Abstract
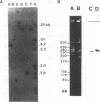
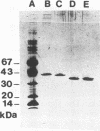
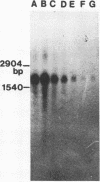
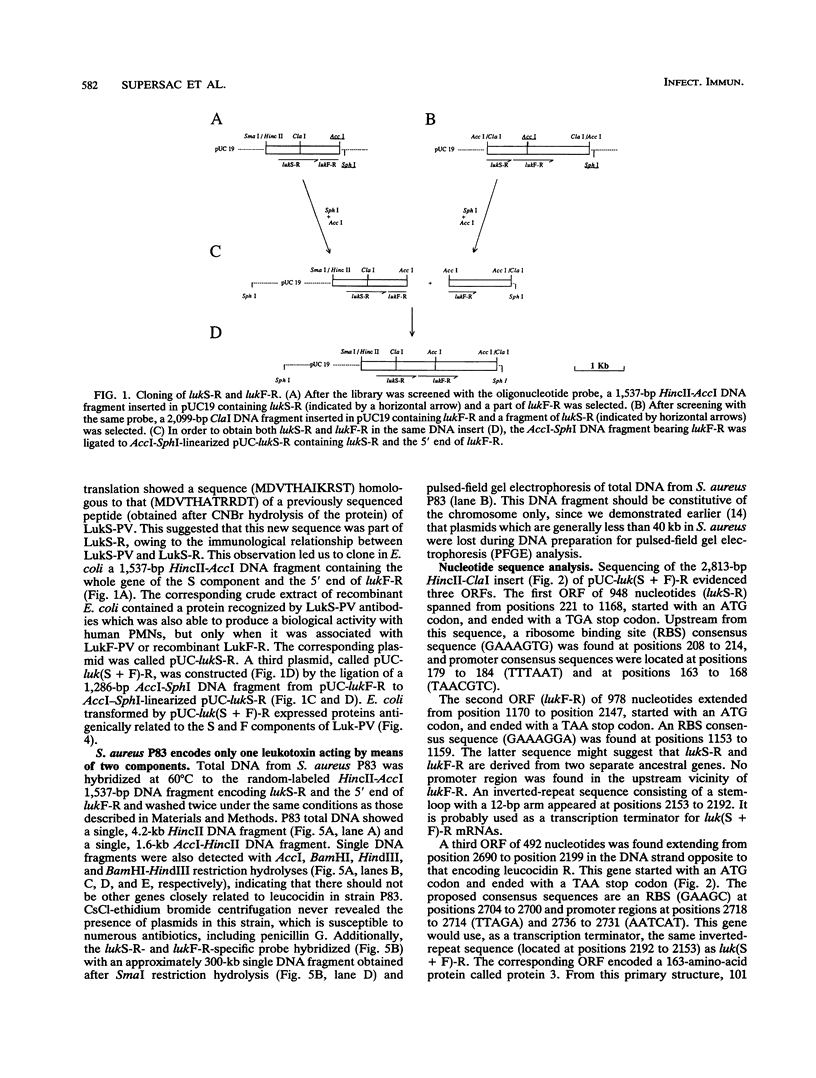
A 2,813-bp HincII-ClaI DNA fragment encodes the two S and F components (LukS-R and LukF-R) of leucocidin R (Luk-R) which are secreted by Staphylococcus aureus P83. The two genes (lukS-R and lukF-R) belong to a single operon. Two peptidic sequences were deduced: LukS-R is a 35,721-Da polypeptide of 315 amino acids, including a signal sequence of 29 residues, and LukF-R is a 36,838-Da polypeptide of 325 amino acids, including a signal sequence of 25 residues. LukS-R and LukF-R were expressed in Escherichia coli and purified from the periplasmic space. Luk-R exerts biological activities on polymorphonuclear cells and on erythrocytes from various animals. Comparison of the amino acid sequence of LukF-R with that of the B component of gamma-hemolysin (HlgB), those of the F and S components of another recently sequenced staphylococcal leucocidin, and those of a few peptides of the F component from Panton-Valentine leucocidin suggests that all four toxins belong to a single, two-component family of toxins.
Full text
PDF


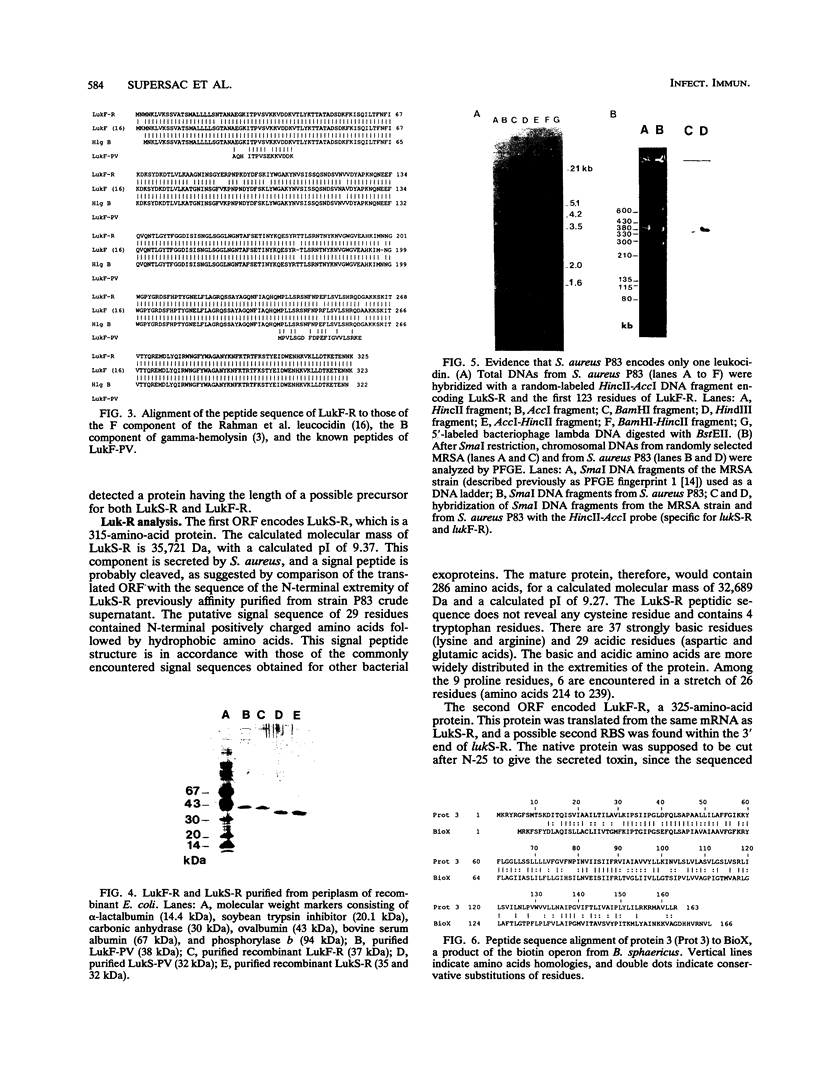
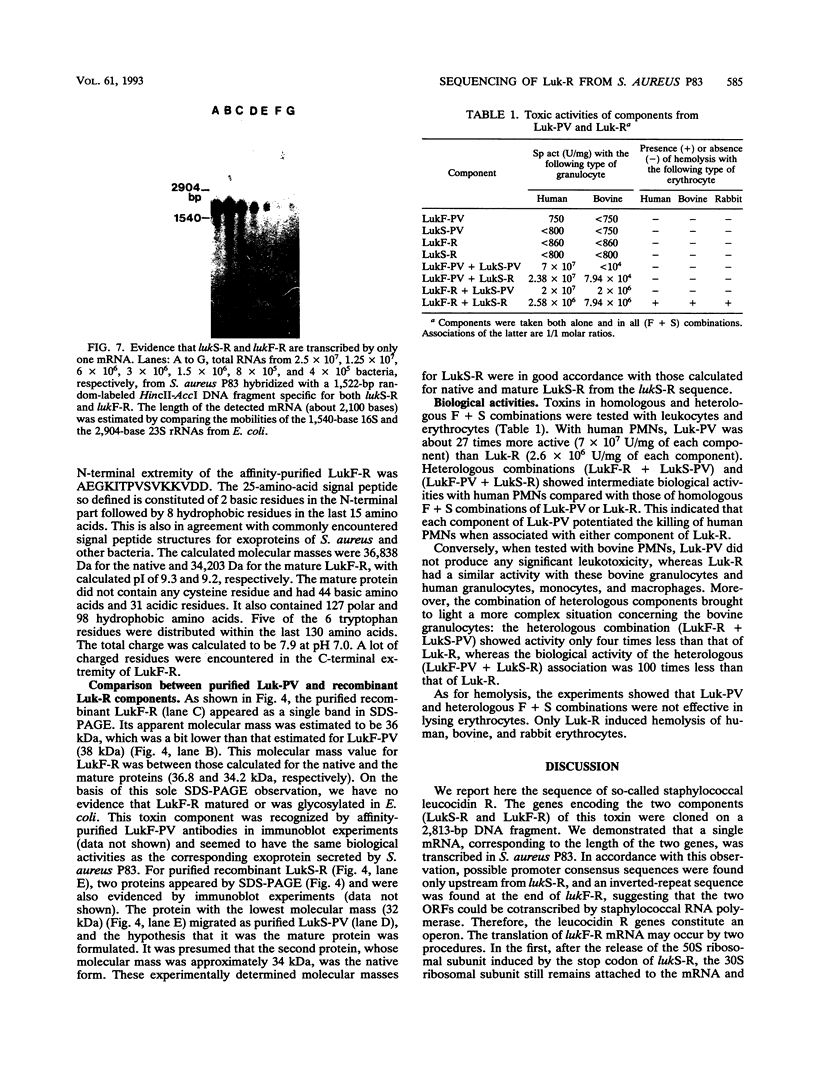


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cribier B., Prévost G., Couppie P., Finck-Barbançon V., Grosshans E., Piémont Y. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology. 1992;185(3):175–180. doi: 10.1159/000247443. [DOI] [PubMed] [Google Scholar]
- Finck-Barbançon V., Prévost G., Piémont Y. Improved purification of leukocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res Microbiol. 1991 Jan;142(1):75–85. doi: 10.1016/0923-2508(91)90099-v. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., VAN HEYNINGEN W. E. Staphylococcal leucocidins. Br J Exp Pathol. 1957 Apr;38(2):123–137. [PMC free article] [PubMed] [Google Scholar]
- Guyonnet F., Plommet M. Hémolysine gamma de staphylococcus aureus: purification et propriétés. Ann Inst Pasteur (Paris) 1970 Jan;118(1):19–33. [PubMed] [Google Scholar]
- Kornblum J. S., Projan S. J., Moghazeh S. L., Novick R. P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63(1):75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeffler D. A., Schat K. A., Norcross N. L. Use of 51Cr release to measure the cytotoxic effects of staphylococcal leukocidin and toxin neutralization on bovine leukocytes. J Clin Microbiol. 1986 Mar;23(3):416–420. doi: 10.1128/jcm.23.3.416-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Hirayama T., Kato I., Matsuda F. Crystallization and properties of staphylococcal leukocidin. Biochim Biophys Acta. 1980 Nov 17;633(1):33–44. doi: 10.1016/0304-4165(80)90035-5. [DOI] [PubMed] [Google Scholar]
- Noda M., Hirayama T., Matsuda F., Kato I. An early effect of the S component of staphylococcal leukocidin on methylation of phospholipid in various leukocytes. Infect Immun. 1985 Oct;50(1):142–145. doi: 10.1128/iai.50.1.142-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phonimdaeng P., O'Reilly M., Nowlan P., Bramley A. J., Foster T. J. The coagulase of Staphylococcus aureus 8325-4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol Microbiol. 1990 Mar;4(3):393–404. doi: 10.1111/j.1365-2958.1990.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Prevost G., Jaulhac B., Piemont Y. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol. 1992 Apr;30(4):967–973. doi: 10.1128/jcm.30.4.967-973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., Izaki K., Kato I., Kamio Y. Nucleotide sequence of leukocidin S-component gene (lukS) from methicillin resistant Staphylococcus aureus. Biochem Biophys Res Commun. 1991 Nov 27;181(1):138–144. doi: 10.1016/s0006-291x(05)81392-0. [DOI] [PubMed] [Google Scholar]
- Rahman A., Nariya H., Izaki K., Kato I., Kamio Y. Molecular cloning and nucleotide sequence of leukocidin F-component gene (lukF) from methicillin resistant Staphylococcus aureus. Biochem Biophys Res Commun. 1992 Apr 30;184(2):640–646. doi: 10.1016/0006-291x(92)90637-z. [DOI] [PubMed] [Google Scholar]
- Rifai S., Barbancon V., Prevost G., Piemont Y. Synthetic exfoliative toxin A and B DNA probes for detection of toxigenic Staphylococcus aureus strains. J Clin Microbiol. 1989 Mar;27(3):504–506. doi: 10.1128/jcm.27.3.504-506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. A., Kaeberle M. L. Evaluation of bovine polymorphonuclear leukocyte function. Vet Immunol Immunopathol. 1981 Apr;2(2):157–174. doi: 10.1016/0165-2427(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Soboll H., Ito A., Schaeg W., Blobel H. Leukozidin von Staphylokokken verschiedener Herkunft. Zentralbl Bakteriol Orig A. 1973 Jul;224(2):184–193. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Speck D., Ohsawa I., Gloeckler R., Zinsius M., Bernard S., Ledoux C., Kisou T., Kamogawa K., Lemoine Y. Isolation of Bacillus sphaericus biotin synthesis control mutants: evidence for transcriptional regulation of bio genes. Gene. 1991 Dec 1;108(1):39–45. doi: 10.1016/0378-1119(91)90485-t. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. G., Bernheimer A. W. Further characterization of staphylococcal gamma-hemolysin. Infect Immun. 1974 Jul;10(1):54–59. doi: 10.1128/iai.10.1.54-59.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODIN A. M. Purification of the two components of leucocidin from Staphylococcus aureus. Biochem J. 1960 Apr;75:158–165. doi: 10.1042/bj0750158. [DOI] [PMC free article] [PubMed] [Google Scholar]