Abstract
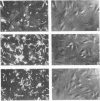
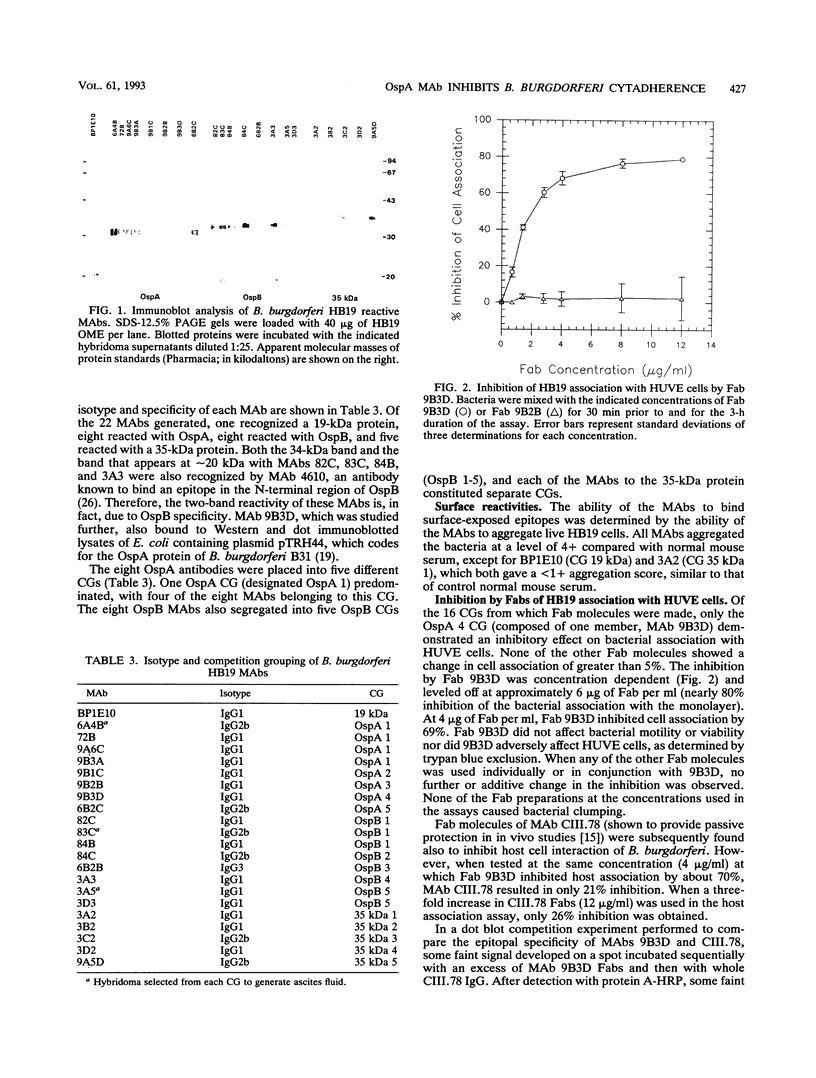
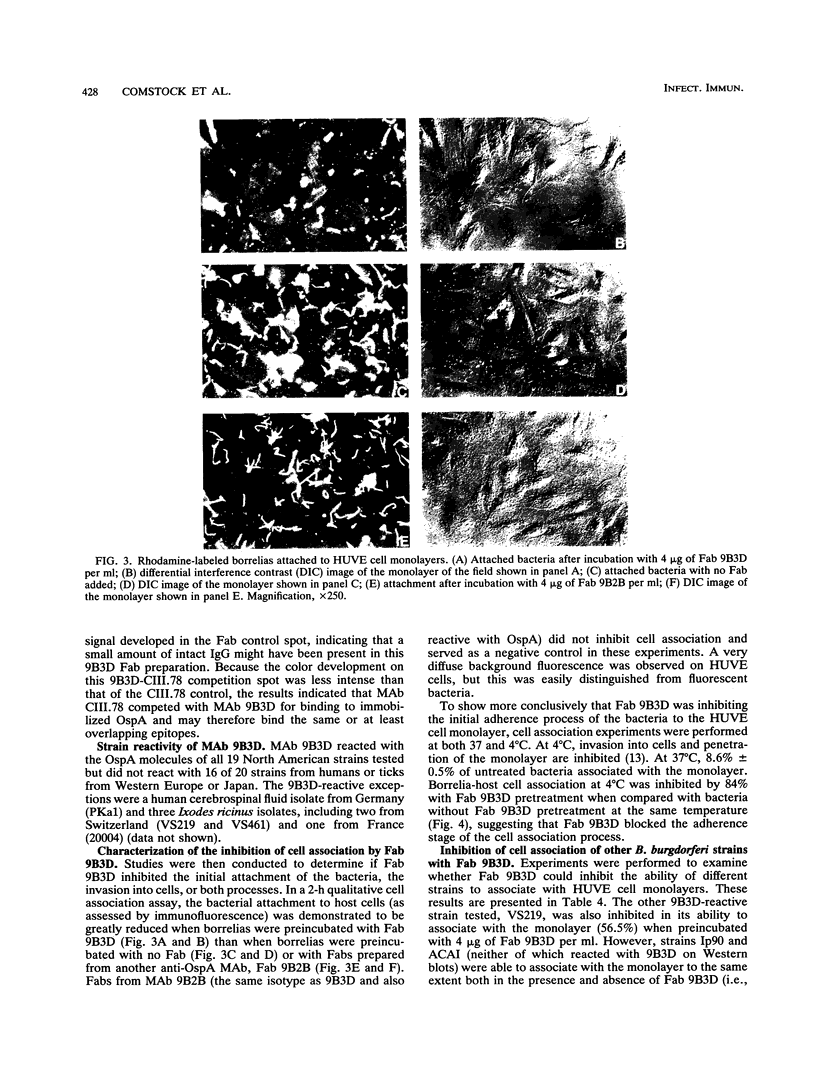
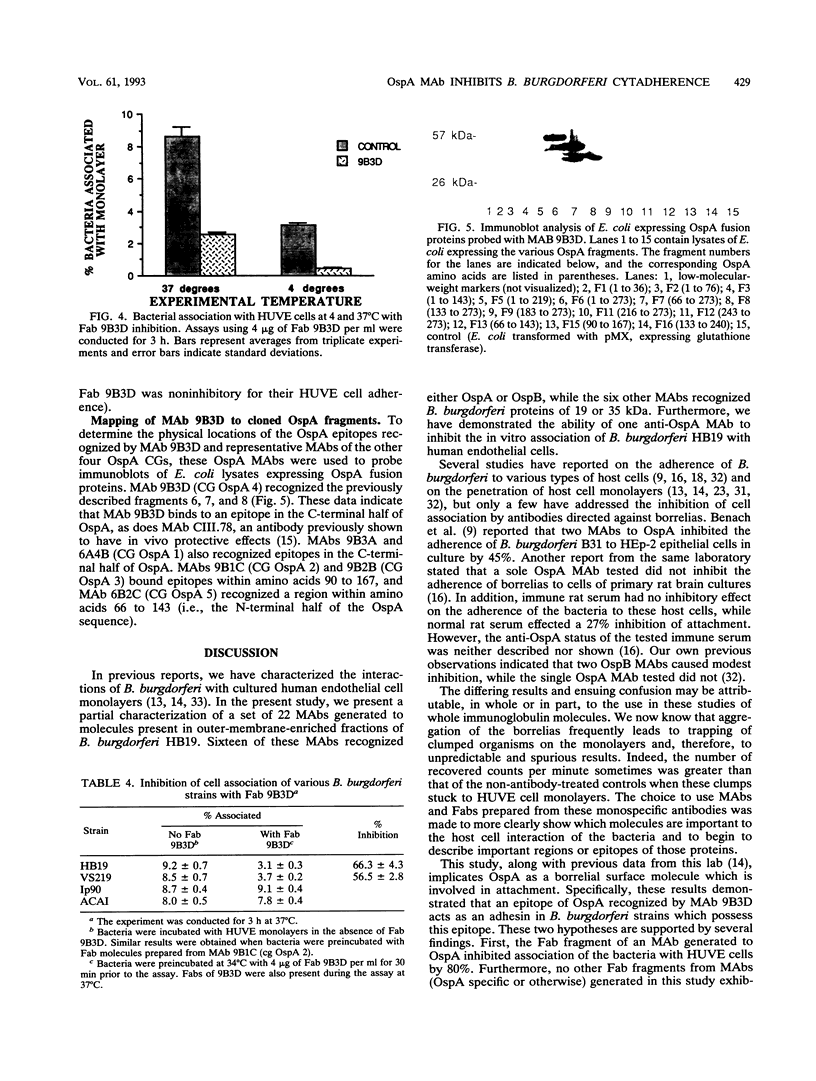
Previously, it has been shown that polyclonal antibodies to Borrelia burgdorferi and some monoclonal antibodies (MAbs) to borrelia major surface proteins caused inhibition of adherence of the bacteria to cultured human umbilical vein endothelial (HUVE) cells. In this study, fragment antigen binding (Fab) molecules generated from the immunoglobulin G fraction of rabbit anti-recombinant OspA serum were found to inhibit the adherence of B. burgdorferi to HUVE cells by 73%. Subsequently, MAbs were generated for use in determining whether or how B. burgdorferi outer surface proteins (Osps) A and/or B are involved in mediating attachment to, and/or invasion of, HUVE cells by B. burgdorferi. Twenty-two MAbs were generated to borrelial proteins with apparent molecular masses (determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) of 19, 31 (OspA), 34 (OspB), and 35 kDa. Fab molecules from one anti-OspA MAb, 9B3D, demonstrated an inhibitory effect on bacterial association with HUVE cells. None of the other MAbs, including the other anti-OspA MAbs, showed an inhibitory effect on cell association of greater than 5%. This effect of Fab 9B3D was concentration dependent and plateaued at approximately 6 micrograms of Fab per ml (nearly 80% inhibition of the bacterial association with the monolayer). Penetration assays and cell association experiments performed by using immunofluorescence also suggested that the inhibitory action of 9B3D occurs at the level of adherence. MAb 9B3D recognized the OspA of every North American strain tested (n = 19) but only 3 [corrected] of 20 strains from western Europe, Russia, and Japan, suggesting that the North American strains and strains from other parts of the world may use different molecules and/or different OspA epitopes to interact with endothelial cells. Immunoblots of Escherichia coli expressing different OspA fusion peptides suggested that the 9B3D epitope resides in the carboxy-terminal half of OspA. MAb 9B3D promises to be a valuable tool for elucidating the domain or domains of OspA involved in the endothelial cell cytadherence of North American strains of B. burgdorferi.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Stoenner H. G. Variable major proteins of Borrellia hermsii. J Exp Med. 1982 Nov 1;156(5):1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Moody K. D., Terwilliger G. A., Jacoby R. O., Steere A. C. An animal model for Lyme arthritis. Ann N Y Acad Sci. 1988;539:264–273. doi: 10.1111/j.1749-6632.1988.tb31860.x. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Garcia-Monco J. C., Deponte P. C. Biological activity of Borrelia burgdorferi antigens. Ann N Y Acad Sci. 1988;539:115–125. doi: 10.1111/j.1749-6632.1988.tb31845.x. [DOI] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Characterization of Borrelia burgdorferi invasion of cultured endothelial cells. Microb Pathog. 1991 Feb;10(2):137–148. doi: 10.1016/0882-4010(91)90074-k. [DOI] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989 May;57(5):1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Garcia-Monco J. C., Fernandez-Villar B., Benach J. L. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989 Sep;160(3):497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- Hechemy K. E., Samsonoff W. A., McKee M., Guttman J. M. Borrelia burgdorferi attachment to mammalian cells. J Infect Dis. 1989 Apr;159(4):805–806. doi: 10.1093/infdis/159.4.805. [DOI] [PubMed] [Google Scholar]
- Howe T. R., LaQuier F. W., Barbour A. G. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect Immun. 1986 Oct;54(1):207–212. doi: 10.1128/iai.54.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R., Wenman W. M. Cyclic AMP inhibits developmental regulation of Chlamydia trachomatis. J Bacteriol. 1986 Nov;168(2):722–727. doi: 10.1128/jb.168.2.722-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Sturrock A., Weis J. J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun. 1991 Feb;59(2):671–678. doi: 10.1128/iai.59.2.671-678.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milch L. J., Barbour A. G. Analysis of North American and European isolates of Borrelia burgdorferi with antiserum to a recombinant antigen. J Infect Dis. 1989 Aug;160(2):351–353. doi: 10.1093/infdis/160.2.351. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T., Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992 Oct;6(20):3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears J. E., Fikrig E., Nakagawa T. Y., Deponte K., Marcantonio N., Kantor F. S., Flavell R. A. Molecular mapping of Osp-A mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991 Sep 15;147(6):1995–2000. [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Szczepanski A., Furie M. B., Benach J. L., Lane B. P., Fleit H. B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990 May;85(5):1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Comstock L. E. Interaction of Lyme disease spirochetes with cultured eucaryotic cells. Infect Immun. 1989 Apr;57(4):1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Navab M., Haake D. A., Fogelman A. M., Miller J. N., Lovett M. A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. 1988 May;85(10):3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]