Abstract
Objective
The aim of this study was to determine the rate of infection at which it is cost-effective to treat dog bite wounds with antibiotics.
Methods
Our study was composed of two parts. First we performed a randomized, double-blind controlled trial (RCT) to compare the infection rates of dog bite wounds in patients given amoxicillin-clavulanic acid versus placebo. Subjects were immunocompetent patients presenting to the emergency department (ED) with dog bite wounds less than 12 hours old without suspected neurovascular, tendon, joint or bone injury, and who had structured follow-up after two weeks. Second, we developed a cost model with sensitivity analysis to determine thresholds for treatment.
Results
In the RCT, primary outcomes were obtained in 94 patients with dog bites. The overall wound infection rate at two weeks was 2% [95% CI 0 to 7%]. Two of 46 patients (4%) receiving no antibiotics developed infections, while none of the 48 patients (0%) receiving prophylactic antibiotics developed an infection (absolute reduction 4% [95% CI −1.0 to 4.5%]). Using a sensitivity analysis across a rate of infections from 0–10%, our cost model determined that prophylactic antibiotics were cost effective if the risk of wound infection was greater than 5% and antibiotics could decrease that risk by greater than 3%.
Conclusion
Our wound infection rate was lower than older studies and more in line with current estimates. Assuming that prophylactic antibiotics could provide an absolute risk reduction (ARR) of 3%, it would not be cost effective to treat wounds with an infection rate of less than 3% and unlikely that the ARR would be achievable unless the baseline rate was greater than 5%, suggesting that only wounds with greater than 5% risk of infection should be treated. Future work should focus on identifying wounds at high-risk of infection that would benefit from antibiotic prophylaxis.
INTRODUCTION
Background
Dog bites are a common public health problem, and nearly five million people are bitten each year in the United States.1 Although only one-fifth of bite victims seek medical care, these patients account for nearly one thousand daily emergency department (ED) visits in this country.1,2
The primary morbidity associated with dog bites is their infection rate, which is generally higher than normal wounds.3–7 However, it is unclear whether bite wounds should be treated with prophylactic antibiotics. There are numerous contradictory studies regarding the exact incidence and true risk of infection. A meta-analysis of prior studies recommended the administration of prophylactic antibiotics, yet this study had several significant limitations.5 For example, studies included in the meta-analysis had small sample sizes, different methodologies, lacked a standardized definition for wound infection, used a variety of antibiotics (often those to which the most common organisms from bites are not susceptible), and contained large range of infection rates in the control groups (from 3.2% to 45.8%).3,5,8–9 Conversely, a more recent Cochrane review9 of the same randomized controlled trials concluded there was no strong evidence for treating wounds with prophylactic antibiotics. The routine use of antimicrobials for such a common but controversial indication is concerning given the risks of medication side effects, increased resistance, and cost of approximately $30 million dollars per year.10
Goals of this investigation
The first objective was to determine the infection rate at which antibiotics are clinically warranted and cost-effective using a cost model and sensitivity analysis based on existing data from the literature and known costs associated with various treatments. The second objective was to estimate current rates of infection and verify previously published infection rates used in our model by conducting a randomized controlled trial of dog bite wounds treated with and without oral prophylactic antibiotics.
METHODS
Decision Tree Cost Model
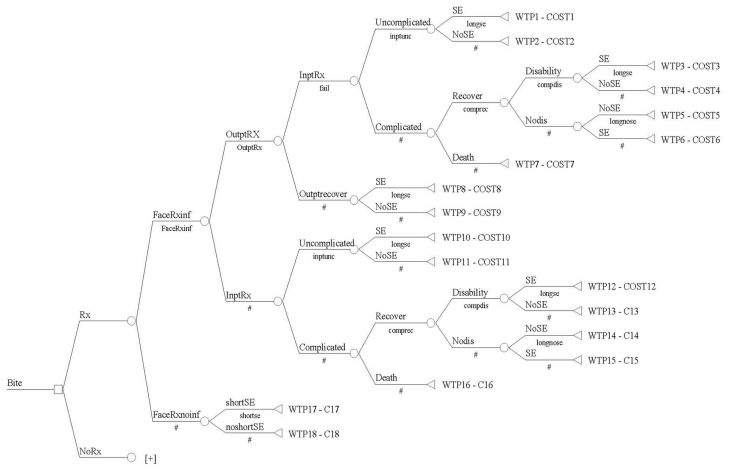
A cost decision tree model based on all clinical outcomes and scenarios was developed using TreeAge Data 4.0 (Figure 1). This tree model mapped out all inpatient and outpatient treatment options, outcomes and side effects for patients sustaining dog bite wounds. The following pathways and assumptions were made: 1) patients would have the same chance of being treated as an outpatient or an inpatient once diagnosed with an infection regardless of whether they were on prophylactic antibiotics; 2) wound infections in patients on prophylactic antibiotics did not alter the chance of outpatient recovery, inpatient recovery, inpatient complication rates, chance of death, chance of disability or side effects; and 3) subsequent outpatient care and follow-up was included in the cost of inpatient care given the high cost of inpatient care and the low cost and variability of subsequent outpatient follow-up. Table 1 lists the estimates made regarding effectiveness and clinical outcomes in the cost model. It also outlines the source/reason for these estimates as well as the frequencies and ranges tested in the analysis. The legal costs resulting from liability of untreated wounds that become infected were omitted from this analysis since wound infection is an expected complication and the treatment unproven. Of note, the highest liability from wound treatment is missed foreign body.11
Figure 1.
Cost model for the use of prophylactic antibiotics for dog bites
Inpt, Inpatient; Inf, infection; Outpt, Outpatient; Rx, perscription
Table 1.
Estimates of antibiotic effectiveness and clinical outcomes in the cost model
| Variable | Estimate | Evidence | Range Analyzed | Source |
|---|---|---|---|---|
| Baseline infection rate facial bites (FaceNoRxInf) | 10% | Retrospective review | 5% – 10% | 1[3] [6] |
| Infection Rate - prophylactic antibiotics – facial bites (FaceRxInf) | 5.6% (95% CI 3.8 – 8.2%) | Meta-analysis of RCT | 3.2% – 10% | [5] |
| Baseline infection rate hand bites (HandNoRxInf) | 36% | Retrospective Review | 10% – 50% | [3] [6] |
| Infection rate - prophylactic antibiotics – hand bites (HandRxInf) | 8.3% (95% CI 1.8 – 34.2%) | Meta-analysis of RCT | 18% – 34.2% | [5] |
| Baseline infection rate other area (extremity and trunk) (OtherNoRxInf) | 17% | Retrospective Review | 10% – 30% | [3] [6] |
| Infection rate - prophylactic antibiotics – other areas | 9.5% (95% CI 6.5% – 13.95) | Meta-analysis of RCT | 6.5% – 14% | [5] |
| % Facial bites | 50% | Survey estimates | N/A | [2] |
| % Hand bites | 30% | Survey estimates | N/A | [2] |
| % Other | 20% | Survey estimates | N/A | [2] |
| % of infected wounds considered for outpt Rx (OutPtRx) | 90% | Physician Opinion | 50% – 90% | |
| Failure rate of outpt antibiotics (Fail) | 5 % – 15% | RCT for outpt treatment of cellulites | 10% – 30% | [17], [18] |
| Risk of complicated inpt course (1-Inptunc) | 1– 4% | Retrospective review | 2% – 10% | [19] |
| Risk of disability after hand infection/surgery (CompDis) | 5% | Retrospective review | 1% – 10% | [20] |
| Risk of death w/ complicated infection (1-CompRec) | 3% | Survey Estimate | 2% – 3% | [2] |
| Antibiotic side effects (ShortSE; LongSE) | 5% – 34% | RCT | 5% – 34% | [17], [18] |
Inpt, Inpatient; Inf, infection; Outpt, Outpatient; RCT, randomized, double-blinded control trial; Rx, perscription
The costs of cellulitis, septicemia, scar revision and incision and drainage of the skin were gathered from the Healthcare Costs and Utilization Project 2003 Nationwide Inpatient Sample and the costs of Outpatient Visit level 3 on the 2005 Medicare Fee Schedule.12 These were then adjusted for inflation to 2006. The average price of amoxicillin-clavulanic acid is $5 a pill (range $2–7)13 The costs of these items are detailed in Table 2.
Table 2.
Cost data used in model
| HCUP Inpatient Charge | 2003 Charge (Dollar) | |
|---|---|---|
| Diagnosis | ||
| 682.0 | Cellulitis of face | 10,644.00 |
| 682.4 | Cellulitis of hand | 12,252.00 |
| 682.9 | Cellulitis, other | 14,837.00 |
| 038.9 | Septicemia | 29,654.00 |
| Procedure | ||
| 86.04 | Other Skin & Subq Incision and Drainage | 16,648.00 |
| 86.89 | Skin Repair & Plastic Reconstruction | 25,868.00 |
| Medicare Charge | 2005 Charge (Dollars) | |
| Outpatient Visit- Level 3 | 1,143.00 |
HCUP, Healthcare Costs and Utilization Project
Sensitivity Analysis
The sensitivity analysis was performed using this decision tree. It calculated and compared the baseline treatment costs of all dog bite wound infections with the collective treatment costs of administering prophylactic antibiotics and treating the non-preventable infections. This calculation was run over varying baseline infection rates and percentage of wound complications prevented. Costs were based on the rate of each infection and the cost of each intervention needed to treat the infection. The analysis assumed that a broad-spectrum antibiotic such as amoxicillin-clavulanic acid would be used for three days as the method of prophylactic treatment. All clinical outcomes were considered to their completion, and it was assumed that all costs and benefits occurred within one year of the bite and thus were not subject to discounting.
Randomized controlled study design
A randomized, double-blind controlled trial was conducted to compare the outcomes of dog bite wounds in patients given three days of prophylactic amoxicillin-clavulanic acid versus placebo. The study was approved by both the University of California San Francisco (UCSF) and Stanford University Institutional Review Boards.
Study Setting and Population
This study was conducted at the ED of the UCSF and Stanford University Medical Center (SUMC), with a combined annual census of 75, 000 visits per year. The study periods were from August 2003 – March 2006 (UCSF) and October 2004 – March 2006 (SUMC).
All dog bites, regardless of site were considered for enrollment. Patients were excluded if they met any of these criteria: 1) wounds over 12 hours old at presentation or already infected; 2) patients with immunosuppression; 3) patients with a penicillin allergy; and 4) wounds with suspected neurovascular, tendon, joint or bone injury.
Randomized controlled study protocol
In order to consider all eligible wounds and maximize patient recruitment, patients with dog bites were identified through a real time notification and tracking system of all ED patients, previously described.14 The notification system screened all chief complaints on a tracking system 24/7, and the research coordinator was paged when a patient registered with “dog” or “bite” in the complaint. The coordinator then called to verify it was a dog bite and request patient enrollment. The emergency medicine physician obtained informed consent from the patient and enrolled the patient into a secure web-based system. The requested demographic, insurance, and visit data for this study was automatically sent to the research database and the appropriate fields populated. Once the consent was printed, signed and verified, the form randomized the patient to a treatment group based on patient weight via a computerized randomization code based in blocks of eight, and stratified according to weight to include children. Three-day courses of blinded medication was prepared by pharmacy and distributed to the patient in the ED with the first medication dose given in the ED. The physician completed the structured web-based data entry form. Printed standardized custom discharge sheets with instructions for taking medications, signs and symptoms of infection and when to follow-up were provided to every patient.
Outcome Measures
Patients were to call investigators or return to the ED if signs of infection developed. All patients underwent structured phone follow-up after 14 days during which they were queried as to: 1) if they had developed signs of infection (redness or discharge) and 2) whether any practitioner treated the wound with antibiotics for wound infection. This determination of infection and treatment was confirmed with the treating physician and was used as the primary outcome for the study for several reasons. The physicians making those determinations were blind to randomization and initial treatment, and in our cost model treatment decisions are what drive costs. Patient opinion as to whether they thought the wound was infected was not considered an outcome if it healed without any further treatment.
Data Analysis
Demographic and clinical characteristics in the randomized, double-blind controlled trial (RCT) were determined by t-tests, chi-square and Fisher’s exact tests as appropriate. The RCT analyses were conducted using SPSS 11.0 (Chicago, Ill). The cost model and related sensitivity analysis was done in TreeAge Software 4.0 (Williamstown, Mass).
RESULTS
Study subjects
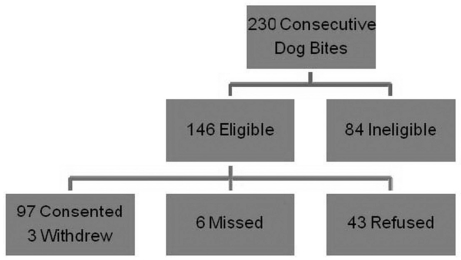
Figure 2 is a CONSORT diagram of patient enrollment. A total of 230 consecutive patients with dog bites presented during the study period. Of these, 84 patients (36.5%) were ineligible due to the exclusion criteria outlined above. Out of the remaining 146 (63.5%), six were missed and 43 refused enrollment. Ninety-seven patients (42% overall) were consented for the study and three subsequently withdrew or were not available for follow-up (Figure 2).
Figure 2.
CONSORT diagram for the randomized control trial on the use of prophylactic antibiotics for dog bites
Results of Randomized Controlled Trial
Table 3 compares the patient demographic and wound characteristics of those that received prophylactic amoxicillin-clavulanic acid with those that received a placebo. The patients were of similar age and gender. The wounds of the two groups were similar with regard to length and time from injury, though the placebo group tended to have a greater but non-significant proportion of full thickness, sutured and facial wounds (Table 3). None of the 48 wounds treated with antibiotics became infected, whereas two of the 46 wounds in the placebo group became infected for a difference of 4% (95% CI 4%–14%) between the two groups. The overall infection rate was 2% (95% CI 0–7%) and both infected wounds occurring in the placebo group were sutured, including one on the face and the other on the neck. Both were diagnosed two days after enrollment.
Table 3.
Characteristics and outcomes of study patients in the randomized, double-blind controlled trial
| Antibiotics N=48 | Placebo N=46 | Difference(95% CI) | |
|---|---|---|---|
| Age (years) | 34 | 31 | 3 (−5 – 11) |
| % Male | 56 | 61 | 5 (−2 – 2) |
| Length (centimeter) | 2.1 | 1.6 | 0.5 (−0.2 – 1.2) |
| Time from Injury (minutes) | 176 | 188 | 12 (−109 – 84) |
| % Full thickness (through dermis) | 56 | 66 | 10 (−3 – 10) |
| % Non-facial | 81 | 64 | 17 (−0.6 – 33) |
| % Closed | 17 | 32 | 15 (−2 – 30) |
| % Infected | 0 | 4 | 4 (−1 – 4.5) |
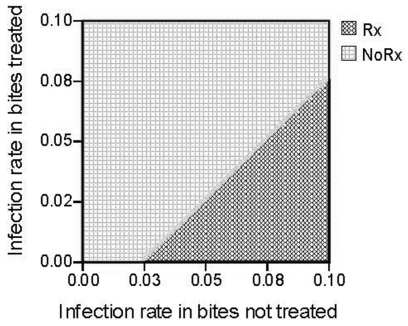
Results of the Sensitivity Analysis
Results of the sensitivity analysis are summarized in Figure 3. This graph shows the cost effectiveness of antibiotics at each baseline rate of infection from 0 to 10% given the corresponding rate of infection in the treated group. The diagonal line is the threshold for which it becomes beneficial to treat with prophylactic antibiotics. The cost model recommends treating wounds at greater than 5% risk of infection (even if they had only a small benefit of about 1–2%). It is likely not cost effective to treat wounds with less than 5% risk of infection and never at levels below 3%.
Figure 3.
Sensitivity analysis of cost analysis comparing benefits of prophylactic antibiotics (Rx) at different infection rates
LIMITATIONS
The high number of eligible patients who refused to participate and the fact we excluded wounds with suspected tendon injuries or fractures could have affected the low number of infections we found in our study. However we did include certain high-risk wounds such as those that were punctures or on extremities.4 There is also no clear validated outcome measure to determine traumatic wound infection. Failure to use a consistent validated outcome measure has limited previous studies and corresponding systematic reviews. The Centers for Disease Control (CDC) has a set of clinical and lab criteria to try to standardize the reporting of surgical site infections. This criterion includes the treating physician’s assessment.15 It was impractical for us to get all patients follow-up so that we could apply the objective CDC criterion, but for our study we did use the determination of the treating physician since it was a blinded assessment and also fit the outcome in our cost model.
Cost models are based on assumptions and tested across a range of sensitivity analysis as outlined in table 1. In the end, the assumptions need to make clinical sense and are estimated based on available data and tested in a sensitivity analysis (Figure 3). For example, we surmised that 90% of infections would initially get treated as outpatients after looking at published data and our institutional numbers.10 It may be possible that more than 10% would get admitted for an infection, and if the number was higher the threshold to treat with prophylactic antibiotics would be higher (i.e., more money could be saved by preventing both infections and the increased costs of hospital admission).
Finally, while our clinical trial is small and not sufficiently powered by itself to find a difference, it will add to the existing literature to allow for more concordant and accurate systematic reviews and meta-analyses. The results are also useful to make treatment recommendations when they are applied through the cost model with sensitivity analysis (equivalent to 95% CI).
DISCUSSION
Our study showed that the current rate of infection from dog bite wounds is lower than reported in most prior studies, and our associated cost model showed that if the rate of infection is less than 3–5% there is little value in the use of prophylactic antibiotics. This has led us to believe that we should focus on determining factors associated with high-risk wounds so that we can appropriately use prophylactic antibiotics.
From our analysis it is clear that the value of antibiotic use is related to the actual rate of infection and the drug’s effect on preventing infections. For example, three days of amoxicillin-clavulanic acid costs about $30 (assuming $5 a pill), whereas the cost of developing an infection that requires a return visit to the ED, probable intravenous antibiotics and possible admission costs thousands of dollars. At the extremes, if 20% of patients develop infections and even 1% could be prevented with a short course of prophylactic antibiotics, the front-end cost of antibiotics in all cases is minimal compared to the large cost of treating infections that do occur. In that case only a small benefit (1%) would be needed to be cost effective. However, as the baseline rate of infection becomes lower in the population, it is harder to achieve a benefit with antibiotics. Figure 3 demonstrates that if the infection rate is 8%, there must to be a 3% decrease in the infection rate (to 5%) to be cost-effective to treat people with antibiotics. At a 5% baseline rate, there would need to be a 3% decrease to be cost-effective, which translates to an impractical 60% relative reduction in infections. At levels of infection less than 3% it is never cost effective to treat since the threshold line is already reached.
Our randomized controlled trial found that the overall infection rate of dog bite wounds was 2% (95% CI 0–7%) with a difference of 4% (95% CI −1–4.5%) between treatment and placebo groups. Based on the cost-model, it is not cost effective to give prophylactic antibiotics at 2%, though it may be justifiable at the upper end of the 95% CI of 7%. Our rate of 2% is lower than the most current reported infection rates of 5–10%4 but similar to that found in other studies.2,5 Several factors may account for this. We used a standardized definition of wound infection and a single agent known to be effective against the pathogens in dog bites. Previous studies used a variety of antibiotics (trimethoprim-sulfamethoxazole, cephalexin and erythromycin) with questionable efficacy against organisms such as Pasteurella multocida.5,9 These studies also did not use a consistent method of determining outcomes. Furthermore, the studies included in a prior meta-analysis5 and Cochrane review9 were limited by design and lack of techniques related to improvement in wound cleansing that have occurred over the last 30 years, particularly irrigation. While the value, timing and route of antibiotic prophylaxis can be argued, the benefit of antibiotics is probably dwarfed by proper irrigation and its effect on wound colonization, which is the prime determinant of infection.16 Since the publication of most of these studies there have been great improvements in wound irrigation and care.
A randomized controlled multi-center trial of sufficient power may address the issue of antibiotic effectiveness. If an absolute risk reduction of 3% can be shown, prophylactic antibiotic use would be cost-effective. However, given the low rate of wound infection, conducting a randomized controlled trial to determine if antibiotics could significantly decrease infection would require nearly 7,000 patients and a cost too great to warrant such a trial. It is also clear that different dog bite wounds have different risks, and assuming them to be of equal risk in treatment decisions makes little clinical sense.
We believe further research should focus on identifying factors associated with high-risk wounds. Our trial and previous literature suggest a small trend of benefit from antibiotic prophylaxis regardless of trial limitations. The results of this trial indicate that the baseline rate of wound infection is low, especially among low-risk wounds. Future studies should try to identify wounds that have a high risk of infection (above 3–5%) that would benefit from prophylactic antibiotics. For example, 13% of untreated sutured wounds got infected in this study suggesting they are at high-risk. There are currently no tools or decision guidelines to predict which bites are high-risk and likely to become infected. A set of clinical predictors to identify wounds at high risk of infection is needed and would be the next logical step to address the controversial issue of prophylactic antibiotics and guide physician management decisions.
Footnotes
Reprints available through open access at http://escholarship.org/uc/uciem_westjem
Conflicts of Interest: By the WestJEM article submission agreement, all authors are required to disclose all affiliations, funding sources, and financial or management relationships that could be perceived as potential sources of bias. The authors disclosed none.
REFERENCES
- 1.Sacks J, Kresnow M, Houston B. Dog bites: how big a problem? Inj Prev. 1996;2:52. doi: 10.1136/ip.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss HB, Friedman DI, Coben JH. Incidence of dog bite injuries treated in emergency departments. JAMA. 1998;279:51–3. doi: 10.1001/jama.279.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Callaham M. Dog bite wounds. JAMA. 1980;244:2327–8. [PubMed] [Google Scholar]
- 4.Morgan M, Palmer J. Dog bites. BMJ. 2007;334:413–7. doi: 10.1136/bmj.39105.659919.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings P. Antibiotics to prevent infection in patients with dog bite wounds: a meta-analysis of randomized trials. Ann Emerg Med. 1994;23(3):577–9. doi: 10.1016/s0196-0644(94)70073-7. [DOI] [PubMed] [Google Scholar]
- 6.Callaham M. Treatment of common dog bites: infection risk factors. JACEP. 1978;7:83–7. doi: 10.1016/s0361-1124(78)80063-x. [DOI] [PubMed] [Google Scholar]
- 7.Maimaris C, Quinton D. Dog-bite lacerations: A controlled trial of primary wound closure. Arch Emerg Med. 1988;5:156–61. doi: 10.1136/emj.5.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dire D, Hogan D, Walker J. Prophylactic oral antibiotics for low-risk dog bite wounds. Pediatr Emerg Care. 1992;8:194–9. doi: 10.1097/00006565-199208000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros I, Saconato H. Antibiotic prophylaxis for mammalian bites. Cochrane Database Syst Rev. 2001;2:CD001738. doi: 10.1002/14651858.CD001738. [DOI] [PubMed] [Google Scholar]
- 10.Talan DA, Citron DM, Abrahamian FM, et al. Bacteriologic analysis of infected dog and cat bites. Emergency Medicine Animal Bite Infection Study Group. N Engl J Med. 1999;340:85–92. doi: 10.1056/NEJM199901143400202. [DOI] [PubMed] [Google Scholar]
- 11.Karcz A, Holbrook J, Auerbach BS, et al. Preventability of malpractice claims in emergency medicine: a closed claims study. Ann Emerg Med. 1990;19:865–73. doi: 10.1016/s0196-0644(05)81559-8. [DOI] [PubMed] [Google Scholar]
- 12.HCUP: Healthcare Cost and Utilization Project. [Accessed October 2, 2008]. Available at: http:// www.hcup-us.ahrq.gov/databases.jsp.
- 13.Pillbot. [Accessed May 12, 2006]. Available at: http://www.pillbot.com.
- 14.Quinn J, Durski K. A real-time tracking, notification, and web-based enrollment system for emergency department research. Acad Emerg Med. 2004;11:1245–8. doi: 10.1197/j.aem.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 16.Bansal BC, Wiebe RA, Perkins SD, et al. Tap water for irrigation of lacerations. Am J Emerg Med. 2002;20:469–72. doi: 10.1053/ajem.2002.35501. [DOI] [PubMed] [Google Scholar]
- 17.Pien F. Double-blind comparative study of two dosage regimens of cefaclor and amoxicillin-clavulanic acid in the outpatient treatment of soft tissue infections. Antimicrob Agents Chemother. 1983;24:856–9. doi: 10.1128/aac.24.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers R. Soft tissue infections in the emergency department: the case for the use of ‘simple’ antibiotics. South Med J. 1991;84:1313–5. doi: 10.1097/00007611-199111000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kullberg BJ, Westendorp RG, van’t Wout JW, et al. Purpura fulminans and symmetrical peripheral gangrene caused by Capnocytophaga canimorsus - formerly df-2 septicemia - a complication of dog bite. Medicine. 1991;70:287–92. doi: 10.1097/00005792-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Benson LS, Edwards SL, Schiff AP, et al. Dog and cat bites to the hand: treatment and cost assessment. J Hand Surg. 2006;31:468–473. doi: 10.1016/j.jhsa.2005.12.011. [DOI] [PubMed] [Google Scholar]