Abstract
Heterodimerization of G-protein coupled receptors has become increasingly recognized as a valuable mechanism to increase receptor diversity. Heterodimers have been observed in the opioid receptor family, but one of the most intriguing is that formed between mu and delta opioid receptors. In this issue of Neuron He et al. present evidence further implicating these heterodimers in morphine tolerance.
Understanding opioid tolerance has long been a goal in the opioid field. Recent years have revealed many new and exciting observations regarding the underlying the processes. These involve many different and unrelated mechanisms, making the integration of these pathways very difficult. Opioid tolerance is the diminished response seen with chronic administration of a drug, or put another way, the need to progressively increase drug doses to maintain a response. Tolerance is the final common pathway for a wide range of divergent mechanisms, much like a tug of war with many different people pulling on the same rope. Each is contributing to the final effort and the loss of any one of them can have a similar effect. In the current issue, He and colleagues (He et al., 2011) describe results that support the concept that one aspect of tolerance is mediated through mu/delta heterodimers and present a mechanism explaining the ability of delta receptor antagonists to prevent tolerance to morphine.
Morphine tolerance involves many distinct systems and can be influenced in many ways. The first was put forward by Collier, who proposed what he referred to as an “hypertrophy of the cyclic AMP system” (Collier, 1980). This was followed by the identification of the role of other neurotransmitter systems, as illustrated by the loss of morphine tolerance with blockade of the NMDA receptor/nitric oxide cascade. Many classes of NMDA receptor antagonists can effectively prevent or reverse morphine tolerance (Trujillo and Akil, 1991), as can inhibition of nitric oxide synthase (Kolesnikov et al., 1997). The importance of dispositional issues was established by studies on P-glycoprotein (King et al., 2001). Chronic administration of morphine upregulates of P-glycoprotein, which in turn decreases morphine penetration into the brain. Knocking out Pgp prevents morphine tolerance. Most recently, investigators have explored receptor trafficking (Von Zastrow, 2010) and suggested a role for mu/delta receptor heterodimers (Gupta et al., 2010). These various different mechanisms are not exclusive and all likely contribute to the overall response.
The role of delta systems in morphine tolerance was first proposed by Takemori and co-workers who showed that the delta receptor antagonist naltrindole prevents morphine tolerance (Abdelhamid et al., 1991). The importance of delta receptors was confirmed by studies in delta receptor knockout mice and antisense downregulation models which also revealed the loss of morphine tolerance. In the current paper by He and co-workers (He et al., 2011), the authors find that spinal delivery of the delta ligand deltorphin I diminished morphine actions, consistent with an inhibitory modulation of morphine analgesia. The opioid field has long had controversies and data that appeared contradictory and the role of delta systems in morphine action is no exception. Soon after their discovery, enkephalins, endogenous delta receptor ligands, were shown to be potent analgesics given either spinally or supraspinally. Furthermore, Porreca and co-workers demonstrated that delta ligands given supraspinally, but not spinally, potentiated morphine analgesia in naive and tolerant mice (Porreca et al., 1987). Thus, delta drugs can both potentiate and diminish morphine analgesia. A number of potential explanations for these conflicting results are possible, including the site of action (i.e. spinal versus supraspinal), since potentiation was previously seen only supraspinally while the decreased effect in the current paper was documented at the spinal level. However, it clearly shows the complexity of opioid systems and the need to reconcile a range of findings.
How delta receptors might influence morphine tolerance has been debated: Is the effect mediated through independent, but interacting, neuronal circuits or by a direct molecular interaction between the receptors? The possibility of a direct interaction arose with the demonstration by heterodimerization of mu and delta receptors and the demonstration that chronic morphine administration upregulates these heterodimers (Gupta et al., 2010). In the current issue, He and colleagues extends these findings (He et al., 2011) , building upon a strong foundation of work on opioid receptor dimerization and trafficking (Gupta et al., 2010;van Rijn et al., 2010;Von Zastrow, 2010).
A role of mu/delta heterodimers in modulating morphine actions requires their co-expression in a single cell, a concept that is controversial. It had long been accepted that mu and delta receptors are co-expressed in small DRG neurons, but recent work documenting the limited selectivity of many of the earlier antisera used to map delta receptors and the inability to observe a fluorescent-tagged delta receptor in the small dorsal root ganglia neurons containing the mu opioid receptor MOR-1 raised important questions about this concept. With these results, the question was recently revisited and evidence presented to support their co-expression in these neurons (Wang et al., 2010). This work is further buttressed by additional studies in the current paper. However, we are still left with the question of why the GFP-tagged DOR-1 was not visualized in these neurons.
He and co-workers further propose that activation of delta receptors within the mu/delta heterodimer leads to the degradation of the mu receptors and a diminished response (He et al., 2011), as opposed to the recycling normally seen (Von Zastrow, 2010). In the paper, they presented strong evidence for the existence of the heterodimers and the trafficking, both 6 in cell lines and in tissue. However, they looked only at MOR-1 itself. MOR-1 gene undergoes extensive alternative splicing, with over two dozen splice variants identified in mice (Pan and Pasternak, 2011). It is not yet clear whether all these variants form heterodimers with delta receptors and, if so, whether their trafficking mimics that of MOR-1. Indeed, evidence has been presented that alternative splicing can markedly impact trafficking patterns (Tanowitz et al., 2008). Clearly, these issues need further investigation in the future.
The major novelty of the paper comes from their work with MORTM1-TAT, which corresponds to the first transmembrane domain of MOR-1. Their ability to use the TAT domain to insert the peptide into the membrane in the correct orientation where it can interrupt the dimerization process is particularly innovative. Here, they observe that systemic administration of the MORTM1-TAT led to its presence within the neurons of the DRG and dorsal horn of the spinal cord. This is quite surprising in view of the general difficulties peptides have traversing the blood-brain barrier. Its presence in the spinal cord, however, raises the question of whether it also is present within the brain and whether it may be active there as well.
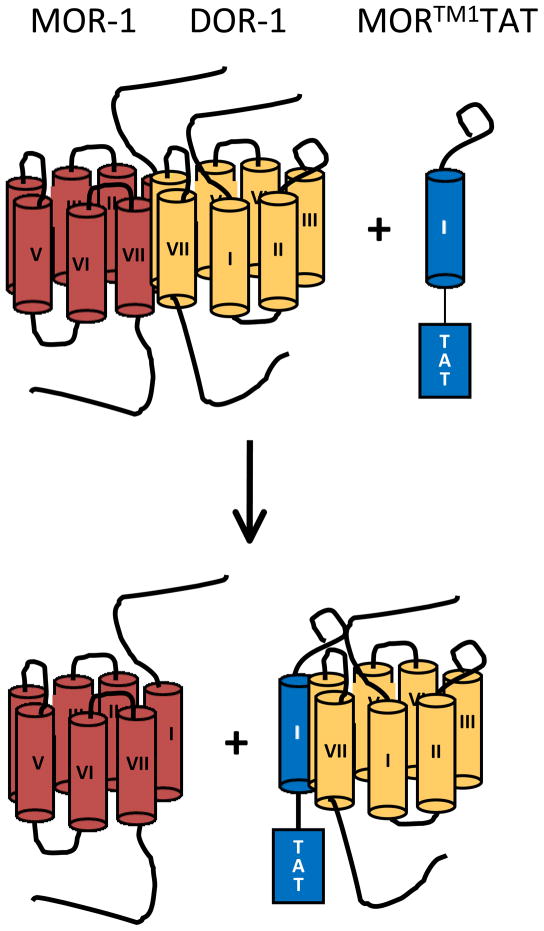
Administration of MORTM1-TAT disrupts the mu/delta heterodimers (Fig 1), but not MOR-1 heterodimers containing α2A or NK-1 receptors. This implies a specific site of interaction between the delta and mu receptors involving TM1 of MOR-1, but not others. When administered systemically to naive animals, MORTM1-TAT increased the response of morphine given systemically and blocked the development of tolerance. The results are quite dramatic and consistent with their hypothesis. However, a number of questions remain. First is the question of the site of action of MORTM1-TAT protein. While the authors provide evidence for activity at the spinal level, it is equally possible that the responses might involve supraspinal 7 heterodimers. Indeed, supraspinal sites are more sensitive to systemic morphine than spinal ones, as shown by the decreased potency of morphine following spinal transaction in the tailflick assay. A more basic question is whether MORTM1-TAT might alter other types of associations as well. The authors examined α2 and NK-1 receptors, but MOR-1 will dimerize with additional receptors, such as ORL1, and even the other MOR-1 splice variants.
Figure 1.
Schematic on the interaction of the engineered MORTM1-TAT with mu/delta receptor heterodimers
In this schematic, morphine (MOR-1; red) and delta (DOR-1; orange) form heterodimers. Addition of the engineered MORTM1-TAT, which includes the sequence of the first transmembrane domain of MOR-1, leads to the disruption of the heterodimer.
The activity of the single MORTM1-TAT also raises a very interesting question. At least three alternatively spliced MOR-1 variants generate truncated proteins corresponding to the first transmembrane domain of MOR-1 (Du et al., 1997;Pan and Pasternak, 2011), a structure very similar to MORTM1-TAT . At least one of the single TM variants has mRNA levels similar to those of MOR-1 itself, implying a relatively high level of expression. Are the actions of the engineered MORTM1-TAT providing insights into the actions of the endogenous single TM variants? While the evidence is strong that MORTM1-TAT can disrupt the mu/delta heterodimer, might it also have activity by blocking or mimicking the naturally occurring TM1 variants?
The current article by He and colleagues (He et al., 2011) presents an intriguing hypothesis on the modulation of morphine analgesia by heterodimerization of delta receptors with mu receptors. It pulls together and confirms prior observations and extends them to provide an explanation for how delta receptors modulate morphine actions. It represents a significant step forward in our understanding of the basic mechanisms underlying various aspects of opioid tolerance. Like most good science, it also raises a number of issues that need to be addressed in the future. Some of these involve MOR-1 splice variants, both the full length ones which can potentially dimerize with delta receptors and the truncated single TM ones which may have actions similar to those seen with MORTM1-TAT. However, it is important to remember that tolerance is like a tug of war, with heterodimerization representing only a single person pulling on the rope.
Acknowledgments
We would like to acknowledge support from grants from the National Institute on Drug Abuse to GWP (DA02615, DA06241, DA7242) and to YXP (DA013997), grants from the National Genetics Foundation and the Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center to GWP and a core grant from the National Cancer Institute to MSKCC (CA08748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- Collier HO. Cellular site of opiate dependence. Nature. 1980;283:625–629. doi: 10.1038/283625a0. [DOI] [PubMed] [Google Scholar]
- Du YL, Elliot K, Pan YX, Pasternak GW, Inturrisi CE. A splice variant of the mu opioid receptor is present in human SHSY-5Y cells. Soc Neurosci. 1997;23:1206. [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, Lim M, Maillet E, Junek M, Cahill CM, Harkany T, Devi LA. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S-Q, Zhang A-N, Guan J-S, Liu H-R, Zhao B, Want H-B, Li Q, Yang H, Luo J, Li Z-L, Wang Q, Lu YF, Bao L, Zhang X. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated co-degradation. Neuron. 2011 doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci. 2001;4:268–274. doi: 10.1038/85115. [DOI] [PubMed] [Google Scholar]
- Kolesnikov YA, Pan YX, Babey AM, Jain S, Wilson R, Pasternak GW. Functionally differentiating two neuronal nitric oxide synthase isoforms through antisense mapping: Evidence for opposing NO actions on morphine analgesia and tolerance. Proc Natl Acad Sci USA. 1997;94:8220–8225. doi: 10.1073/pnas.94.15.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y-X, Pasternak GW. Molecular Biology of Mu Opioid Receptors. In: Pasternak GW, editor. The Opiate Receptors. New York: Springer; 2011. pp. 121–160. [Google Scholar]
- Porreca F, Heyman JS, Mosberg HI, Omnaas FR, Vaught JL. Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse. J Pharmacol Exp Ther. 1987;241:393–398. [PubMed] [Google Scholar]
- Tanowitz M, Hislop JN, von ZM. Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptors. J Biol Chem. 2008;283:35614–35621. doi: 10.1074/jbc.M806588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor anagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol. 2010;10:73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Zastrow M. Regulation of opioid receptors by endocytic membrane traffic: mechanisms and translational implications. Drug Alcohol Depend. 2010;108:166–171. doi: 10.1016/j.drugalcdep.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, Hokfelt TG, Bao L, Zhang X. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]