Abstract
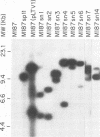
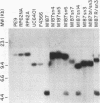
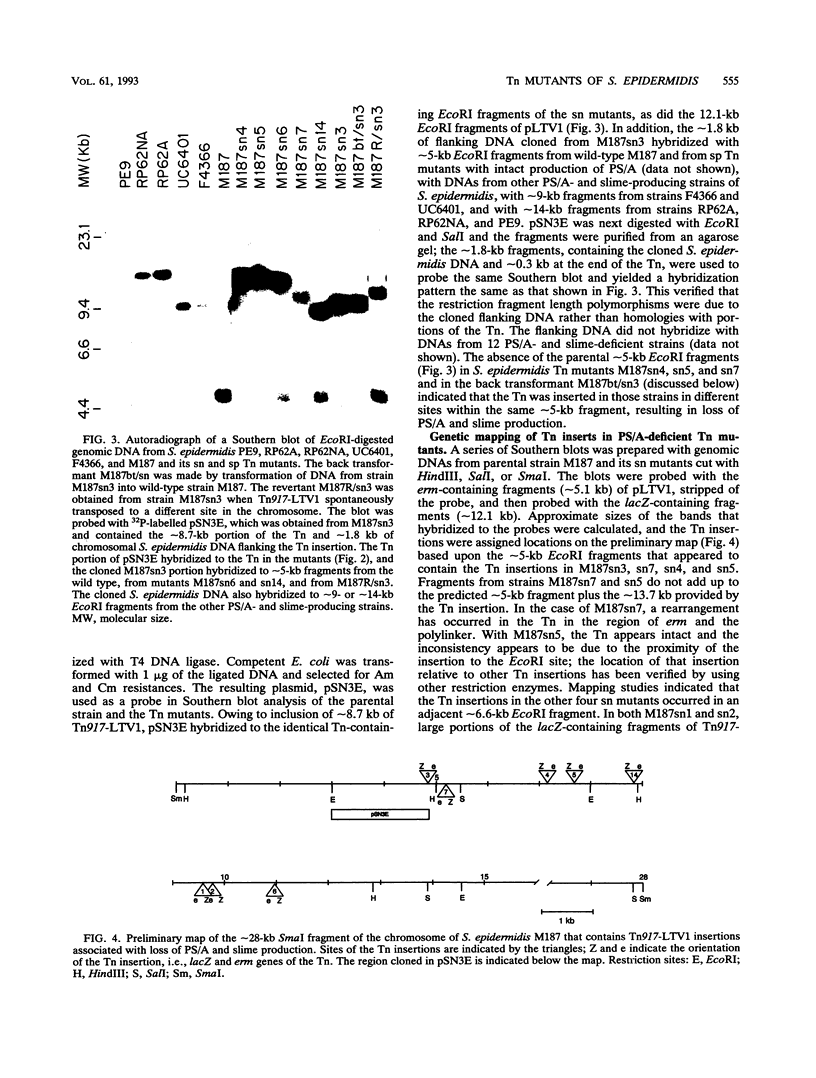
We used transposon (Tn) mutagenesis to study the role of capsular polysaccharide/adhesin (PS/A) and slime in adherence of Staphylococcus epidermidis to catheters. pLTV1, containing Tn917-LTV1, was transformed into S. epidermidis M187 by protoplast fusion with S. aureus RN4220(pLTV1), creating M187(pLTV1). Tn mutants were isolated following growth at 42 degrees C; mutants deficient in PS/A and slime production were selected. PS/A- and slime-deficient Tn mutants had a 10-fold decrease in vitro in the initial phase of adherence to catheters, comparable to levels of strains that do not produce PS/A. Introduction of Tn917-LTV1-interrupted DNA from PS/A-deficient mutant M187sn3 into the parental strain via transformation of protoplasts yielded recipients with inserts identical to those of the Tn mutant that were PS/A and slime deficient. Chromosomal DNA flanking the Tn in mutant M187sn3 was cloned into Escherichia coli. The cloned DNA was found to hybridize to approximately 5-kb EcoRI fragments from the parental strain and from control Tn mutants that express parental levels of PS/A and to either approximately 9- or approximately 14-kb EcoRI fragments from other highly adherent, PS/A-producing strains. Mapping studies demonstrated that in the eight PS/A-deficient mutants that have been isolated, the Tn insertions all occur within a region of approximately 11.6 kb that is defined by three EcoRI sites. These results support previous findings indicating that in S. epidermidis PS/A is involved with in vitro adherence to plastic biomaterials and elaboration of PS/A is closely associated with slime production.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin J., Götz F. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):203–207. doi: 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- Baddour L. M., Christensen G. D., Hester M. G., Bisno A. L. Production of experimental endocarditis by coagulase-negative staphylococci: variability in species virulence. J Infect Dis. 1984 Nov;150(5):721–727. doi: 10.1093/infdis/150.5.721. [DOI] [PubMed] [Google Scholar]
- Buret A., Ward K. H., Olson M. E., Costerton J. W. An in vivo model to study the pathobiology of infectious biofilms on biomaterial surfaces. J Biomed Mater Res. 1991 Jul;25(7):865–874. doi: 10.1002/jbm.820250706. [DOI] [PubMed] [Google Scholar]
- Camilli A., Portnoy A., Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990 Jul;172(7):3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Madison B. M., Parisi J. T., Abraham S. N., Hasty D. L., Lowrance J. H., Josephs J. A., Simpson W. A. Colonial morphology of staphylococci on Memphis agar: phase variation of slime production, resistance to beta-lactam antibiotics, and virulence. J Infect Dis. 1990 Jun;161(6):1153–1169. doi: 10.1093/infdis/161.6.1153. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987 Dec;55(12):2870–2877. doi: 10.1128/iai.55.12.2870-2877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Parisi J. T., Bisno A. L., Simpson W. A., Beachey E. H. Characterization of clinically significant strains of coagulase-negative staphylococci. J Clin Microbiol. 1983 Aug;18(2):258–269. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983 Apr;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., Beachey E. H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985 Dec;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport D. S., Massanari R. M., Pfaller M. A., Bale M. J., Streed S. A., Hierholzer W. J., Jr Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986 Feb;153(2):332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- Dickinson G. M., Bisno A. L. Infections associated with indwelling devices: concepts of pathogenesis; infections associated with intravascular devices. Antimicrob Agents Chemother. 1989 May;33(5):597–601. doi: 10.1128/aac.33.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber B. F., Kaplan M. H., Clogston A. G. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis. 1990 Jan;161(1):37–40. doi: 10.1093/infdis/161.1.37. [DOI] [PubMed] [Google Scholar]
- Fidalgo S., Vázquez F., Mendoza M. C., Pérez F., Méndez F. J. Bacteremia due to Staphylococcus epidermidis: microbiologic, epidemiologic, clinical, and prognostic features. Rev Infect Dis. 1990 May-Jun;12(3):520–528. doi: 10.1093/clinids/12.3.520. [DOI] [PubMed] [Google Scholar]
- Franson T. R., Sheth N. K., Rose H. D., Sohnle P. G. Scanning electron microscopy of bacteria adherent to intravascular catheters. J Clin Microbiol. 1984 Sep;20(3):500–505. doi: 10.1128/jcm.20.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E. D., Peters G., Verstegen M., Regelmann W. E. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984 Feb 18;1(8373):365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Grueter L., Koenig O., Laufs R. Transposon mutagenesis in Staphylococcus epidermidis using the Enterococcus faecalis transposon Tn917. FEMS Microbiol Lett. 1991 Aug 1;66(2):215–218. doi: 10.1016/0378-1097(91)90335-8. [DOI] [PubMed] [Google Scholar]
- Ishak M. A., Gröschel D. H., Mandell G. L., Wenzel R. P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985 Dec;22(6):1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Tojo M., Goldmann D. A., Tosteson T. D., Pier G. B. Antibody to the capsular polysaccharide/adhesin protects rabbits against catheter-related bacteremia due to coagulase-negative staphylococci. J Infect Dis. 1990 Aug;162(2):435–441. doi: 10.1093/infdis/162.2.435. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Betley M. J., Hopkins C. A., Perez N. E., Pier G. B. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J Infect Dis. 1987 Nov;156(5):741–750. doi: 10.1093/infdis/156.5.741. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Weise C. E., Sarafin H. W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977 Jun 9;296(23):1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- Muller E., Takeda S., Goldmann D. A., Pier G. B. Blood proteins do not promote adherence of coagulase-negative staphylococci to biomaterials. Infect Immun. 1991 Sep;59(9):3323–3326. doi: 10.1128/iai.59.9.3323-3326.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Iglewski B. H. Isolation and characterization of transposon-induced mutants of Pseudomonas aeruginosa deficient in production of exoenzyme S. Infect Immun. 1984 Aug;45(2):470–474. doi: 10.1128/iai.45.2.470-474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattee P. A. Distribution of Tn551 insertion sites responsible for auxotrophy on the Staphylococcus aureus chromosome. J Bacteriol. 1981 Jan;145(1):479–488. doi: 10.1128/jb.145.1.479-488.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Tojo M., Yamashita N., Goldmann D. A., Pier G. B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988 Apr;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- Wessels M. R., Moses A. E., Goldberg J. B., DiCesare T. J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J. J., Christensen G. D., Bartley D. L., Simmons J. C., Barrett F. F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987 Oct;156(4):548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]