. doi: 10.1017/s0031182000065410. [DOI] [PubMed] [Google Scholar]
- Barnwell J. W. Cytoadherence and sequestration in falciparum malaria. Exp Parasitol. 1989 Nov;69(4):407–412. doi: 10.1016/0014-4894(89)90190-2. [DOI] [PubMed] [Google Scholar]
- Carlson J., Helmby H., Hill A. V., Brewster D., Greenwood B. M., Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990 Dec 15;336(8729):1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- Carlson J., Holmquist G., Taylor D. W., Perlmann P., Wahlgren M. Antibodies to a histidine-rich protein (PfHRP1) disrupt spontaneously formed Plasmodium falciparum erythrocyte rosettes. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2511–2515. doi: 10.1073/pnas.87.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David P. H., Handunnetti S. M., Leech J. H., Gamage P., Mendis K. N. Rosetting: a new cytoadherence property of malaria-infected erythrocytes. Am J Trop Med Hyg. 1988 Mar;38(2):289–297. doi: 10.4269/ajtmh.1988.38.289. [DOI] [PubMed] [Google Scholar]
- Ellis J., Irving D. O., Wellems T. E., Howard R. J., Cross G. A. Structure and expression of the knob-associated histidine-rich protein of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Nov;26(1-2):203–214. doi: 10.1016/0166-6851(87)90144-7. [DOI] [PubMed] [Google Scholar]
- Forsyth K. P., Philip G., Smith T., Kum E., Southwell B., Brown G. V. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am J Trop Med Hyg. 1989 Sep;41(3):259–265. [PubMed] [Google Scholar]
- Handunnetti S. M., David P. H., Perera K. L., Mendis K. N. Uninfected erythrocytes form "rosettes" around Plasmodium falciparum infected erythrocytes. Am J Trop Med Hyg. 1989 Feb;40(2):115–118. doi: 10.4269/ajtmh.1989.40.115. [DOI] [PubMed] [Google Scholar]
- Ho M., Davis T. M., Silamut K., Bunnag D., White N. J. Rosette formation of Plasmodium falciparum-infected erythrocytes from patients with acute malaria. Infect Immun. 1991 Jun;59(6):2135–2139. doi: 10.1128/iai.59.6.2135-2139.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel M., David P. H., Oligino L. D. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J Exp Med. 1983 Apr 1;157(4):1137–1148. doi: 10.1084/jem.157.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Gilladoga A. D. Molecular studies related to the pathogenesis of cerebral malaria. Blood. 1989 Dec;74(8):2603–2618. [PubMed] [Google Scholar]
- Kaul D. K., Roth E. F., Jr, Nagel R. L., Howard R. J., Handunnetti S. M. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood. 1991 Aug 1;78(3):812–819. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Miller L. H., Howard R. J. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984 Jun 1;159(6):1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- Marsh K., Howard R. J. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986 Jan 10;231(4734):150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- Mendis K. N., David P. H., Hommel M., Carter R., Miller L. H. Immunity to malarial antigens on the surface of Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 1983 Sep;32(5):926–930. doi: 10.4269/ajtmh.1983.32.926. [DOI] [PubMed] [Google Scholar]
- Miller L. H. Distribution of mature trophozoites and schizonts of Plasmodium falciparum in the organs of Aotus trivirgatus, the night monkey. Am J Trop Med Hyg. 1969 Nov;18(6):860–865. doi: 10.4269/ajtmh.1969.18.860. [DOI] [PubMed] [Google Scholar]
- Miller L. H. The ultrastructure of red cells infected by Plasmodium falciparum in man. Trans R Soc Trop Med Hyg. 1972;66(3):459–462. doi: 10.1016/0035-9203(72)90277-5. [DOI] [PubMed] [Google Scholar]
- Oo M. M., Aikawa M., Than T., Aye T. M., Myint P. T., Igarashi I., Schoene W. C. Human cerebral malaria: a pathological study. J Neuropathol Exp Neurol. 1987 Mar;46(2):223–231. doi: 10.1097/00005072-198703000-00009. [DOI] [PubMed] [Google Scholar]
- Raventos-Suarez C., Kaul D. K., Macaluso F., Nagel R. L. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3829–3833. doi: 10.1073/pnas.82.11.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riganti M., Pongponratn E., Tegoshi T., Looareesuwan S., Punpoowong B., Aikawa M. Human cerebral malaria in Thailand: a clinico-pathological correlation. Immunol Lett. 1990 Aug;25(1-3):199–205. doi: 10.1016/0165-2478(90)90115-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Parra M., Chapman G. B., Stearns M. E., Rener J., Aikawa M., Uni S., Aley S. B., Panton L. J., Howard R. J. Localization of Plasmodium falciparum histidine-rich protein 1 in the erythrocyte skeleton under knobs. Mol Biochem Parasitol. 1987 Sep;25(2):165–174. doi: 10.1016/0166-6851(87)90005-3. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Treutiger C. J., Hedlund I., Helmby H., Carlson J., Jepson A., Twumasi P., Kwiatkowski D., Greenwood B. M., Wahlgren M. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992 May;46(5):503–510. doi: 10.4269/ajtmh.1992.46.503. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Miller L. H., McGregor I. A., Jensen J. B. Plasmodium falciparum strain-specific antibody blocks binding of infected erythrocytes to amelanotic melanoma cells. Nature. 1983 Jun 2;303(5916):429–431. doi: 10.1038/303429a0. [DOI] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R., Wåhlin B., Carlson J., Berzins K., Torii M., Aikawa M., Perlmann P., Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989 May 1;169(5):1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd E., Greenan J. R., Sherman I. W. Expression of senescent antigen on erythrocytes infected with a knobby variant of the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1931–1935. doi: 10.1073/pnas.84.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]