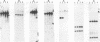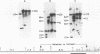Abstract
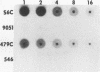
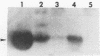
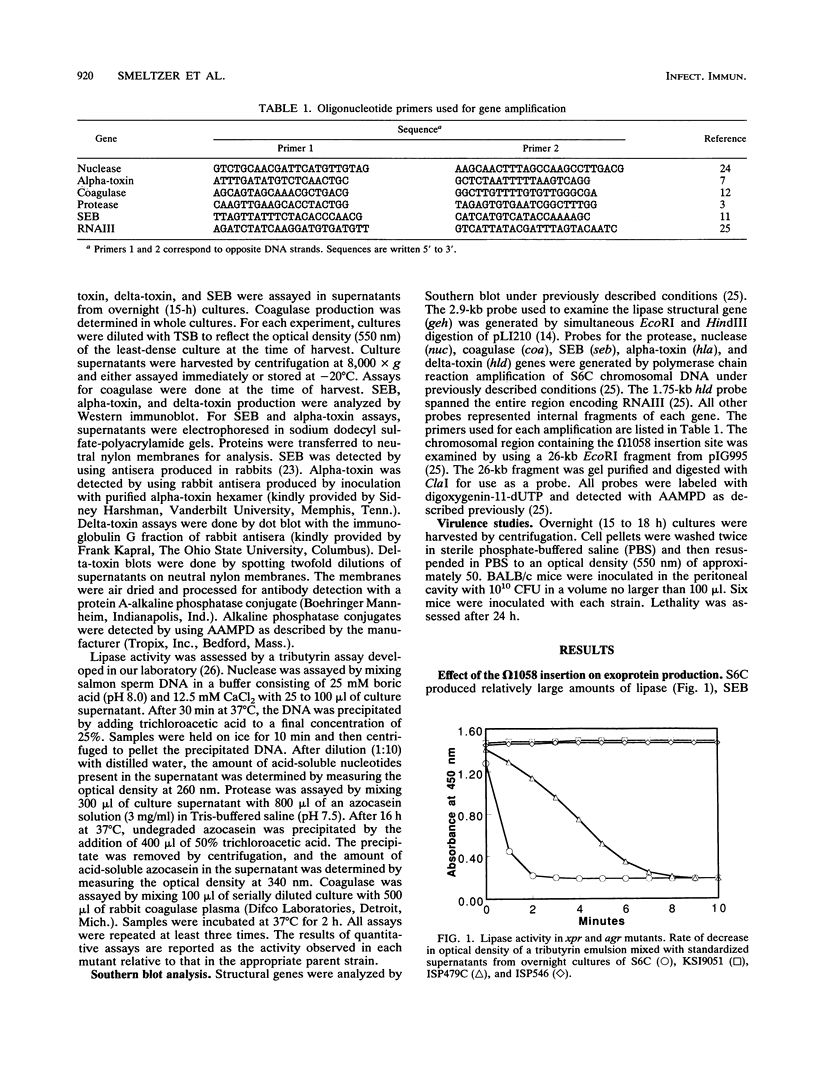
We recently described a Tn551 insertion in the chromosome of Staphylococcus aureus S6C that resulted in drastically reduced expression of extracellular lipase (M. S. Smeltzer, S. R. Gill, and J. J. Iandolo, J. Bacteriol. 174:4000-4006, 1992). The insertion was localized to a chromosomal site (designated omega 1058) distinct from the lipase structural gene (geh) and the accessory gene regulator (agr), both of which were structurally intact in the lipase-negative (Lip-) mutants. In this report, we describe a phenotypic comparison between strains S6C, a hyperproducer of enterotoxin B; KSI9051, a derivative of S6C carrying the Tn551 insertion at omega 1058; ISP546, an 8325-4 strain that carries a Tn551 insertion in the agr locus; and ISP479C, the parent strain of ISP546 cured of the Tn551 delivery plasmid pI258repA36. Compared with their respective parent strains, ISP546 and KSI9051 produced greatly reduced amounts of lipase, alpha-toxin, delta-toxin, protease, and nuclease. KSI9051 also produced reduced amounts of staphylococcal enterotoxin B. Coagulase production was increased in ISP546 but not in KSI9051. Using a mouse model, we also demonstrated that ISP546 and KSI9051 were far less virulent than ISP479C and S6C. We have designated the genetic element defined by the Tn551 insertion at omega 1058 xpr to denote its role as a regulator of extracellular protein synthesis. We conclude that xpr and agr are similar and possibly interactive regulatory genes that play an important role in pathogenesis of staphylococcal disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas-ali B., Coleman G. The characteristics of extracellular protein secretion by Staphylococcus aureus (Wood 46) and their relationship to the regulation of alpha-toxin formation. J Gen Microbiol. 1977 Apr;99(2):277–282. doi: 10.1099/00221287-99-2-277. [DOI] [PubMed] [Google Scholar]
- Campbell I. M. Secondary metabolism and microbial physiology. Adv Microb Physiol. 1984;25:1–60. doi: 10.1016/s0065-2911(08)60290-8. [DOI] [PubMed] [Google Scholar]
- Carmona C., Gray G. L. Nucleotide sequence of the serine protease gene of Staphylococcus aureus, strain V8. Nucleic Acids Res. 1987 Aug 25;15(16):6757–6757. doi: 10.1093/nar/15.16.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. L., Koomey J. M., Butler C. A., Projan S. J., Fischetti V. A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone-Post P., Malyankar U., Khan S. A. Role of host factors in the regulation of the enterotoxin B gene. J Bacteriol. 1991 Mar;173(5):1827–1830. doi: 10.1128/jb.173.5.1827-1830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S. D., Iandolo J. J., Chapes S. K. Murine macrophage activation by staphylococcal exotoxins. Infect Immun. 1991 Nov;59(11):4049–4055. doi: 10.1128/iai.59.11.4049-4055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill M. E., Khan S. A. Regulation of the enterotoxin B gene in Staphylococcus aureus. J Biol Chem. 1988 May 5;263(13):6276–6280. [PubMed] [Google Scholar]
- Gray G. S., Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus wood 46. Infect Immun. 1984 Nov;46(2):615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzon L., Arvidson S. The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. EMBO J. 1990 May;9(5):1391–1399. doi: 10.1002/j.1460-2075.1990.tb08254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzon L., Löfdahl S., Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989 Nov;219(3):480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida S., Miyata T., Yoshizawa Y., Kawabata S., Morita T., Igarashi H., Iwanaga S. Nucleotide sequence of the staphylocoagulase gene: its unique COOH-terminal 8 tandem repeats. J Biochem. 1987 Nov;102(5):1177–1186. doi: 10.1093/oxfordjournals.jbchem.a122156. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Iandolo J. J. Lysogenic conversion of staphylococcal lipase is caused by insertion of the bacteriophage L54a genome into the lipase structural gene. J Bacteriol. 1986 May;166(2):385–391. doi: 10.1128/jb.166.2.385-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. H., Kornblum J., Kreiswirth B., Novick R., Foster T. J. Regulation of the protein A-encoding gene in Staphylococcus aureus. Gene. 1992 May 1;114(1):25–34. doi: 10.1016/0378-1119(92)90703-r. [DOI] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regassa L. B., Betley M. J. Alkaline pH decreases expression of the accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1992 Aug;174(15):5095–5100. doi: 10.1128/jb.174.15.5095-5100.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regassa L. B., Couch J. L., Betley M. J. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect Immun. 1991 Mar;59(3):955–962. doi: 10.1128/iai.59.3.955-962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regassa L. B., Novick R. P., Betley M. J. Glucose and nonmaintained pH decrease expression of the accessory gene regulator (agr) in Staphylococcus aureus. Infect Immun. 1992 Aug;60(8):3381–3388. doi: 10.1128/iai.60.8.3381-3388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollof J., Braconier J. H., Söderström C., Nilsson-Ehle P. Interference of Staphylococcus aureus lipase with human granulocyte function. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):505–510. doi: 10.1007/BF01962601. [DOI] [PubMed] [Google Scholar]
- Rollof J., Hedström S. A., Nilsson-Ehle P. Lipolytic activity of Staphylococcus aureus strains from disseminated and localized infections. Acta Pathol Microbiol Immunol Scand B. 1987 Apr;95(2):109–113. doi: 10.1111/j.1699-0463.1987.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):902–911. doi: 10.1128/iai.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983 May-Jun;22(2-3):181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Smeltzer M. S., Gill S. R., Iandolo J. J. Localization of a chromosomal mutation affecting expression of extracellular lipase in Staphylococcus aureus. J Bacteriol. 1992 Jun;174(12):4000–4006. doi: 10.1128/jb.174.12.4000-4006.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer M. S., Hart M. E., Iandolo J. J. Quantitative spectrophotometric assay for staphylococcal lipase. Appl Environ Microbiol. 1992 Sep;58(9):2815–2819. doi: 10.1128/aem.58.9.2815-2819.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F., Kornblum J., Novick R. P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991 Oct;173(20):6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. C., Bergdoll M. S. Effect of environmental conditions on production of toxic shock syndrome toxin 1 by Staphylococcus aureus. Infect Immun. 1990 Apr;58(4):1026–1029. doi: 10.1128/iai.58.4.1026-1029.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]