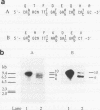
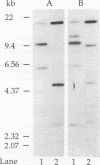
Abstract
A hemagglutinin with an M(r) of 67,000 (pMGA) from Mycoplasma gallisepticum S6 was purified by using monoclonal antibody affinity chromatography. Purified pMGA was treated with a number of enzymes, the resultant peptides were purified, and their amino acid sequence was determined by using an Applied Biosystems (model 471A) protein sequencer. The DNA sequence encoding two peptides was used to dictate the sequences of synthetic oligonucleotides which were used to screen a library of EcoRI-cut M. gallisepticum DNA in pUC18. A clone reactive to both probes was isolated and found to contain a recombinant insert of 10 kb. The clone was mapped by using restriction endonucleases and fragments subcloned into pUC18 for DNA sequencing. Analysis of part of the DNA sequence revealed an open reading frame containing 1,941 nucleotides which encoded 647 amino acids. The amino terminus was preceded by a putative leader sequence of 25 amino acids. A promoter region preceding the putative start codon GUG was also located. This gene would encode a mature protein of 67,660 Da. There were a number of differences between the predicted amino acid sequence and that determined by direct peptide sequencing. Also, two tryptic peptides of pMGA were not found in the DNA sequence. This suggested that the cloned gene did not encode pMGA but did encode a homolog (pMGA1.2). Furthermore, downstream of pMGA1.2 was a region of DNA encoding a leader sequence followed by an amino acid sequence with high homology to that encoded by the pMGA1.2 gene. The presence within M. gallisepticum of a family of pMGA genes is inferred from the DNA sequence and Southern transfer data. A possible role for this gene family in immune evasion is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frey M. L., Hanson R. P., Andrson D. P. A medium for the isolation of avian mycoplasmas. Am J Vet Res. 1968 Nov;29(11):2163–2171. [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Mechanism of signal peptide cleavage in the biosynthesis of the major lipoprotein of the Escherichia coli outer membrane. J Biol Chem. 1982 May 10;257(9):5177–5182. [PubMed] [Google Scholar]
- Inamine J. M., Denny T. P., Loechel S., Schaper U., Huang C. H., Bott K. F., Hu P. C. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene. 1988 Apr 29;64(2):217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Ho K. C., Loechel S., Hu P. C. Evidence that UGA is read as a tryptophan codon rather than as a stop codon by Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum. J Bacteriol. 1990 Jan;172(1):504–506. doi: 10.1128/jb.172.1.504-506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisohn S., Dykstra M. J. A quantitative study of single and mixed infection of the chicken trachea by Mycoplasma gallisepticum. Avian Dis. 1987 Jan-Mar;31(1):1–12. [PubMed] [Google Scholar]
- Levisohn S., Kleven S. H. Vaccination of chickens with nonpathogenic Mycoplasma gallisepticum as a means for displacement of pathogenic strains. Isr J Med Sci. 1981 Jul;17(7):669–673. [PubMed] [Google Scholar]
- Loechel S., Inamine J. M., Hu P. C. A novel translation initiation region from Mycoplasma genitalium that functions in Escherichia coli. Nucleic Acids Res. 1991 Dec 25;19(24):6905–6911. doi: 10.1093/nar/19.24.6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham P. F., Glew M. D., Brandon M. R., Walker I. D., Whithear K. G. Characterization of a major hemagglutinin protein from Mycoplasma gallisepticum. Infect Immun. 1992 Sep;60(9):3885–3891. doi: 10.1128/iai.60.9.3885-3891.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. F., Kirszbaum L., Walker I. D., d'Apice A. J. SP-40,40, a newly identified normal human serum protein found in the SC5b-9 complex of complement and in the immune deposits in glomerulonephritis. J Clin Invest. 1988 Jun;81(6):1858–1864. doi: 10.1172/JCI113531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L. D., Shane S. W., Karpas A. A., Cunningham T. M., Probst P. S., Barile M. F. Monoclonal antibodies to surface antigens of a pathogenic Mycoplasma hominis strain. Infect Immun. 1991 May;59(5):1683–1689. doi: 10.1128/iai.59.5.1683-1689.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. The Vlp system of Mycoplasma hyorhinis: combinatorial expression of distinct size variant lipoproteins generating high-frequency surface antigenic variation. J Bacteriol. 1991 Aug;173(15):4782–4793. doi: 10.1128/jb.173.15.4782-4793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruland K., Wenzel R., Herrmann R. Analysis of three different repeated DNA elements present in the P1 operon of Mycoplasma pneumoniae: size, number and distribution on the genome. Nucleic Acids Res. 1990 Nov 11;18(21):6311–6317. doi: 10.1093/nar/18.21.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Su C. J., Chavoya A., Dallo S. F., Baseman J. B. Sequence divergency of the cytadhesin gene of Mycoplasma pneumoniae. Infect Immun. 1990 Aug;58(8):2669–2674. doi: 10.1128/iai.58.8.2669-2674.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Tryon V. V., Baseman J. B. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect Immun. 1987 Dec;55(12):3023–3029. doi: 10.1128/iai.55.12.3023-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. L., McDaniel L. S., Blalock D. K., Fallon M. T., Cassell G. H. Heterogeneity among strains and a high rate of variation within strains of a major surface antigen of Mycoplasma pulmonis. Infect Immun. 1988 May;56(5):1358–1363. doi: 10.1128/iai.56.5.1358-1363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whithear K. G., Bowtell D. D., Ghiocas E., Hughes K. L. Evaluation and use of a micro-broth dilution procedure for testing sensitivity of fermentative avian mycoplasmas to antibiotics. Avian Dis. 1983 Oct-Dec;27(4):937–949. [PubMed] [Google Scholar]
- von Heijne G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989 May;2(7):531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]