Abstract
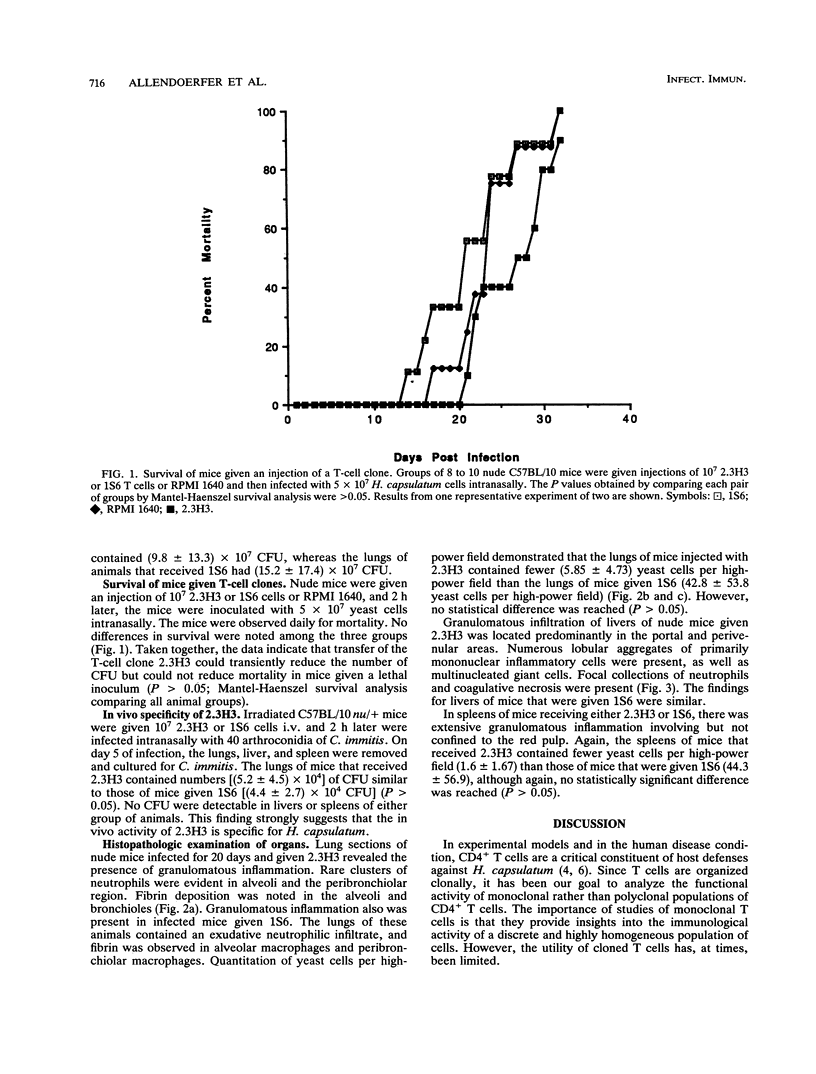
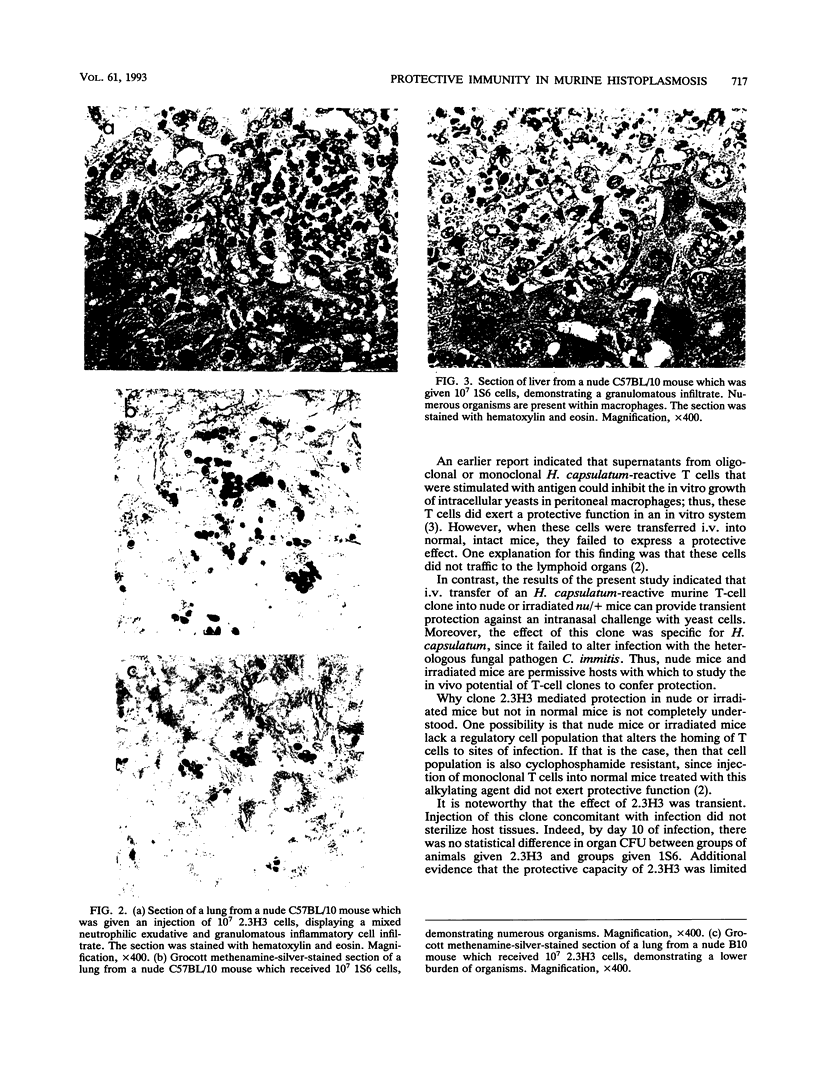
We have reported that a murine Histoplasma capsulatum-reactive CD4+ T-cell line and clones thereof did not adoptively transfer protection against H. capsulatum infection in normal or cyclophosphamide-treated C57BL/6 mice. One explanation for the results was that the T cells failed to traffic to lymphoid organs in these animals. In this study, we have sought to determine whether one of these clones, 2.3H3, could mediate protection in nude (C57BL/10) or irradiated (5 Gy) heterozygous nude (nu/+) C57BL/6 mice. Mice were inoculated intravenously with 10(7) resting 2.3H3 cells or with an equal number of cells of the ovalbumin-reactive clone 1S6; 2 h later, the mice were challenged intranasally with 5 x 10(6) yeast cells. By day 5 of infection, lungs, livers, and spleens of nude and irradiated nu/+ mice given 2.3H3 contained significantly fewer (P < 0.05) CFU than the same organs from mice inoculated with 1S6. This effect was specific for H. capsulatum, since 2.3H3 did not reduce the number of Coccidioides immitis CFU in lungs, livers, and spleens of irradiated nu/+ mice. By day 10, the amounts of H. capsulatum CFU in lungs, livers, or spleens of nude and irradiated nu/+ mice inoculated with 2.3H3 were smaller than those in 1S6-inoculated mice, but these differences did not reach statistical significance (P > 0.05). The mortality rate of mice inoculated with 2.3H3 and that of mice inoculated with 1S6 were similar. Histopathological examination of tissues from 2.3H3- and 1S6-inoculated mice demonstrated the presence of granulomatous inflammation in organs from both groups. Tissues from 2.3H3-treated mice contained fewer yeasts per high-power field than tissues from 1S6-treated mice. Thus, irradiated or nude mice are permissive for the expression of protective immunity by a CD4+ T-cell clone. Although the protective capacity of T cells in these animals is transient, these animals will be useful for differentiating protective from nonprotective T-cell clones.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991 Mar 8;251(4998):1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Deepe G. S., Jr Protective immunity in murine histoplasmosis: functional comparison of adoptively transferred T-cell clones and splenic T cells. Infect Immun. 1988 Sep;56(9):2350–2355. doi: 10.1128/iai.56.9.2350-2355.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Smith J. G., Sonnenfeld G., Denman D., Bullock W. E. Development and characterization of Histoplasma capsulatum-reactive murine T-cell lines and clones. Infect Immun. 1986 Dec;54(3):714–722. doi: 10.1128/iai.54.3.714-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A. M., Bullock W. E., Taylor C. L., Deepe G. S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988 Jul;56(7):1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R. A., Jr, Des Prez R. M. Pathogenesis and clinical spectrum of histoplasmosis. South Med J. 1973 Jan;66(1):13–25. doi: 10.1097/00007611-197301000-00004. [DOI] [PubMed] [Google Scholar]
- Graybill J. R. Histoplasmosis and AIDS. J Infect Dis. 1988 Sep;158(3):623–626. doi: 10.1093/infdis/158.3.623. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Reay P. A., Ehrich E. W., Davis M. M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell W., Goldberg D. M., Neu H. C. Histoplasmosis in patients with the acquired immune deficiency syndrome. Am J Med. 1986 Dec;81(6):974–978. doi: 10.1016/0002-9343(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Gootee L., Bucher C., Bullock W. E. Inhibition of intracellular growth of Histoplasma capsulatum yeast cells by cytokine-activated human monocytes and macrophages. Infect Immun. 1991 Feb;59(2):737–741. doi: 10.1128/iai.59.2.737-741.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat L. J., Slama T. G., Norton J. A., Kohler R. B., Eitzen H. E., French M. L., Sathapatayavongs B. Risk factors for disseminated or fatal histoplasmosis. Analysis of a large urban outbreak. Ann Intern Med. 1982 Feb;96(2):159–163. doi: 10.7326/0003-4819-96-2-159. [DOI] [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol. 1984 May;132(5):2593–2597. [PubMed] [Google Scholar]
- Wu-Hsieh B., Zlotnik A., Howard D. H. T-cell hybridoma-produced lymphokine that activates macrophages to suppress intracellular growth of Histoplasma capsulatum. Infect Immun. 1984 Jan;43(1):380–385. doi: 10.1128/iai.43.1.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]