Abstract
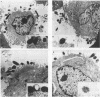
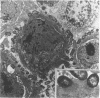
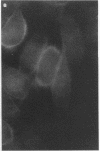
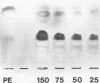
Adhesion of Helicobacter pylori was reported previously to be morphologically identical to "attaching and effacing" Escherichia coli. Therefore, the aim of the present study was to define the adhesion phenotype of H. pylori LC-11 to HEp-2, KATO-III, HEL, and CHO tissue culture cells. By using both staining of F-actin with fluorescein-labeled phalloidin and ultrastructural analysis, diffuse bacterial adhesion to discrete microvillus-denuded regions of the plasma membrane was observed in each of the infected cell lines. However, strain LC-11 did not induce formation of F-actin adhesion pedestals on the eukaryotic cells. H. pylori was negative by colony blot hybridization with an E. coli attaching and effacing gene probe. Elevations in inositol triphosphates followed infection of HEp-2 cells with H. pylori (405% of control values +/- 147%; P < 0.05). To correlate the observed histopathology with expression of the H. pylori phosphatidylethanolamine receptor, a thin-layer chromatography overlay-binding assay was used to identify receptors in each of the cell lines. H. pylori adhered to eukaryotic cells regardless of the presence (HEp-2, KATO-III, and CHO cells) or absence (HEL cells) of the lipid receptor as detected under the assay conditions. However, in comparison to cell lines that possess the phosphatidylethanolamine receptor, HEL cells demonstrated less quantitative H. pylori binding. These findings suggest that mechanisms distinct from E. coli enteropathogens underlie the adhesion of H. pylori to mucosal surfaces. In addition to the phosphatidylethanolamine H. pylori receptor, another host factor(s) likely mediates the attachment of H. pylori to human eukaryotic cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beebakhee G., Louie M., De Azavedo J., Brunton J. Cloning and nucleotide sequence of the eae gene homologue from enterohemorrhagic Escherichia coli serotype O157:H7. FEMS Microbiol Lett. 1992 Feb 1;70(1):63–68. doi: 10.1016/0378-1097(92)90563-4. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Bode G., Malfertheiner P., Ditschuneit H. Pathogenetic implications of ultrastructural findings in Campylobacter pylori related gastroduodenal disease. Scand J Gastroenterol Suppl. 1988;142:25–39. [PubMed] [Google Scholar]
- Cook J. A., Mitchell J. B. Viability measurements in mammalian cell systems. Anal Biochem. 1989 May 15;179(1):1–7. doi: 10.1016/0003-2697(89)90191-7. [DOI] [PubMed] [Google Scholar]
- Drumm B., Sherman P. Long-term storage of Campylobacter pylori. J Clin Microbiol. 1989 Jul;27(7):1655–1656. doi: 10.1128/jcm.27.7.1655-1656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Adherence and internalization of Helicobacter pylori by HEp-2 cells. Gastroenterology. 1992 May;102(5):1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Hemalatha S. G., Drumm B., Sherman P. Adherence of Helicobacter pylori to human gastric epithelial cells in vitro. J Med Microbiol. 1991 Oct;35(4):197–202. doi: 10.1099/00222615-35-4-197. [DOI] [PubMed] [Google Scholar]
- Hessey S. J., Spencer J., Wyatt J. I., Sobala G., Rathbone B. J., Axon A. T., Dixon M. F. Bacterial adhesion and disease activity in Helicobacter associated chronic gastritis. Gut. 1990 Feb;31(2):134–138. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Kaper J. B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991 Dec;59(12):4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood C. A., Huesca M., Kuksis A. The glycerolipid receptor for Helicobacter pylori (and exoenzyme S) is phosphatidylethanolamine. Infect Immun. 1992 Jun;60(6):2470–2474. doi: 10.1128/iai.60.6.2470-2474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood C. A., Law H., Pellizzari A., Sherman P., Drumm B. Gastric glycerolipid as a receptor for Campylobacter pylori. Lancet. 1989 Jul 29;2(8657):238–241. doi: 10.1016/s0140-6736(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Kaper J. B., Robins-Browne R., Prado V., Vial P., Levine M. M. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987 Sep;6(9):829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Neman-Simha V., Mégraud F. In vitro model for Campylobacter pylori adherence properties. Infect Immun. 1988 Dec;56(12):3329–3333. doi: 10.1128/iai.56.12.3329-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Robins-Browne R. M. Traditional enteropathogenic Escherichia coli of infantile diarrhea. Rev Infect Dis. 1987 Jan-Feb;9(1):28–53. doi: 10.1093/clinids/9.1.28. [DOI] [PubMed] [Google Scholar]
- Segal E. D., Shon J., Tompkins L. S. Characterization of Helicobacter pylori urease mutants. Infect Immun. 1992 May;60(5):1883–1889. doi: 10.1128/iai.60.5.1883-1889.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariff A., Luna E. J. Diacylglycerol-stimulated formation of actin nucleation sites at plasma membranes. Science. 1992 Apr 10;256(5054):245–247. doi: 10.1126/science.1373523. [DOI] [PubMed] [Google Scholar]
- Sherman P., Cockerill F., 3rd, Soni R., Brunton J. Outer membranes are competitive inhibitors of Escherichia coli O157:H7 adherence to epithelial cells. Infect Immun. 1991 Mar;59(3):890–899. doi: 10.1128/iai.59.3.890-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Fleming N., Forstner J., Roomi N., Forstner G. Bacteria and the mucus blanket in experimental small bowel bacterial overgrowth. Am J Pathol. 1987 Mar;126(3):527–534. [PMC free article] [PubMed] [Google Scholar]
- Smith J. I., Drumm B., Neumann A. W., Policova Z., Sherman P. M. In vitro surface properties of the newly recognized gastric pathogen Helicobacter pylori. Infect Immun. 1990 Sep;58(9):3056–3060. doi: 10.1128/iai.58.9.3056-3060.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick M. J., Madara J. L., Fields B. N., Normark S. J. Molecular cross talk between epithelial cells and pathogenic microorganisms. Cell. 1991 Nov 15;67(4):651–659. doi: 10.1016/0092-8674(91)90061-3. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafriri D., Oron Y., Eisenstein B. I., Ofek I. Growth advantage and enhanced toxicity of Escherichia coli adherent to tissue culture cells due to restricted diffusion of products secreted by the cells. J Clin Invest. 1987 Apr;79(4):1210–1216. doi: 10.1172/JCI112939. [DOI] [PMC free article] [PubMed] [Google Scholar]