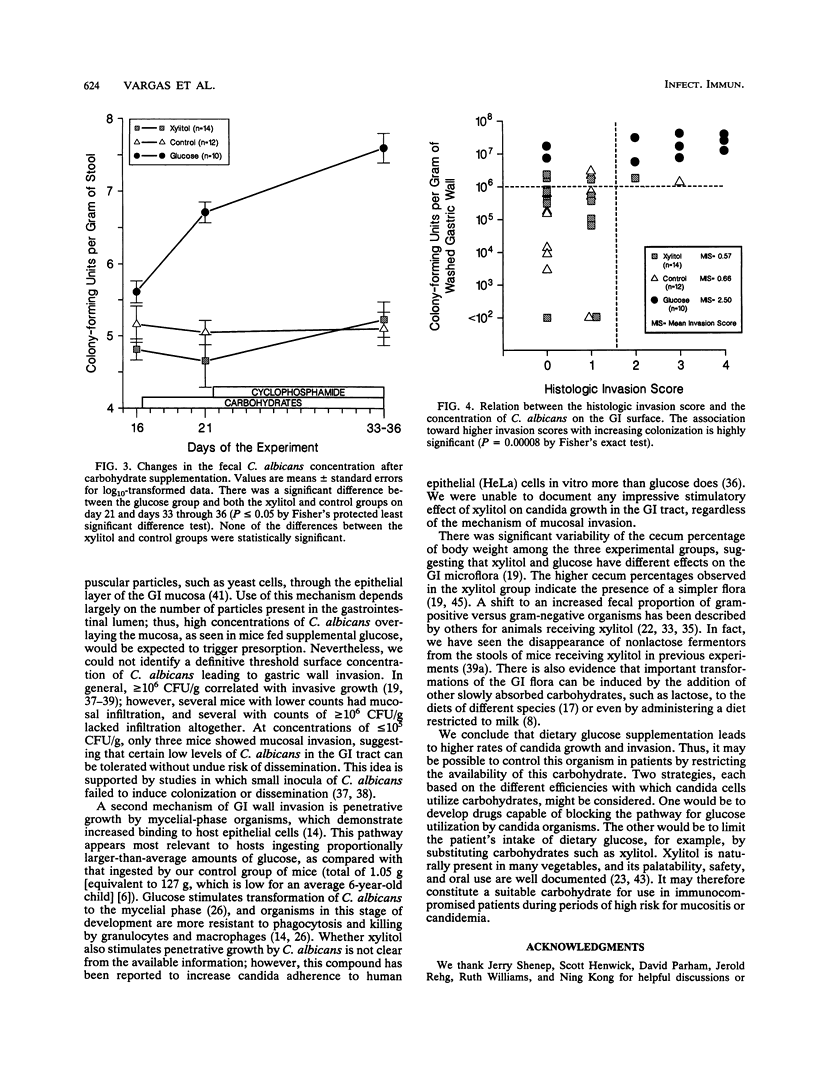
Abstract
We studied the effect of dietary carbohydrate supplementation on Candida albicans colonization and invasion of the gastrointestinal tract in a neutropenic mouse model. Mice inoculated with C. albicans were allowed free access to standard chow and drinking water supplemented with either glucose or xylitol or no carbohydrates (control). On days 33 through 36 postinoculation, the mean +/- standard error log10 CFU of C. albicans per gram on the mucosal surface, determined by quantitating CFU dislodged in the first wash of the gastric wall, was significantly higher in mice given the glucose supplement: 7.20 +/- 0.09 (glucose) versus 5.38 +/- 0.28 (xylitol) and 5.11 +/- 0.33 (control) CFU/g (P < or = 0.05 for each comparison by Fisher's protected least-significant-difference test). Fecal cultures also yielded the highest quantities of C. albicans in the glucose group. Invasion of the gastric wall by C. albicans correlated well with surface colonization in glucose-supplemented animals. Eight of 10 mice in this group, all with > 10(6) CFU/g, showed extensive invasive growth, as compared with only 2 of 26 mice in the remaining groups (P = 0.00006 by Fisher's exact test). These results indicate that dietary glucose intake is a key determinant of C. albicans growth in the gastrointestinal tract. The data provide an experimental rationale for clinical trials to decrease the intake of glucose or its utilization by C. albicans in immunocompromised patients.
Full text
PDF

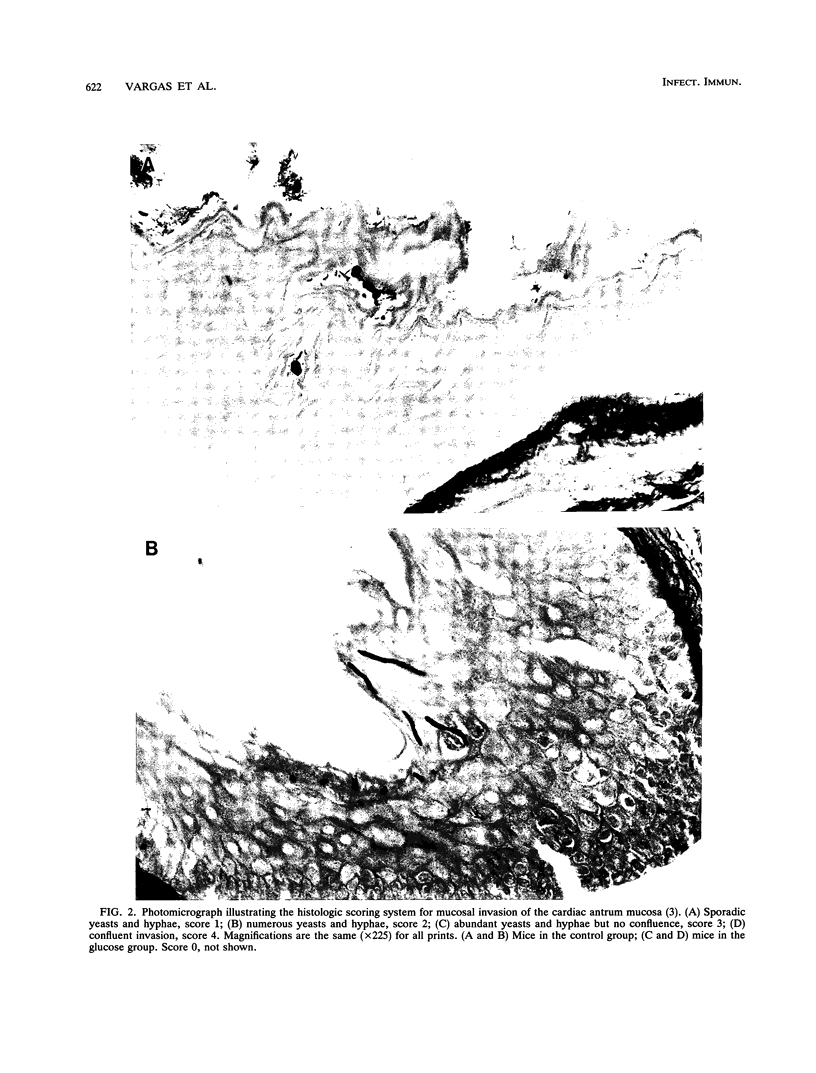
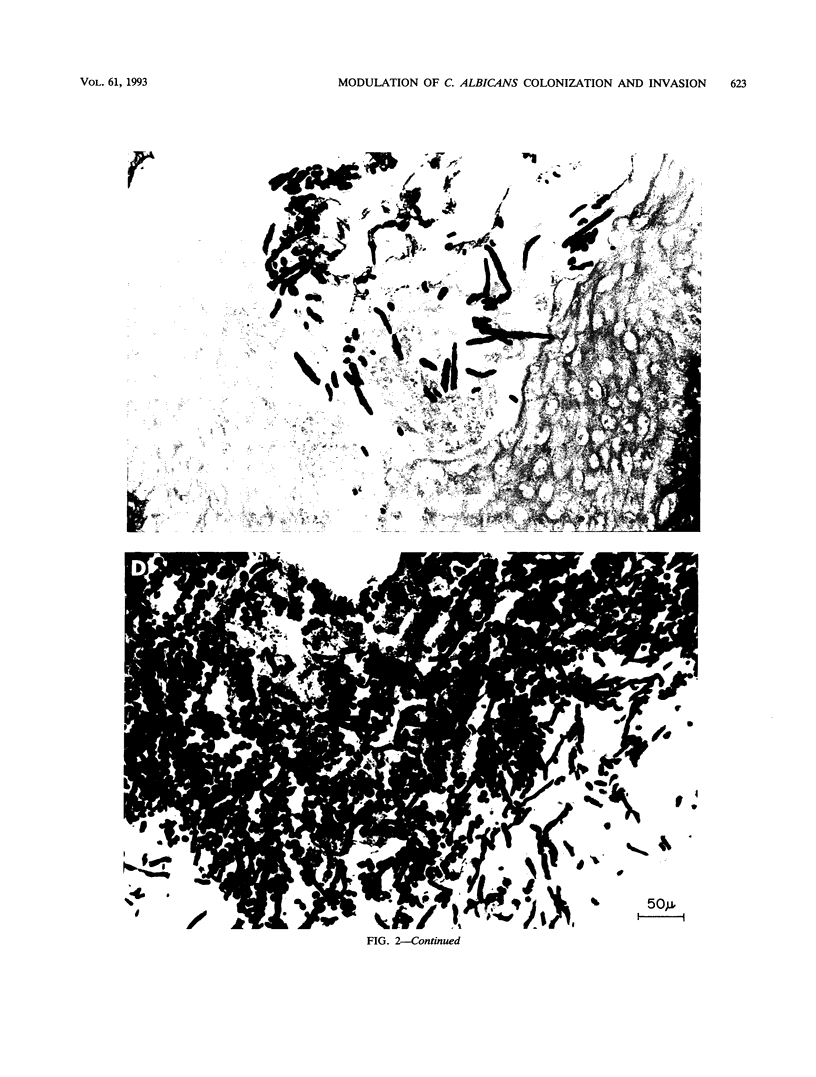


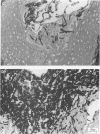
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P. Candidiasis in cancer patients. Am J Med. 1984 Oct 30;77(4D):13–19. [PubMed] [Google Scholar]
- Bowen W. H., Cornick D. E. The microbiology of gingival-dental plaque recent findings from primate research. Int Dent J. 1970 Sep;20(3):382–395. [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990 Apr;58(4):1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Lynn K. T., Seshan K. R. An animal model for oropharyngeal, esophageal and gastric candidosis. Mycoses. 1990 Jan;33(1):7–19. doi: 10.1111/myc.1990.33.1.7. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Lynn K. T., Seshan K. R., Pope L. M. Gastrointestinal and systemic candidosis in immunocompromised mice. J Med Vet Mycol. 1989;27(6):363–380. doi: 10.1080/02681218980000491. [DOI] [PubMed] [Google Scholar]
- Cormane R. H., Goslings W. R. Factors influencing the growth of Candida albicans (in vivo and in vitro studies). Sabouraudia. 1963 Oct;3(1):52–63. [PubMed] [Google Scholar]
- Escherich T. The intestinal bacteria of the neonate and breast-fed infant. 1885. Rev Infect Dis. 1989 Mar-Apr;11(2):352–356. doi: 10.1093/clinids/11.2.352. [DOI] [PubMed] [Google Scholar]
- Field L. H., Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Persistence and spread of Candida albicans after intragastric inoculation of infant mice. Infect Immun. 1981 Feb;31(2):783–791. doi: 10.1128/iai.31.2.783-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freireich E. J., Gehan E. A., Rall D. P., Schmidt L. H., Skipper H. E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966 May;50(4):219–244. [PubMed] [Google Scholar]
- Freter R., Abrams G. D. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun. 1972 Aug;6(2):119–126. doi: 10.1128/iai.6.2.119-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Botney M., Cleven D., Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983 Feb;39(2):676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore B. J., Retsinas E. M., Lorenz J. S., Hostetter M. K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988 Jan;157(1):38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Herrera C. Effects of compromising agents on candidosis in mice with persistent infections initiated in infancy. Infect Immun. 1982 Jan;35(1):222–228. doi: 10.1128/iai.35.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan O. E., Jones J. H., Russell C. Experimental oral candidal infection and carriage of oral bacteria in rats subjected to a carbohydrate-rich diet and tetracycline treatment. J Med Microbiol. 1985 Dec;20(3):291–298. doi: 10.1099/00222615-20-3-291. [DOI] [PubMed] [Google Scholar]
- Hull T. G., Rettger L. The Influence of Milk and Carbohydrate Feeding on the Character of the Intestinal Flora: IV. Diet versus Bacterial Implantation. J Bacteriol. 1917 Jan;2(1):47–71. doi: 10.1128/jb.2.1.47-71.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON S. A. Candida (Monilia) albicans: effect of amino acids, glucose, pH, chlortetracycline (aureomycin), dibasic sodium and calcium phosphates, and anaerobic and aerobic conditions on its growth. AMA Arch Derm Syphilol. 1954 Jul;70(1):49–60. doi: 10.1001/archderm.1954.01540190051003. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A. Ecology of Candida albicans gut colonization: inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect Immun. 1985 Sep;49(3):654–663. doi: 10.1128/iai.49.3.654-663.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight L., Fletcher J. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteroids, and diabetes mellitus. J Infect Dis. 1971 Apr;123(4):371–377. doi: 10.1093/infdis/123.4.371. [DOI] [PubMed] [Google Scholar]
- Krause W., Matheis H., Wulf K. Fungaemia and funguria after oral administration of Candida albicans. Lancet. 1969 Mar 22;1(7595):598–599. doi: 10.1016/s0140-6736(69)91534-7. [DOI] [PubMed] [Google Scholar]
- Krishnan R., Wilkinson I., Joyce L., Rofe A. M., Bais R., Conyers R. A., Edwards J. B. The effect of dietary xylitol on the ability of rat caecal flora to metabolise xylitol. Aust J Exp Biol Med Sci. 1980 Dec;58(6):639–652. doi: 10.1038/icb.1980.66. [DOI] [PubMed] [Google Scholar]
- Larmas M., Mäkinen K. K., Scheinin A. Turku sugar studies. III. An intermediate report on the effect of sucrose, fructose and xylitol diets on the numbers of salivary lactobacilli, candida and streptococci. Acta Odontol Scand. 1974;32(6):423–433. doi: 10.3109/00016357409026551. [DOI] [PubMed] [Google Scholar]
- Maleszka R., Schneider H. Fermentation of D-xylose, xylitol, and D-xylulose by yeasts. Can J Microbiol. 1982 Mar;28(3):360–363. doi: 10.1139/m82-054. [DOI] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun. 1984 Jul;45(1):6–12. doi: 10.1128/iai.45.1.6-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen K. K., Ojanotko A., Vidgren H. Effect of xylitol on the growth of three oral strains of Candida albicans. J Dent Res. 1975 Nov-Dec;54(6):1239–1239. doi: 10.1177/00220345750540062901. [DOI] [PubMed] [Google Scholar]
- Olsen I., Birkeland J. M. Initiation and aggravation of denture stomatitis by sucrose rinses. Scand J Dent Res. 1976 Mar;84(2):94–97. doi: 10.1111/j.1600-0722.1976.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Pizzo P. A. Considerations for the prevention of infectious complications in patients with cancer. Rev Infect Dis. 1989 Nov-Dec;11 (Suppl 7):S1551–S1563. doi: 10.1093/clinids/11.supplement_7.s1551. [DOI] [PubMed] [Google Scholar]
- Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979 Aug;25(2):702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell C., Jones J. H. The effects of oral inoculation of the yeast and mycelial phases of Candida albicans in rats fed on normal and carbohydrate rich diets. Arch Oral Biol. 1973 Mar;18(3):409–412. doi: 10.1016/0003-9969(73)90165-9. [DOI] [PubMed] [Google Scholar]
- Salminen E. K., Salminen S. J., Porkka L., Kwasowski P., Marks V., Koivistoinen P. E. Xylitol vs glucose: effect on the rate of gastric emptying and motilin, insulin, and gastric inhibitory polypeptide release. Am J Clin Nutr. 1989 Jun;49(6):1228–1232. doi: 10.1093/ajcn/49.6.1228. [DOI] [PubMed] [Google Scholar]
- Salminen S., Salminen E., Koivistoinen P., Bridges J., Marks V. Gut microflora interactions with xylitol in the mouse, rat and man. Food Chem Toxicol. 1985 Nov;23(11):985–990. doi: 10.1016/0278-6915(85)90248-0. [DOI] [PubMed] [Google Scholar]
- Samaranayake L. P., MacFarlane T. W. The effect of dietary carbohydrates on the in-vitro adhesion of Candida albicans to epithelial cells. J Med Microbiol. 1982 Nov;15(4):511–517. doi: 10.1099/00222615-15-4-511. [DOI] [PubMed] [Google Scholar]
- Samonis G., Anaissie E. J., Rosenbaum B., Bodey G. P. A model of sustained gastrointestinal colonization by Candida albicans in healthy adult mice. Infect Immun. 1990 Jun;58(6):1514–1517. doi: 10.1128/iai.58.6.1514-1517.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone H. H., Geheber C. E., Kolb L. D., Kitchens W. R. Alimentary tract colonization by Candida albicans. J Surg Res. 1973 Apr;14(4):273–276. doi: 10.1016/0022-4804(73)90028-0. [DOI] [PubMed] [Google Scholar]
- Stone H. H., Kolb L. D., Currie C. A., Geheber C. E., Cuzzell J. Z. Candida sepsis: pathogenesis and principles of treatments. Ann Surg. 1974 May;179(5):697–711. doi: 10.1097/00000658-197405000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkheimer G., Schulz F. H., Aurich I., Strauch S., Beuthin K., Wendlandt H. Persorption of particles. Digestion. 1968;1(2):78–80. doi: 10.1159/000196836. [DOI] [PubMed] [Google Scholar]
- Walbaum S., Dujardin L. Individual evolution of digestive tract colonization of holoxenic mice by Candida albicans. Infect Immun. 1985 May;48(2):433–438. doi: 10.1128/iai.48.2.433-438.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. M., van Eys J. Nutritional significance of fructose and sugar alcohols. Annu Rev Nutr. 1981;1:437–475. doi: 10.1146/annurev.nu.01.070181.002253. [DOI] [PubMed] [Google Scholar]
- Wekell M. M., Hartman W. J., Dong F. M. Incidence of increased numbers of Clostridium perfringens in the intestinal tract of rats fed xylitol. J Nutr. 1980 Oct;110(10):2103–2108. doi: 10.1093/jn/110.10.2103. [DOI] [PubMed] [Google Scholar]
- Wells C. L., Balish E. Gastrointestinal ecology and histology of rats monoassociated with anaerobic bacteria. Appl Environ Microbiol. 1980 Jan;39(1):265–267. doi: 10.1128/aem.39.1.265-267.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. H., Perini F. Role of competition for nutrients in suppression of Clostridium difficile by the colonic microflora. Infect Immun. 1988 Oct;56(10):2610–2614. doi: 10.1128/iai.56.10.2610-2614.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]