Abstract
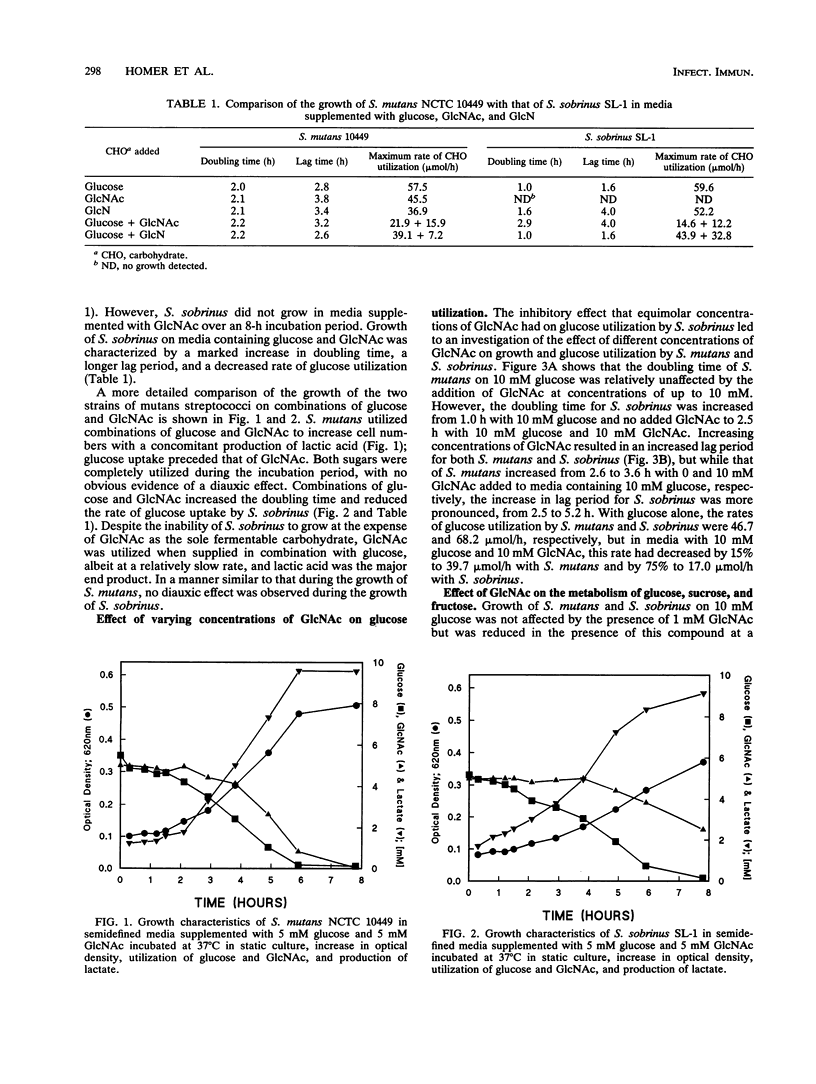
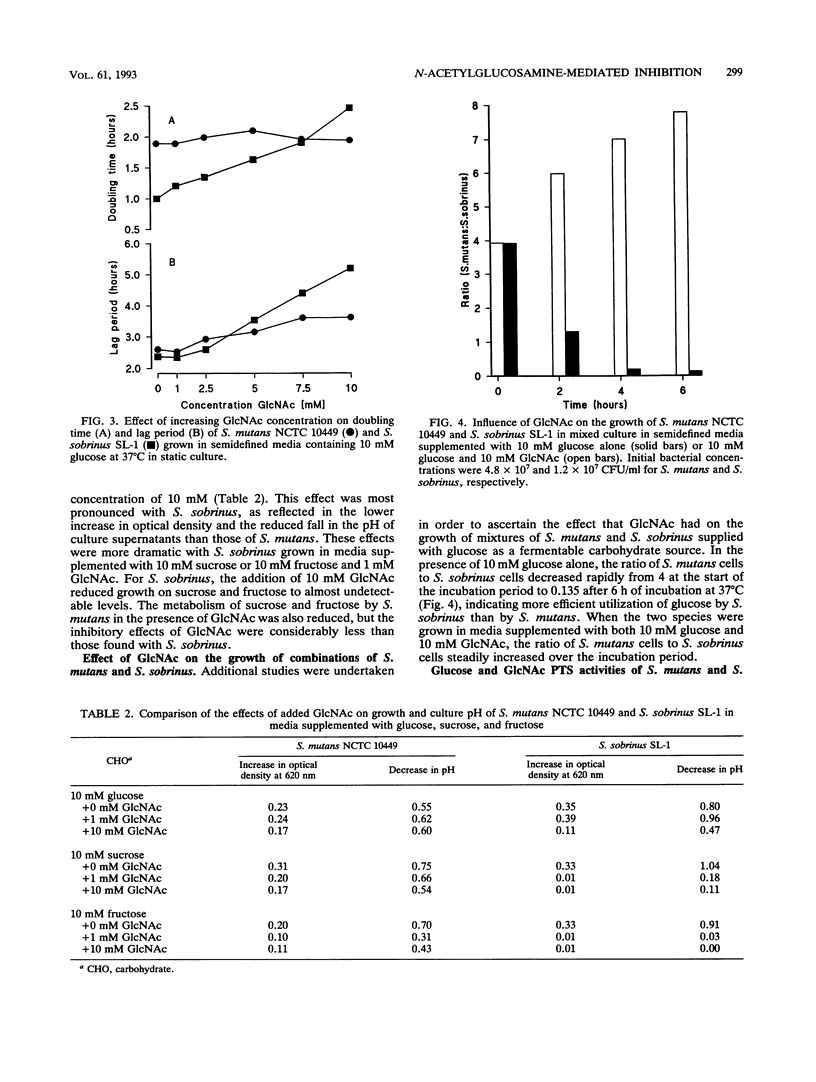
We have investigated the ability of two species of streptococci isolated from the human oral cavity (Streptococcus mutans NCTC 10449 and Streptococcus sobrinus SL-1) to metabolize N-acetylglucosamine (GlcNAc), a naturally occurring amino sugar present in saliva and human glycoproteins, when provided as the sole fermentable carbohydrate and determined the effects of the presence of GlcNAc on the fermentation of other carbohydrates. S. mutans used GlcNAc at concentrations of up to 10 mM to increase cell numbers, but S. sobrinus was unable to ferment the amino sugar alone and its uptake only occurred in the presence of a fermentable carbohydrate. GlcNAc had a marked inhibitory effect on the ability of S. sobrinus to produce lactic acid from glucose, sucrose, and fructose, at the same time increasing the lag period and doubling time of batch-grown cells. Such patterns of inhibition were found with S. mutans, but the effects were less than those seen in S. sobrinus. In mixed culture studies of the two species, S. sobrinus became the predominant organism when 10 mM glucose was supplied as the sole fermentable carbohydrate, with a concomitant decrease in the numbers of S. mutans cells, but supplementation of the broth with 10 mM glucose and 10 mM GlcNAc resulted in the emergence of S. mutans as the predominant organism. S. mutans and S. sobrinus grown in media containing glucose possessed the ability to transport glucose and GlcNAc, probably via the same glucose-phosphotransferase system at similar rates. However, intracellular levels of N-acetylglucosamine-6-phosphate deacetylase and glucosamine-6-phosphate deaminase were markedly higher in S. mutans grown on glucose and GlcNAc than in S. sobrinus: 34 and 398 and 8 and 17 nmol of NADPH formed per mi per mg of protein for S. mutans and S. sobrinus, respectively. We propose that GlcNAc inhibited growth of S. sobrinus in media containing glucose and GlcNAc by competing with glucose for the glucose phosphotransferase, depleting intracellular levels of phosphoenolpyruvate, and possessing, in contrast to S. mutans, low levels of N-acetyl-glucosamine-6-phosphate deacetylase and glucosamine-6-phosphate deaminase activity. Together, these data suggest that in dental plaque, S. sobrinus when exposed to GlcNAc will have a reduced ability to compete with S. mutans for dietary carbohydrates, contributing to the greater frequency of isolation of S. mutans from human populations.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arglebe C. Biochemistry of human saliva. Adv Otorhinolaryngol. 1981;26:97–234. doi: 10.1159/000395291. [DOI] [PubMed] [Google Scholar]
- BATES C. J., PASTERNAK C. A. FURTHER STUDIES ON THE REGULATION OF AMINO SUGAR METABOLISM IN BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:147–154. doi: 10.1042/bj0960147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beighton D., Hardie J. M., Whiley R. A. A scheme for the identification of viridans streptococci. J Med Microbiol. 1991 Dec;35(6):367–372. doi: 10.1099/00222615-35-6-367. [DOI] [PubMed] [Google Scholar]
- Beighton D., Hayday H. The effects of fluoride on the growth of oral streptococci. Microbios. 1980;27(108):117–124. [PubMed] [Google Scholar]
- Beighton D., Russell R. R., Whiley R. A. A simple biochemical scheme for the differentiation of Streptococcus mutans and Streptococcus sobrinus. Caries Res. 1991;25(3):174–178. doi: 10.1159/000261363. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Curtis M. A., Griffiths G. S., Price S. J., Coulthurst S. K., Johnson N. W. The total protein concentration of gingival crevicular fluid. Variation with sampling time and gingival inflammation. J Clin Periodontol. 1988 Nov;15(10):628–632. doi: 10.1111/j.1600-051x.1988.tb02263.x. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd 1984 Kreshover lecture. Genetic analysis of Streptococcus mutans virulence and prospects for an anticaries vaccine. J Dent Res. 1986 Aug;65(8):1034–1045. doi: 10.1177/00220345860650080101. [DOI] [PubMed] [Google Scholar]
- Emilson C. G., Carlsson P., Bratthall D. Strains of mutans streptococci isolated in a population with extremely low caries prevalence are cariogenic in the hamster model. Oral Microbiol Immunol. 1987 Dec;2(4):183–186. doi: 10.1111/j.1399-302x.1987.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper D. S., Loesche W. J. Effect of pH upon sucrose and glucose catabolism by the various genogroups of Streptococcus mutans. J Dent Res. 1983 May;62(5):526–531. doi: 10.1177/00220345830620050101. [DOI] [PubMed] [Google Scholar]
- Kral T. A., Daneo-Moore L. Biochemical differentiation of certain oral streptococci. J Dent Res. 1981 Sep;60(9):1713–1718. doi: 10.1177/00220345810600091301. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist B., Emilson C. G. Interactions between and within Streptococcus mutans and Streptococcus sobrinus isolated from humans harboring both species. Scand J Dent Res. 1991 Dec;99(6):498–504. doi: 10.1111/j.1600-0722.1991.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A. Sequence of the nagBACD operon in Escherichia coli K12 and pattern of transcription within the nag regulon. Mol Microbiol. 1989 Apr;3(4):505–515. doi: 10.1111/j.1365-2958.1989.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Phosphoenolpyruvate-dependent sucrose phosphotransferase activity in Streptococcus mutans NCTC 10449. Infect Immun. 1979 Jun;24(3):821–828. doi: 10.1128/iai.24.3.821-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Enzymatic characterization of some oral and nonoral gram-negative bacteria with the API ZYM system. J Clin Microbiol. 1981 Sep;14(3):288–294. doi: 10.1128/jcm.14.3.288-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A. C., Strzempko M. N., Belsky C. A., McKinley G. A. API ZYM and API An-Ident reactions of fastidious oral gram-negative species. J Clin Microbiol. 1985 Sep;22(3):333–335. doi: 10.1128/jcm.22.3.333-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem J. 1968 Feb;106(4):847–858. doi: 10.1042/bj1060847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Pasternak C. A. The purification and properties of N-acetylglucosamine 6-phosphate deacetylase from Escherichia coli. Biochem J. 1967 Oct;105(1):121–125. doi: 10.1042/bj1050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Soet J. J., Toors F. A., de Graaff J. Acidogenesis by oral streptococci at different pH values. Caries Res. 1989;23(1):14–17. doi: 10.1159/000261148. [DOI] [PubMed] [Google Scholar]
- de Stoppelaar J. D., van Houte J., de Moor C. E. The presence of dextran-forming bacteria, resembling Streptococcus bovis and Streptococcus anguis, in human dental plaque. Arch Oral Biol. 1967 Oct;12(10):1199–1202. doi: 10.1016/0003-9969(67)90069-6. [DOI] [PubMed] [Google Scholar]
- van Houte J., Russo J. Effect of oral nutrient limitation of gnotobiotic rats on acidogenic properties of dental plaque formed by oral streptococci. J Dent Res. 1985 May;64(5):815–817. doi: 10.1177/00220345850640050601. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., van Steenbergen T. J., Kippuw N., de Graaff J. Enzymatic characterization of oral and non-oral black-pigmented Bacteroides species. Antonie Van Leeuwenhoek. 1986;52(2):163–171. doi: 10.1007/BF00429320. [DOI] [PubMed] [Google Scholar]
- van der Hoeven J. S., van den Kieboom C. W., Camp P. J. Utilization of mucin by oral Streptococcus species. Antonie Van Leeuwenhoek. 1990 Apr;57(3):165–172. doi: 10.1007/BF00403951. [DOI] [PubMed] [Google Scholar]


