Abstract
Indoleamine 2,3-dioxygenase (IDO) suppresses the functions of CD4+ T cells through its ability to metabolize the essential amino acid tryptophan. Although the activity of IDO is required for the immunosuppression of allergic airway disease by the Toll-Like-Receptor 9 (TLR9) agonist, oligonucleotides comprised of cytosine and guanine nucleotides linked by phosphodiester bonds (CpG) DNA, it is unclear whether IDO expression by resident lung epithelial cells is sufficient to elicit these effects. Therefore, we created a transgenic mouse inducibly overexpressing IDO within nonciliated airway epithelial cells. Upon inhalation of formalin-fixed Aspergillus fumigatus hyphal antigens, the overexpression of IDO from airway epithelial cells of these mice reduced the number of CD4+ T cells within the inflamed lung and impaired the capacity of antigen-specific splenic CD4+ effector T cells to secrete the cytokines IL-4, IL-5, IL-13, and IFN-γ. Despite these effects, allergic airway disease pathology was largely unaffected in mice expressing IDO in airway epithelium. In support of the concept that dendritic cells are the major cell type contributing to the IDO-inducing effects of CpG DNA, mice expressing TLR9 only in the airway epithelium did not augment IDO expression subsequent to the administration of CpG DNA. Furthermore, the systemic depletion of CD11c+ cells rendered mice incapable of CpG DNA-induced IDO expression. Our results demonstrate that an overexpression of IDO within the airway epithelium represents a novel mechanism by which the number of CD4+ T cells recruited to the lung and their capacity to produce cytokines can be diminished in a model of allergic airway disease, and these results also highlight the critical role of dendritic cells in the antiasthmatic effects of IDO induction by CpG DNA.
Keywords: asthma, inflammation, lung, epithelial
CLINICAL RELEVANCE.
This study demonstrates the role of airway epithelial indoleamine 2,3-dioxygenase (IDO) activity in a model of allergic airway disease. Although IDO reduces CD4+ T-cell numbers and activities in the lung, additional cells, particularly CD11c+ dendritic cells, are a more effective cell type that functions in the IDO-inducing response to the antiallergic effects of Toll-like receptor (TLR)9 agonists.
Allergic asthma is characterized by airway eosinophilia, hyperresponsiveness to bronchoconstricting agents, and airway remodeling. In allergic asthma, activated CD4+ T cells largely drive the pathophysiology of the disease (1) by producing significant amounts of IL-4, IL-5, and IL-13 (2–4). These cytokines are responsible for many of the features associated with allergic airway disease, including class-switching in B cells to produce IgE and IgG1 (5), eosinophilia (6), and mucous metaplasia (7). Although several therapies are available for the treatment of asthma, none appear to be as effective as corticosteroids (8). However, the use of corticosteroids may promote undesirable side effects, and many patients are refractory to this treatment (9, 10). Because the incidence of allergic asthma is increasing in the Western world (11) and current therapies are not totally effective (12), the development of novel treatments for the disease is necessary.
CD4+ T cells isolated from patients with severe asthma express markers indicative of activation, compared with those from normal control subjects or patients with mild asthma (13). CD4+ T cells were also found to proliferate within the peribronchiolar regions of the lungs of patients with asthma (14) and within the lungs of mice after exposure to ultrafine carbon and ovalbumin (15). Therefore, inhibiting the activities of these cells within the lung may be imperative in controlling the disease (16, 17). Capitalizing on the peribronchiolar location of activated CD4+ T cells in allergic asthma may provide an opportunity to control the pathogenic effects of these cells via the airway epithelium.
Indoleamine 2,3-dioxygenase (IDO) is an enzyme capable of limiting the proliferation of CD4+ T cells through its ability to metabolize tryptophan (18, 19). The consequences of tryptophan metabolism are twofold. First, by depleting the local microenvironment of tryptophan, the least abundant but an essential amino acid, cells are unable to synthesize proteins (18). Second, the downstream catabolites of tryptophan metabolism, including kynurenine and 3-hydroxyanthranilic acid (3-HAA), induce the apoptosis of cells (20, 21), most notably those undergoing proliferation, such as activated lymphocytes. IDO is expressed in many cell types, and particularly in subsets of antigen-presenting cells (18, 21), but can be induced in lung structural cells (22). However, the ability of IDO to limit the proliferation of CD4+ T cells when expressed by mouse airway epithelial cells, specifically the nonciliated airway epithelial cells of the lung, has not been described.
The activity of IDO was demonstrated to be largely responsible for reducing features associated with allergic airway disease in a mouse model in which IDO expression was induced through the administration of oligonucleotides comprised of cytosine and guanine nucleotides linked by phosphodiester bonds (CpG) DNA, a Toll-Like-Receptor 9 (TLR9) agonist (23). Although several studies documented the substantial expression of IDO in plasmacytoid dendritic cells (24), the consequences of IDO expression in other pulmonary cell types remain incompletely studied. The expression of IDO by airway epithelial cells may render proliferating cells that are in proximity to the airway, such as CD4+ T cells (25), susceptible to the consequences of IDO activity.
We created a line of triple-transgenic mice in which IDO is inducibly overexpressed only within the nonciliated airway epithelial cells of the lung (CC10-IDO). We used the CC10-IDO transgenic mouse in a model of allergic airway disease in which a formalin-fixed extract of hyphae from the fungi Aspergillus fumigatus was inhaled to promote antigen sensitization via the lung. The allergic sensitization that occurs via inhalation was reported to promote the clonal expansion of CD4+ T cells to a similar extent in both the lung and mediastinal lymph node (15). We report that airway epithelial IDO activity significantly reduced the number of lymphocytes in the bronchoalveolar lavage (BAL) fluid, the number of CD4+ T cells within the lung, and the concentrations of antigen-specific cytokines produced from ex vivo restimulated splenic CD4+ T cells. Despite these cellular effects, the pathophysiologic alterations in this model of allergic airway disease were unaffected. We provide additional data demonstrating that dendritic cells are a more relevant target of CpG DNA and a more relevant inducer of lung IDO expression than are airway epithelial cells. This study provides evidence for a novel means through which the activities of CD4+ T cells can be inhibited in a tissue-specific manner, as an alternative to global immune suppression, which can systemically compromise the ability of the immune system to function properly.
MATERIALS AND METHODS
Airway Epithelial Cell Culture and IDO Activity Assay
Mouse transformed airway epithelial cells (MTCCs) were obtained from Francisco DeMayo (Baylor College of Medicine, Houston, TX) and plated at a density of 3 × 104 cells per well in a 96-well plate with 200 μl media (RPMI 1640; ATCC, Manassas, VA). Adenovirus expressing either β-galactosidase (LacZ) or IDO, obtained from the University of Pittsburgh Vector Core, was added 24 hours after plating the cells. To measure kynurenine production, a high tryptophan-containing medium (RPMI 1640 containing 600 μM tryptophan) was added to the cells with the virus. Some wells were also treated with 1-methyl tryptophan (1-MT) (Sigma, St. Louis, MO). The 1-MT was dissolved in 1 M NaOH to create a stock concentration of 20 mM in media. Immediately before adding the cells, the 1-MT was further diluted to 1.5 mM. Cells were incubated at 37°C with virus for 48 hours, after which kynurenine was measured in the medium. We removed 160 μl of medium from each well and added 10 μl of 30% trichloracetic acid, and this was incubated at 50°C for 30 minutes to hydrolyze N-formyl–kynurenine to kynurenine. After centrifugation at 600 g for 10 minutes, the supernatant was transferred to a 96-well plate, and 100 μl of 1.2% (wt/vol) 4-(dimethylamine) benzaldehyde (Ehrlich reagent; Sigma) in glacial acetic acid were added. After 10 minutes at room temperature, the absorbance was read at 492 nm. A standard curve of kynurenine was generated to quantitate the concentration in virus-treated samples.
Whole Lung Extraction for IDO Activity
Whole lung was excised and frozen at −80°C until the assay was performed. Frozen lung was crushed using a liquid nitrogen–chilled mortar and pestle, and subsequently homogenized in 2 volumes of 0.14 M potassium chloride/0.02 M potassium phosphate buffer, pH 7.0. The homogenate was centrifuged at 30,000 × g for 30 minutes. The supernatant was removed and added to assay buffer (1:1), which contained 50 mM potassium phosphate buffer, 20 mM ascorbate, 200 μg/ml catalase, 10 μM methylene blue, and 400 μM L-tryptophan. The reaction was incubated at 37°C for 30 minutes, at which time the reaction was stopped and proteins were precipitated by the addition of 30% trichloracetic acid. The solution was then centrifuged at 1,000 × g for 5 minutes, and HPLC was performed to measure the production of kynurenine and the depletion of tryptophan.
HPLC
HPLC was performed to measure kynurenine production, using samples prepared as already described. We injected 100 μl of sample into a C-18 column (250 × 4.6 mm, 5 μm; Waters, Milford, MA). The mobile phase consisted of 5 mM ZnCl2, 5 mM acetic acid, and 4% acetonitrile, pH 4.9, and samples were eluted at 1 ml/minute. The production of kynurenine was measured at an absorbance of 254 nm, whereas the depletion of tryptophan was measured in fluorescence (excitation, 360 nm; emission, 460 nm). Standards of kynurenine and tryptophan were run with the assay to establish the retention times and to allow for the determination of concentrations in the samples.
Semiquantitative and Quantitative RT-PCR
Total RNA was isolated using RNeasy Columns (Qiagen, Valencia, CA). The RNA was DNase-treated and reverse-transcribed into cDNA, using SuperscriptII (Invitrogen, Carlsbad, CA). Conventional RT-PCR was performed using SYBR PCR Master Mix (Bio-Rad, Hercules, CA). Real-time quantitative RT-PCR was performed using the iQ Supermix (Bio-Rad) and intron-spanning primers and probes (Applied Biosystems, Foster City, CA) for IDO, CC chemokine ligand 20 (CCL20), and mouse TLR9 (mTLR9), or SYBR PCR Master Mix and intron-spanning primers for human TLR9 (hTLR9) and β-actin. Forty cycles of PCR were performed using the Bio-Rad Chromo4 under universal cycling conditions: denaturation at 95°C for 15 seconds, and annealing/extension at 60°C for 1 minute. The level of gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels, and relative mRNA levels were determined according to the comparative cycle threshold method (ABI Prism 7700 Sequence Detection System, User Bulletin No. 2; Applied Biosystems). Briefly, the threshold cycle (CT) was determined for the gene of interest and GAPDH in each sample. The ΔCT was calculated for each sample by subtracting the CT of GAPDH from the CT of IDO. The ΔΔCT values were calculated by subtracting the ΔCT of Tg+ mice from the ΔCT of Tg− mice. The ΔΔCT values were transformed into absolute values, using the equation: 2T−ΔΔC. Primer sequences for assessing the expression of the human TLR9 (hTLR9) transgene were hTLR9 forward, 5′-CAGGGACAACCACCACTTCT-3′; and human growth hormone (hGH) reverse, 5′-GAGCAGGCCAAAAGCCAGGA-3′; and for β-actin, forward 5′-TCCTTCGTTGCCGGTCCACA-3′, and reverse 5′-CGTCTCCGGAGTCCATCACA-3′.
Mice
We purchased 6–8-week-old female BALB/cJ mice from Jackson Laboratories (Bar Harbor, ME). We purchased 6–8-week-old male B6.FVB-Tg(Itgax-DTR/EGFP)57LanJ mice from Jackson Laboratories and bred them with C57BL/6J mice. Transgene-positive pups were identified by PCR. All mice were housed in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility at the University of Vermont. Mice were maintained on a 12-hour light/dark cycle and received food and water ad libitum. Our Institutional Animal Care and Use Committee granted approval for all studies.
Generation of Doxycycline-Inducible CC10-IDO Transgenic Mice
Transgenic mice were generated using three constructs: rCC10-tTS-hGHpA (suppressor; with rat CC10-tTS-human growth hormone for RNA stability), rCC10-M2-hGHpA (activator), and pTetOP-hGHpA. These constructs were a kind gift of Dr. Prabir Ray at the University of Pittsburgh. The hemagglutinin (HA)-tagged IDO sequence was created through PCR amplification of whole-lung cDNA generated from BALB/cJ mice. The primers used included: forward, 5′-CCGTCGACATGTACCCATACGACGTCCCAGACTACGCTGCACTCAGTAAAATATCTCCTAC-3′; and reverse, 5′-CGCGATATCCTAAGGCCAACTCATAAGAGCTTTCTC -3′. The forward primer contained a SalI site followed by the HA tag, and the reverse primer contained an EcoRV site followed by a stop codon. The DNA sequence created through PCR was then cloned into the TA cloning vector (Invitrogen), the HA-IDO sequence was removed using SalI and EcoRV, and the HA-IDO was subsequently placed in the multiple cloning site of the pTetOP-hGH vector, using the same enzymes. After all three constructs had been generated and the sequences verified, each was digested with BssHII to remove the promoter, transgene, and hGH polyA tail. The three separate transgenes were purified by three rounds of dialysis in injection buffer using 0.22-μm membranes (Millipore, Bedford, MA), and all three constructs were simultaneously microinjected into fertilized (C57BL/6 X C3H/HeN)F2 eggs. Transgenic mice were generated as previously described by the transgenic mouse facility at the University of Vermont (26). Three lines of founders were obtained. However, only one of the three founders had inducible expression, and this founder was further backcrossed onto the BALB/cJ strain for at least eight generations. Initially, the founders were identified by slot blot, as previously described (27), in which probes were generated by a restriction digest specific for tetracycline-controlled reverse transactivator (M2), tetracycline-controlled transcriptional silencer (tTS), and the tetracycline-regulated operator (TetOP). The probes for M2 were generated by digesting the CC10-M2-hGH plasmid with HindIII and BamHI restriction enzymes, for tTS by digesting the CC10-tTS-hGH plasmid with HindIII and BamHI, and for TetOP using XhoI and BamHI. A 1% agarose gel was run, and the band of interest was excised and extracted from the gel, using a gel extraction kit from Qiagen. PCR was established subsequent to the slot blot procedure, and transgenic mice were identified through the PCR of genomic DNA for the incorporation of each transgene tTS, M2, and IDO. Primer sequences included: IDO forward, GAAGAGCCCTCAAATGTGGA; and hGH reverse, GAGCAGGCCAAAAGCCAGGA. For IDO, the forward primer was generated within the IDO sequence, and the reverse primer was specific for hGH, to differentiate between endogenous IDO and transgene IDO. Primer sequences for M2 included: forward, GACCAAAGTCATAAACGGCGC; M2 reverse, CGCGATGTGAGAGGAGAGCA; tTS forward, GAGCACAGCCACATCTTCAA; and tTS reverse, GAGTTGGCAGTTTCTCC.
Generation of TLR9−/− x CC10-hTLR9 Mice
CC10-hTLR9 mice were generated by excising the hTLR9 cDNA from the pCMV-SPORT6 plasmid (IMAGE clone identification 5,495,717), purchased from Invitrogen, with EcoRV and NotI. The ends of the hTLR9 fragment were filled in using a Klenow fragment. A plasmid containing the rat CC10 promoter, mutant IκBα, and hGHpolyA (27) was cut with SmaI and BamHI to liberate the IkBα fragment. The ends of the plasmid containing the rat CC10 promoter and hGHpolyA were filled in using a Klenow fragment, treated with calf intestinal alkaline phosphatase, and ligated with the hTLR9 fragment. The correct orientation of the hTLR9 insert was confirmed using HindIII. The rCC10-hTLR9-hGHpolyA fragment was excised using BssHII, resolved on a 1% agarose gel, and purified using Elutip-D minicolumns, according to the manufacturer's instructions (Schleicher and Schuell, Keene, NH). The transgene was further purified by three rounds of dialysis against the injection buffer, using 0.22-μm membranes (Millipore), and microinjected into fertilized (C57BL/6 × C3H/HeN)F2 eggs. Transgenic mice were generated, and transgene integration was analyzed by slot blot, using a 502-base pair (bp) fragment from the human growth hormone sequence. Six transgenic founders were obtained, and three lines from these founders were backcrossed for six generations into TLR9−/− mice on C57BL/6J. Transgenic mice were subsequently identified from genomic DNA obtained by ear-punch biopsy, using the following PCR primers: rCC10 forward, 5′-CACATTACAACATCAGCCCACATC-3′; and hTLR9 reverse, 5′-CTATTCGGCCGTGGGTCCCTGGC-3′.
Aspergillus fumigatus Crude Hyphal Extract Production
A. fumigatus lysates were generously provided by Dr. Kieren Marr (University of Washington School of Medicine, Seattle, WA), and were generated in a culture of Af293 isolate for 5 days at 37°C in RPMI-1640 plus 10% FCS. The hyphal mat was harvested, sequentially washed in PBS, and disrupted by vortexing with glass beads. To inactivate the extract, the slurry was subjected to 1% paraformaldehyde, and the protein concentration was measured using the Bradford Assay (Bio-Rad) according to the manufacturer's instructions. The product was concentrated using endotoxin-free dialysis membrane with a 10-kD pore size (Pierce Biotechnology, Rockford, IL) to achieve a final protein concentration of 800–1,000 μg/ml.
Aspergillus fumigatus Antigen Airway Exposure
Mice were anesthetized with isofluorane and administered 1 μg of A. fumigatus hyphal extract in 40 μl of PBS via oropharyngeal aspiration on days 0, 7, and 14. Mice were analyzed on day 19. For oropharyngeal aspiration, mice were anesthetized with inhaled isoflurane and suspended by their incisors on a 60° incline board with their tongues gently extended, and 25 μl of extract were delivered into the distal part of the oropaharynx and aspirated into the lower respiratory tract.
Assessment of Pulmonary Function to Measure Airway Hyperresponsiveness
Mice were anesthetized with 90 mg/kg of pentobarbital, tracheotomized, and mechanically ventilated for the assessment of pulmonary function using the forced oscillation technique, as previously described (28, 29). Briefly, the tracheotomy tube was connected to a computer-controlled, volume-cycled ventilator (FlexiVent; SCIREQ, Inc., Montreal, PQ, Canada), and mice were ventilated at 160–200 breaths/minute with a tidal volume of 0.2 ml and 3 cm H2O positive end-expiratory pressure. Pressure, flow, and volume were measured to calculate the baseline and peak responses for airway resistance, tissue damping, and tissue stiffness (30) after challenge with inhaled doses of saline or aerosolized methacholine (Sigma-Aldrich, St. Louis, MO) in saline, ranging from 3.125–50 mg/ml in half-log increments, as previously described (31). The percentage of the response relative to baseline after each methacholine dose is reported.
Bronchoalveolar Lavage and Cell Enumeration
BAL fluid was collected after assessment of pulmonary function from euthanized mice by the instillation and recovery of 1 ml of 0.9% NaCl plus protease inhibitor cocktail (Sigma-Aldrich) into the lungs through the tracheal cannula, using a tuberculin syringe. BAL fluid was centrifuged at 400 × g, and the total cells in the pellet were resuspended in PBS and enumerated by counting with an Advia 120 Hematology System (Bayer HealthCare, Tarrytown, NY). For differential cell counts, 2 × 104 cells were centrifuged onto glass slides at 800 rpm. Cytospins were stained using the Hema3 kit (Biochemical Sciences, Inc., Swedesboro, NJ), and at least 500 cells were counted.
Histopathology and Morphometry
After euthanasia and bronchoalveolar lavage, the left lobes of lungs were instilled with 4% paraformaldehyde in PBS (4% PFA) for 10 minutes at a pressure of 25 cm H2O, and placed into 4% PFA at 4°C overnight for the fixation of tissue. Fixed lungs were then mounted in paraffin, and 7-μm sections were cut, affixed to glass microscope slides, and deparaffinized. Antigen unmasking was performed by boiling for 10 minutes in 10 mM sodium citrate buffer (pH 6.0). Slides were washed three times in distilled water and incubated in 3% hydrogen peroxide for 10 minutes, followed by two washes in water for 5 minutes each and then in PBS for 5 minutes each, and incubated in normal serum (Vectastain ABC Kit; Vector Laboratories, Burlingame, CA) for 20 minutes. Slides were washed with PBS and stained with HA antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:500 dilution in normal horse serum overnight at 4°C, and 100–400 μl of diluted antibody were added to each section. The remainder of the protocol was followed according to the manufacturer of the Vectastain ABC Kit. Multiple airways of similar size with a length/diameter ratio of less than 2:1 were assessed in each section by individuals blinded to the identity of sections. Sections were assessed for overall architecture, inflammation, and epithelial appearance.
Preparation, Stimulation, and ELISA Analysis of CD4+ Lymphocyte Suspensions
Single-cell suspensions were generated from spleens by passing the tissues through a 70-μm mesh, and lymphocytes were enriched in LSM Lymphocyte Separation Medium (MP Biomedicals, Irvine, CA). CD4+ T cells were isolated by positive selection, using CD4 magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol. Isolated CD4+ T cells were > 95% pure, as assessed by CD4 surface staining and FACS analysis. CD4+ T cells (4 × 106 cells/ml) were activated with 1 μg/ml of Aspergillus hyphael extract in the presence of antigen-presenting cells (APCs) (4 × 106 cells/ml) from naive BALB/cJ mice, obtained through splenic T-cell depletion by negative selection, using antibodies to CD4, CD8, and Thy-1 and treatment with rabbit complement and mitomycin C, as previously described (32). After 72 hours of stimulation in RPMI-1640 media (ATCC) containing 50 μM 2-mercaptoethanol and 10 μg/ml folic acid, supernatants were collected and analyzed by ELISA for IL-4, IL-5, IL-13, and IFN-γ, according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). Optical densities (ODs) from triplicate samples and duplicate standards were read using a PowerWavex platereader (BioTek Instruments, Winooski, VT) at 450 nm, with background subtraction at 540 nm.
In Vitro Airway Epithelial Cell and CD4+ T-Cell Coculture
MTCCs were irradiated at 5,000 rad for 5 minutes, and 3 × 104 cells/well were plated in a 96-well plate. Twenty-four hours later, the medium was removed, and 1 × 109 viral particles (VP)/ml of adenovirus expressing IDO (AdIDO) or adenovirus expressing LacZ (AdLacZ) were added to each well for 24 hours. CD4+ T cells were generated by positive selection, as already described, and 1.5 × 105 cells/well were added to the MTCC culture after three washes. Anti-CD23 and anti-CD28 antibodies (B.D. Pharmingen, San Jose, CA) were added to a final concentration of 5 μg/ml and 1 μg/ml, respectively, to stimulate the CD4+ T cells polyclonally. Forty-eight hours later, cells were tritium-pulsed with 0.2 μCi/well 3H-thymidine (Perkin-Elmer, Boston, MA) and stimulated with 1 μg/ml each of anti-CD3 and anti-CD28. Twenty-four hours after the tritium pulse, the plates were harvested using a Tomtec Harvester 96 (Tomtec, Hamden, CT) according to the manufacturer's instructions, and liquid scintillation was used to measure CD4+ T-cell proliferation.
Whole-Lung Extraction and Flow Cytometry
The trachea of each mouse was cannulated, and the lungs were removed. Lungs were then inflated with 1 ml of RPMI containing 1 mg/ml of collagenase and 0.025 μl/ml of DNase. Lungs were further cut into pieces in 5 ml of RPMI containing collagenase and DNase, and incubated at 37°C for 30 minutes with gentle shaking. A catheter needle was used to break up the lung further, followed by a 30-minute incubation at 37°C with gentle shaking. The digested lung was then filtered through a 70-μM filter, and the supernatant was spun at 1,600 rpm for 5 minutes. The supernatant was removed, the pellet was resuspended in 1 ml of PBS followed by 12 ml of Gey's buffer, inverted to mix, and centrifuged at 4°C for 5 minutes at 1,600 rpm. The supernatant was removed and the pellet was resuspended in 8 ml of media, passed through a 40-μM filter, and centrifuged at 4°C for 5 minutes at 1,600 rpm. One wash was performed, the resulting pellet was resuspended in 5 ml of media, the cells were counted, and 1 × 106 cells were incubated in Fc block (anti-CD16/anti-CD32; B.D. Biosciences, Franklin Lakes, NJ) for 15 minutes on ice to prevent nonspecific antibody binding via Fc receptors. Cells were stained for 15 minutes on ice in 50 μl of antibody solution at the optimal concentration. Antibodies included CD4-PE-Texas Red (CalTag, Dorchester, UK), CD8α-AlexaFluor 647, TCRβ-FITC, and B220-PE (B.D. Biosciences). CD1-d tetramer, loaded with PBS57 and conjugated to phycoerythrin to stain natural killer T (NKT) lymphocytes, was obtained from the tetramer facility (Emory University Vaccine Center, Atlanta, GA) of the National institutes of Health. After 15 minutes, cells were washed, fixed, and subsequently distinguished using a LSR II FACS (Becton Dickinson, Franklin, NJ).
CpG DNA and Diphtheria Toxin Treatment
Mice were administered CpG DNA oligodeoxynucleotides (ODN 10104) or non-CpG (ODN 1827) (Coley Pharmaceutical, Ottawa, ON, Canada) via intraperitoneal injection or oropharyngeal aspiration. Diphtheria toxin (Sigma) was administered to CD11c-DTR mice at 50 μg/mouse, at a concentration of 1 mg/ml.
Western Blot for IDO and Actin
Lungs were frozen at −80° until analyses were performed. Powdered lungs were resuspended in Ripa buffer (25 mM Tris-Cl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) containing protease inhibitor cocktail (Sigma) and gently mixed for 1 hour at 4°C. The protein content was determined using the Bradford assay (Bio-Rad), according to manufacturer's instructions. Fifty miocrograms of protein were run on a 15% polyacrylamide gel, and proteins were then transferred to nitrocellulose membranes. Membranes were blocked at room temperature for 1 hour in 5% nonfat dry milk. The primary antibody was incubated in 1% nonfat dry milk and 0.03% Tween-20 overnight at a dilution of 1:1,000. The IDO antibody was purchased from Chemicon (Billerica, MA), and the actin antibody was purchased from Santa Cruz Biotechnology. The secondary antibody was used at 1:1,000 in the same buffer as the primary antibody for 1 hour at room temperature.
Statistical Analysis
Data were analyzed using the Student t test and two-way ANOVA, with Bonferroni correction for multiple comparisons. Statistical calculations were performed using GraphPad Prism 5 for Windows (GraphPad Software, Inc., La Jolla, CA). P < 0.05 was considered statistically significant.
RESULTS
Mouse Transformed Airway Epithelial Cells Overexpressing IDO Produce Kynurenine
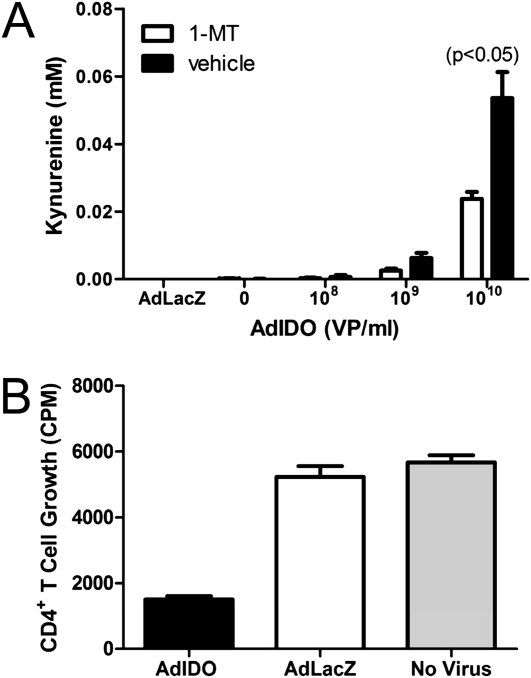
The activity of IDO from antigen-presenting cells such as macrophages and dendritic cells is well-described (18, 21, 33). However, the activity of IDO from mouse nonciliated airway epithelial cells is not. A mouse transformed airway epithelial cell line, MTCC, was transduced with increasing amounts of AdIDO, and the production of kynurenine was measured. As a control, AdLacZ was used at a concentration of 1 × 1010 viral particles (VP)/ml. Increasing amounts of AdIDO promoted increased levels of kynurenine production, whereas AdLacZ did not augment kynurenine production (Figure 1A). Kynurenine production by IDO-transduced MTCC was diminished using a specific inhibitor of IDO, 1-MT. These results demonstrate that IDO expression from airway epithelial cells can promote the production of kynurenine, a molecule implicated in the suppression of CD4+ T cell activities (34).
Figure 1.
Kynurenine production and CD4+ T-cell proliferation attributable to indolamine 2,3-dioxygenase (IDO) expression from transformed mouse airway epithelial cells. Transformed mouse airway epithelial cells (MTCCs) were transduced with a control adenovirus expressing LacZ (AdLacZ) or increasing amounts (VP, viral particles) of adenovirus expressing IDO (AdIDO). (A) We used 600 μM 1-methyl tryptophan (1-MT) to inhibit IDO activity. An IDO activity assay was performed to measure the production of kynurenine. MTCCs transduced with AdIDO, a control adenovirus (AdLacZ), or left untransduced (No Virus) were cocultured with CD4+ T cells isolated from spleens of wild-type mice and stimulated in vitro with anti-CD3 and anti-CD28. (B) CD4+ T-cell proliferation was measured by tritiated thymidine incorporation. Values are means (± SEM) of three wells/condition, and are representative studies performed twice.
IDO Production from Mouse Airway Epithelial Cells Reduces the Proliferation of CD4+ T Cells
We next determined whether the expression of IDO from MTCCs would result in the inhibition of CD4+ T-cell proliferation. Using AdIDO, or AdLacZ as a control, MTCCs were transduced and cocultured with CD4+ T cells that had been isolated from wild-type mice. The expression of IDO from MTCCs reduced the polyclonal proliferation of CD4+ T cells more than twofold (Figure 1B). However, the expression of the control virus, AdLacZ, had no effect on the proliferation of CD4+ T cells, because CD4+ T cells that were cultured alone proliferated at a similar level. These studies demonstrate that the expression of IDO by murine airway epithelial cells in vitro can diminish CD4+ T-cell proliferation and provide a rationale for expressing IDO in nonciliated airway epithelial cells in vivo.
Characterization of CC10-IDO Doxycycline-Inducible Mice
Based on the results of the in vitro studies, a line of transgenic mice was generated that inducibly overexpressed IDO only within nonciliated airway epithelial cells. A suppressor and an activator of a TetOP were placed downstream of the rat CC10 promoter in two separate DNA constructs. The CC10 promoter was chosen because the CC10 protein is expressed exclusively within nonciliated airway epithelial cells (35). A third construct was created in which mouse IDO cDNA was placed downstream of the tetracycline operator. The three DNA constructs (Figure 2A) were injected into mouse oocysts, and after 8–10 generations of backcrossing into BALB/cJ mice, experiments were performed.
Figure 2.
CC10-IDO mouse characterization. CC10-IDO triple-transgenic mice were created by the genomic incorporation of three transgenes. (A) One transgene encodes an activator of transcription, whereby transcription is directed by the rat CC10 (rCC10) promoter, restricting transcription to nonciliated airway epithelial cells. The second transgene encodes a suppressor of transcription also directed by the rat CC10 promoter. The third transgene encodes hemagglutinin (HA)-tagged IDO directed by a doxycycline-sensitive tetracycline promoter. TetOP, tetracycline-regulated operator. (B) After 14 days of doxycycline feeding, RNA was isolated from whole lungs of CC10-IDO transgenic (Tg+) and transgene-negative (Tg−) littermates, and quantitative RT-PCR was performed to measure IDO induction from various tissues. (C) IDO mRNA induction was measured over time from Tg+ and Tg− littermates after consuming doxycycline food for up to 28 days. (D) HPLC was performed to measure IDO activity from whole-lung homogenate of Tg+ and Tg− mice after 28 days of consuming doxycycline food. (E) Representative lung sections from doxycycline-fed CC10-IDO Tg+ (left) and Tg− (right) littermates were analyzed for HA (orange) staining in airway epithelium. Values are means (± SEM) of 5 mice/group.
To demonstrate that transgene expression was restricted to the lung, quantitative PCR was performed on cDNA generated from mRNA isolated from the liver, kidney, spleen, uterus, heart, and whole lung. IDO mRNA levels above baseline were detected only within the lungs of CC10-IDO transgenic mice (Tg+), and were induced ∼ 110-fold after mice consumed a high dose of doxycycline-containing chow (6 g/kg) for 14 days, compared with transgene-negative littermates (Tg−) that only displayed basal levels of IDO expression (Figure 2B).
A time course of IDO mRNA induction was performed to determine how early transgene expression occurred, and for how long transgene expression remained elevated (Figure 2C). CC10-IDO mice and transgene-negative littermates were fed doxycycline-containing chow for up to 28 days. The earliest time point examined was 2 days, when an almost 100-fold induction of IDO mRNA was measured. IDO levels did vary over time with doxycycline treatment, but remained substantially elevated above baseline throughout the 28-day feeding period.
Importantly, the IDO protein that was produced in the CC10-IDO mice was enzymatically active. After 28 days of consuming doxycycline-containing food, an IDO activity assay was performed from whole lung (Figure 2D). As measured by HPLC for the production of kynurenine, the CC10-IDO mice had fourfold more IDO activity than transgene-negative littermates. The IDO activity that was measured in transgene-negative littermates was comparable to the activity previously reported for IDO activity measured from whole lung in normal mice (36). Not only did the CC10-IDO mice exhibit fourfold more IDO activity compared with normal mice, but immunostaining for the HA tag connected to the transgenic IDO protein revealed that the expression of IDO within the lung was limited to the airway epithelium (Figure 2E).
Several other experiments were performed to demonstrate that the CC10-IDO mice were physiologically normal (data not shown). After 18 days of doxycycline feeding, the CC10-IDO mice manifested no change in the number of cells or cell type measured from BAL. Because IDO can induce the apoptosis of cells within the microenvironment proximal to its expression, experiments were performed to determine the integrity of the epithelium. Intravenous injections of FITC-dextran and measurement of fluorescence in BAL fluid indicated that CC10-IDO mice retained intact epithelial barrier integrity. A protein assay was also performed using BAL fluid, which demonstrated that protein was not leaking from the lungs of CC10-IDO transgenic mice fed doxycycline-containing food. Histologic analyses of paraffin-embedded lung sections did not reveal any damage to the lung or inflammation. Importantly, CC10-IDO mice demonstrated no differences in airway physiology as measured by methacholine responsiveness, compared with transgene-negative littermates that had also been fed doxycycline-containing food (data not shown).
CC10-IDO Mice That Are Sensitized and Challenged with Aspergillus fumigatus Hyphal Antigens Have a Significant Induction of IDO within Their Lungs
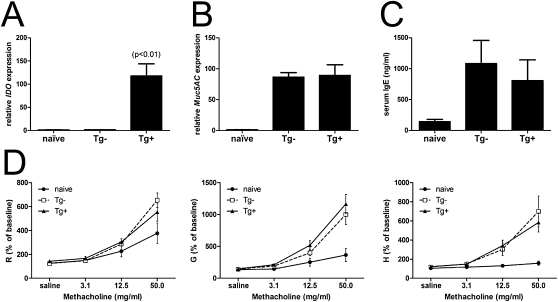
To determine whether the overexpression of IDO would be effective at reducing features in a model of allergic sensitization and challenge, we induced allergy in mice, using 1 μg of a formalin-fixed crude hyphal extract of A. fumigatus to which the mice were sensitized directly via the lung, using oropharyngeal aspiration. CC10-IDO mice and transgene-negative littermates were fed chow containing doxycycline, starting on day 0 and throughout the entire sensitization and challenge protocol. CC10-IDO transgenic and transgene-negative littermates were exposed to A. fumigatus hyphal antigens on days 0, 7, and 14, and were analyzed on day 19. Quantitative RT-PCR was performed from whole lung, and IDO expression above baseline levels was detected only in the transgenic mice. After sensitization with A. fumigatus hyphal antigens and the consumption of doxycycline for 19 days, CC10-IDO transgenic mice exhibited an almost 100-fold induction of IDO, as measured in whole lung. Tg− littermates did not exhibit augmented IDO expression (Figure 3A). These data demonstrate that the transgene-mediated induction of IDO remains robust in the lungs of allergically inflamed mice, an important finding considering that inflammatory cytokines were reported to suppress CC10 expression (37). Despite the elevation in IDO expression in transgenic mice, Muc5AC, a marker of mucus-cell metaplasia, was not significantly reduced (Figure 3B); nor were serum levels of IgE (Figure 3C).
Figure 3.
Pulmonary gene expression, serum IgE, and airway physiology after Aspergillus fumigatus antigen sensitization and challenge. CC10-IDO transgenic (Tg+) and transgene-negative (Tg−) mice aspirated 1 μg of formalin-fixed A. fumigatus hyphal extract on days 0, 7, and 14. Mice were analyzed on day 19. All mice consumed doxycycline-containing food starting on day 0 and throughout the entire protocol. RNA from whole lung was analyzed by quantitative RT-PCR, and IDO (A) and Muc5AC (B) expression were normalized to glyceraldehyle 3-phosphade dehydrogenase (GAPDH), relative to that measured from the lungs of Tg− mice. (C) Serum was analyzed by ELISA to quantitate total IgE concentrations. Tg+ (solid triangles) and Tg− (open squares) mice sensitized to A. fumigatus antigens were assessed on day 19 for airway hyperresponsiveness to increasing amounts of inhaled methacholine and compared with naive BALB/cJ mice (solid circles). Measurements were recorded from forced oscillations and determined as airway resistance (RN), tissue damping (G), and tissue stiffness (H). (D) As derived from the constant phase model, data are expressed as the percent of baseline measurements (± SEM) for each of the parameters measured from 9 Tg+, 8 Tg−, and 7 naive BALB/cJ mice, and are representative of two independent studies.
Aspergillus fumigatus Hyphal Antigen-Exposed CC10-IDO Mice Have No Alterations in Pulmonary Methacholine Responsiveness
The airway hyperresponsiveness of CC10-IDO mice that were sensitized to A. fumigatus hyphal antigens was measured. A FlexiVent analysis was performed to measure airway and peripheral methacholine responsiveness in allergically inflamed CC10-IDO mice, compared with allergically inflamed transgene-negative littermates and naive BALB/cJ mice. Mice were mechanically ventilated through a cannula placed into the trachea, and the smooth muscle agonist methacholine was added to the inspiratory port during ventilation. Although the A. fumigatus antigen-exposed mice demonstrated enhanced responsiveness to methacholine compared with naive mice, no significant differences were evident in airway hyperresponsiveness, tissue elastance, and tissue stiffness, also known as parenchymal responsiveness, between transgenic and transgene-negative mice (Figure 3D).
CC10-IDO Mice Allergically Sensitized to Aspergillus fumigatus Antigens Display Significant Reductions in Lymphocytes Within BAL Fluid
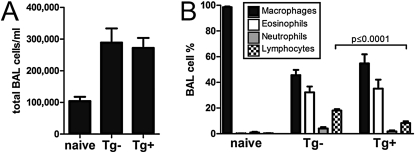
IDO is capable of reducing the proliferation of cells that are in proximity to its activity. Therefore, we hypothesized that the expression of IDO from nonciliated airway epithelial cells within the lung would significantly reduce the number of inflammatory cells present within the airways of allergically inflamed mice. After performing bronchoalveolar lavage on CC10-IDO mice, transgene-negative littermates, and naive BALB/cJ mice that were all allergically sensitized and challenged with A. fumigatus hyphal antigens, we measured the numbers of cells (Figure 4A) and cell types found within the BAL fluid. After the airway challenge, several different cell types were present within the BAL fluid of mice, with a predominance of macrophages and eosinophils, and substantial numbers of neutrophils and lymphocytes (Figure 4B). Although no differences were evident in the numbers of macrophages, eosinophils, or neutrophils, significant reductions in the number of lymphocytes within the BAL fluid were measured in CC10-IDO transgenic mice compared with transgene-negative littermates. A multiplex analysis of cytokines was performed on the BAL fluid from CC10-IDO mice to determine whether signals that recruit cells to the airway were affected by the overexpression of IDO from airway epithelium. No significant differences were measured in chemokines such as granulocyte colony-stimulating factor (G-CSF) and keratinocyte-derived chemokine that recruit neutrophils, MCP-1 that recruits macrophages, and eotaxin that recruits eosinophils (data not shown). Unlike eosinophils, neutrophils, and macrophages that are recruited into the lung during inflammation, lymphocytes are capable of proliferating within the lung (14). Although significant reductions in the number of lymphocytes of transgenic mice were measured, this did not result in significant changes in the total number of cells, because lymphocytes comprise a small population of cells within the BAL fluid in this model of allergic airway disease. These results suggest that the overexpression of IDO within the airway epithelium has the ability to reduce the proliferation of lymphocytes in a model of allergy in which allergic sensitization occurs within the lung.
Figure 4.
BAL cell differential count after Aspergillus fumigatus antigen sensitization and challenge. After sensitization and challenge with 1 μg of formalin-fixed A. fumigatus hyphal extract, saline was instilled into the lungs of tracheotomized, naive, CC10-IDO transgenic (Tg+) and transgene-negative (Tg−) mice that subsequently recovered, and total (A) and differential (B) cell counts were performed. All mice consumed doxycycline-containing food throughout the entire protocol for 19 days. Values are means (± SEM) of 10 Tg+ mice and 8 Tg− mice, and are representative studies performed twice.
CD4+ T Cells, but Not B Cells, Are Significantly Reduced within the Lymphocyte Population of CC10-IDO Mice Exposed to Aspergillus fumigatus Antigens
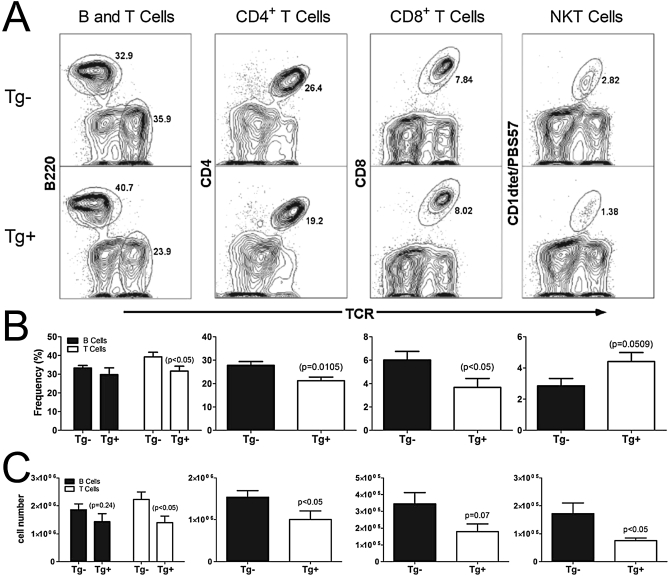
As shown in Figure 4, CC10-IDO mice sensitized to A. fumigatus had a significant reduction in the number of lymphocytes found within the BAL fluid. We determined which cell types within the lymphocyte population recovered from the lung were reduced because of the overexpression of IDO from the airway epithelium. Flow cytometry was performed from whole-lung digest. Of the cell types analyzed, i.e., CD4+, CD8+, B220+, and NKT, only the percentages of total T cells, CD4+ T cells, and CD8+ T cells were diminished. When the cell numbers of each subset in the lung were calculated, the number of T cells, CD4+ T cells, and NKT cells were reduced (Figure 5). Until now, the effect that the overexpression of IDO from airway epithelial cells exerts on the number of lung CD4+ and CD8+ T cells has not been examined. Our results demonstrate that the expression of IDO from a non–antigen-presenting cell has the ability to reduce the number of CD4+ and NKT cells, as well as the frequency of CD8+ T cells, present in the lungs of allergically inflamed mice after an allergic sensitization and challenge regimen in which antigen exposure occurs exclusively via inhalation.
Figure 5.
Lymphocyte populations within the lung after Aspergillus fumigatus antigen sensitization and challenge. Lung digests were performed from CC10-IDO transgenic mice (Tg+) (bottom histograms) and transgene-negative littermates (Tg−) (top histograms) that had been sensitized and challenged with A. fumigatus antigens. Flow cytometry was performed to determine the frequency of B cells, T cells, CD4+ T cells, CD8+ T cells, and natural killer T (NKT) lymphocytes found within the lymphocyte population. Below the representative histograms (A), graphs display the frequency of cells as determined from the gated lymphocyte parent population (B), and cell numbers were calculated from the frequency multiplied by the lymphocyte numbers in the lungs (C). Data from 10 Tg+ and 8 Tg− mice/group (± SEM) are presented in the graphs below the representative histograms.
The activity of IDO was reported to induce regulatory T cells (Tregs) (38–40). Therefore, intracellular staining was performed to determine whether or not the overexpression of IDO within airway epithelial cells in this model of allergic airway disease resulted in a difference in the number of Tregs present within whole lung. However, no difference in the number of cells that stained positive for the forkhead box P3 transcription factor (Foxp3) were observed in CC10-IDO transgenic mice compared with Tg− littermates (data not shown).
Antigen-Specific Splenic CD4+ T Cells from CC10-IDO Mice Produce Significantly Less IFN-γ, IL-4, IL-5, and IL-13 than CD4+ T Cells from Transgene-Negative Mice
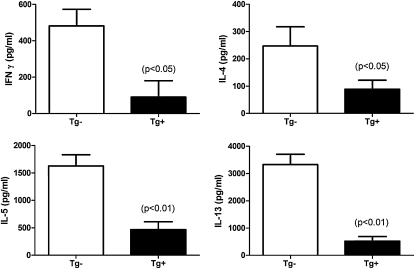
Because the overexpression of IDO in airway epithelial cells diminishes the number of CD4+ T cells in the lung after allergen sensitization and challenge, we measured whether cytokine production by CD4+ T cells was also affected. Positive selection was performed to isolate CD4+ T cells from the spleens of A. fumigatus antigen-sensitized and challenged CC10-IDO transgenic and transgene-negative mice that had all consumed doxycycline-containing chow through the entire protocol. The CD4+ T cells were cultured for 72 hours in the presence of A. fumigatus and wild-type antigen-presenting cells isolated from naive BALB/cJ mice. After sensitization to A. fumigatus, several Th2 cytokines were elicited by CD4+ T cells during the in vitro restimulation. However, sensitization to A. fumigatus also resulted in a large increase in the secretion of the Th1 cytokine, IFN-γ, by antigen-restimulated CD4+ T cells. CC10-IDO mice displayed a significantly reduced production of each of these cytokines from antigen-specific ex vivo restimulated splenic CD4+ T cells (Figure 6). These results demonstrate that in an allergic model in which antigen sensitization occurs via the lung, the overexpression of IDO from the airway epithelium has the capacity to reduce the activities of effector CD4+ T cells, as measured through the production of cytokines.
Figure 6.
Aspergillus-specific CD4+ T-cell cytokine secretion. Positively selected splenic CD4+ T cells from CC10-IDO transgenic (Tg+) and transgene-negative (Tg−) littermate mice were cultured in the presence of antigen-presenting cells isolated from naive BALB/cJ mice and 1 μg of Aspergillus crude hyphal extract for 72 hours. Cytokine concentrations were measured by ELISA. Values are means (± SEM) of 8 Tg+ mice and 8 Tg− mice, and are representative of experiments performed twice.
Activation of TLR9 Exclusively in Airway Epithelial Cells Does Not Induce IDO
Reductions in allergic airway disease features after the administration of the TLR9 agonist, CpG DNA, were suggested to be a consequence of IDO induction within airway epithelial cells (23). Several experiments were performed in which we used a similar model of inducing allergic airway disease in CC10-IDO mice, through exposure to ovalbumin (Ova) in conjunction with the Th2-skewing adjuvant aluminum hydroxide (Alum), followed by aerosolized challenges with Ova. This is a more robust model of Th2-mediated allergic airway disease compared with the Aspergillus fumigatus model. Using the Alum/Ova model in CC10-IDO mice resulted in modest reductions of several features associated with allergic airway disease, although none were statistically significant except for the production of IL-4 by CD4+ T cells restimulated in vitro with Ova (shown in Figure E1 in the online supplement). Given this discrepancy between our model and the model of Hayashi and colleagues (23), in which airway epithelial cells were implicated as a cell type in which IDO induction occurs after CpG DNA administration, we created a transgenic mouse in which hTLR9 is expressed only in airway epithelial cells. These transgenic mice were then bred onto a TLR9-deficient background, thereby rendering TLR9 expression present only in the epithelium of the lung (Figure 7A). CC10-hTLR9 transgenic and transgene-negative littermate mice were exposed to CpG DNA via oropharyngeal aspiration, and lungs were analyzed after 24 hours for gene expression by quantitative RT-PCR. As expected, hTLR9 transgene expression was present only in transgene-positive mice (Figure 7B), and mouse TLR9 was absent from all mice (Figure 7C). Despite finding significant CpG DNA-induced expression of the chemokine CCL20 only in CC10-hTLR9 transgenic mice (Figure 7D), no induction of IDO occurred in transgenic mice after CpG DNA administration (Figure 7E). These results suggest that CpG DNA does not directly induce the expression of IDO within airway epithelial cells.
Figure 7.
Lung IDO expression in CC10-hTLR9 mice exposed to CpG DNA. (A) Tissues from CC10-hTLR9 transgenic mice on a TLR9-deficient background were analyzed by RT-PCR for lung-specific human Toll-Like-Receptor 9 (hTLR9) transgene expression. Transgene-negative (Tg−) and CC10-hTLR9 transgenic (CC10-hTLR9 Tg+) mice were administered 10 μg CpG DNA into their airways by oropharyngeal aspiration. Twenty-four hours later, pulverized lungs were analyzed by quantitative RT-PCR for relative expression levels of hTLR9 transgene (B), mouse TLR9 (mTLR9) (C), CCL20 (D), and IDO (E). Values are means (± SEM) of five mice per group, and are representative of experiments performed twice.
Dendritic Cells Are Required for CpG DNA-Induced IDO Expression
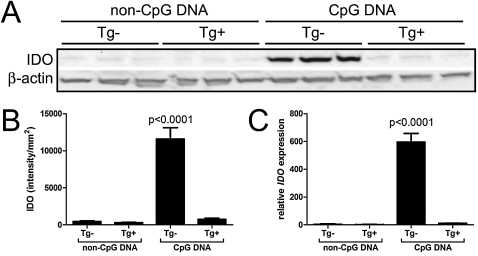
The suppression of T-cell activities because of the expression of IDO in antigen-presenting cells, such as CD11c+ dendritic cells, is well-described (19, 41). Because airway epithelial cells expressing hTLR9 do not induce IDO upon stimulation, we used CD11c-DTR transgenice mice in which the systemic administration of diphtheria toxin results in a transient depletion of CD11c+ cells (42). Diphtheria toxin was administered to CD11c-DTR transgenic and transgene-negative littermate mice via oropharyngeal aspiration, promoting a significant depletion of CD11c+ cells from the lung (data not shown), and 24 hours later, mice received CpG DNA or non-CpG DNA intraperitoneally. Dendritic cell–depleted (CD11c-DTR transgenic) mice that received CpG DNA expressed basal levels of IDO protein compared with transgene-negative littermates, which displayed a significant induction of IDO protein (Figures 8A and 8B) and RNA (Figures 8A and 8B) within the whole lung. Together with the results generated in the CC10-hTLR9 transgenic mice, these data suggest that the cells primarily responsible for the induction of IDO within the lung after exposure to CpG DNA are CD11c+ dendritic cells, and not airway epithelium.
Figure 8.
Lung IDO expression in mice depleted of conventional myeloid dendritic cells and exposed to CpG DNA. Transgene-negative (Tg−) and CD11c-DTR transgenic mice (Tg+) were administered 4 ng/g diphtheria toxin via oropharyngeal aspiration, to deplete dendritic cells locally from the lung. Twenty-four hours later, mice were administered 50 μg non-CpG DNA or CpG DNA into their airways by intraperitoneal injection. (A) Lung homogenates were analyzed 24 hours later by Western blot for IDO expression. (B) IDO band intensities on Western blots were quantitated by luminescence. (C) Pulverized lungs were analyzed by quantitative RT-PCR for relative expression levels of IDO. Values are means (± SEM) of three mice per group, and are representative of experiments performed twice.
DISCUSSION
Allergic asthma is a heterogeneous disease in which antigen-specific immunoglobulins, airway eosinophilia, mucus-cell metaplasia, airway hyperresponsiveness, bronchoconstriction, and Th2 and inflammatory cytokines all contribute to the disease pathology (43). The underlying mechanism of the inflammatory response observed in allergic asthma is attributable primarily to the activities of CD4+ T cells (1, 44, 45). Although several therapies are available for the treatment of asthma, they all have side effects or are ineffective in particular patients (46, 47). Moreover, many asthma medications have systemic immunosuppressive actions that can promote infection (48). Therefore, selectively impeding the activities of CD4+ T cells in a localized manner, such as in proximity to the airway that undergoes substantial alterations in response to Th2 cytokines, is an attractive strategy for suppressing the features associated with allergic asthma and avoiding the pitfalls associated with systemic immune suppression. In attempting to develop a model to reach these goals, we created transgenic mice in which the tryptophan-metabolizing enzyme, IDO, is inducibly overexpressed only in nonciliated airway epithelial cells. As a consequence of IDO-mediated tryptophan catabolism, the limited availability of this essential amino acid within the local microenvironment renders rapidly proliferating cells, such as stimulated lymphocytes, incapable of protein synthesis and unable to proliferate (18). In addition, the toxic byproducts of tryptophan metabolism inhibit the proliferation of CD4+ T cells (21, 49).
We demonstrate here that the expression of IDO within airway epithelial cells reduces the number of lymphocytes found within the BAL fluid of CC10-IDO mice that were sensitized by oropharyngeal aspiration to A. fumigatus hyphal antigens, compared with transgene-negative littermates. Within the lung lymphocyte population, flow cytometry analysis demonstrated that the cell type significantly and consistently reduced was the CD4+ T-cell population. Whereas NKT cells showed a diminished number and CD8+ T cells showed a diminished frequency, B cells were consistently unaffected by the overexpression of IDO within the airway (Figure 5). Similarly, the levels of serum IgE were unaffected by the expression of IDO in the airway epithelium (Figure 3). This suggests that the mechanism through which IDO exerts its effects involves limiting the availability of tryptophan, resulting in the inhibition of cellular proliferation, and not through the production of toxic byproducts of tryptophan metabolism, because not all lymphocyte subsets within the lung were affected by the overexpression of IDO. Importantly, CD8+ T cells (34) and B cells (21) (although not affected in our in vivo model) are susceptible to downstream catabolites of tryptophan metabolism in vitro.
At present, the complete repertoire of enzymes within the airway epithelium that participate in the complex process of tryptophan metabolism is unknown, and we measured only the production of kynurenine, the primary catabolite of IDO-mediated tryptophan degradation. Hayashi and colleagues demonstrated that administering 3-HAA, a product of tryptophan metabolism that occurs downstream of kynurenine, into the lungs of mice induced the apoptosis of T cells within the lung (49). However, 3-HAA is reportedly not produced within the lung as a consequence of IDO induction, whereas its precursor, 3-hydroxykynurenine, is produced, suggesting that the lung does not produce substantial amounts of kynurinase, the enzyme involved in the conversion of 3-HAA to 3-hydroxykynurenine (50, 51). We have yet to explore which downstream catabolites of tryptophan metabolism are produced in the lung in CC10-IDO mice. Perhaps the enhanced expression of additional enzymes in the airway epithelium would promote a more substantial inhibition of the allergic airway disease phenotype.
Although our studies involved the exposure of mice to an extract of killed A. fumigatus hyphal antigens, IDO was also demonstrated to play a critical role in the modulation of allergic and inflammatory responses to infection by live Aspergillus (52, 53). We have not yet explored the effects of IDO expression at different time points within our allergic Aspergillus model. In our experiments, mice were fed doxycycline-containing food throughout both the sensitization and challenge periods. Examining how the expression of IDO by airway epithelium affects A. fumigatus antigen–induced pathophysiology, when expressed only during the challenge phase of the model, would be of great relevance, considering that allergic asthma is treated only after symptoms have arisen. Because effector T cells rapidly proliferate during an allergen challenge, the expression of IDO at this time would likely limit the proliferation of these cells because of the removal of tryptophan from the environment. However, because the primary clonal expansion of rare A. fumigatus–specific CD4+ T-cell clones would not be affected by initiating doxycycline administration until this later time point, the overall effects of IDO expression may in fact be significantly diminished. Furthermore, we do not know which population of CD4-expressing T cells present within the lungs or spleens of CC10-IDO transgenic mice are affected by the activities of IDO. When cultured at equivalent numbers, CD4+ T cells from CC10-IDO transgenic mice secrete significantly reduced levels of cytokines compared with transgene-negative littermates. Whether this reduction is attributable to limiting the proliferation of the CD4+ T cells during the initial antigen exposure in vivo, or to the induction of cells with suppressive activity, remains to be determined.
Pulmonary IDO activity is itself affected in allergic asthma. House dust mite allergens are capable of suppressing dendritic-cell IDO activity in patients with asthma (54), and steroid treatment of patients with asthma increases sputum IDO activity (55). As a consequence, the modulation of IDO activity has been considered for the treatment of allergic airway disease, as reviewed by Le and Broide (56), and was implicated in reversing allergic airway disease through its expression in CD8α+ dendritic cells (57). In addition, the downstream catabolites of tryptophan metabolism were demonstrated to promote tolerance within the lung during allergen immunotherapy, further revealing the significant impact of the activity of IDO in potentially reducing features associated with allergic airway disease (58). Finally, beyond its T cell–modulating effects, the potential antioxidant activity of IDO (59, 60) may also be of benefit in allergic asthma. However, others found that mice globally deficient in IDO from birth and subjected to a model of allergic airway disease exhibited an attenuated Th2 response, attributable to a lack of initial T-cell priming by dendritic cells that are relatively immature compared with those of wild-type mice, thus demonstrating that IDO is also capable of sustaining, rather than opposing, Th2-associated allergy in the airways (61). The effects mediated by IDO appear to be manifold and dependent on the cell type in which the enzyme is expressed.
Our results highlight an important role of airway epithelial IDO in allergic airway disease, but also reveal a critical role of dendritic cells in the induction of IDO in response to CpG DNA administration. Our use of a mouse expressing TLR9, through which CpG DNA induces intracellular signaling exclusively in airway epithelium, reveals that the induction of IDO in epithelial cells does not occur directly after CpG DNA treatment. According to Hayashi and colleagues (23), epithelial-cell IDO induction was dependent on the responsiveness of IFN-γ. Therefore, if IDO is potently induced by CpG, it is likely via an indirect mechanism involving IFN-γ, and not directly via TLR9. Nonetheless, transgenic airway epithelial IDO expression itself is not sufficient to abrogate the allergic asthma phenotype completely in this mouse model. The abilities of dendritic cells to migrate into draining lymph nodes and to engage in intimate contact with T lymphocytes are not shared with airway epithelium, and may be critical for IDO to exert its most potent effect.
We demonstrated that the expression of IDO specifically from the airway epithelium limits the activities of effector CD4+ T cells, which are a major cell type implicated in the pathogenesis of allergic airway diseases. However, pulmonary methacholine hyperresponsiveness, a diagnostic tool in allergic asthma, as manifested in A. fumigatus hyphal antigen–exposed mice, was not diminished by the overexpression of IDO and by reducing CD4+ T-cell numbers and activities. We previously reported that a substantial reduction in inflammatory cells and cytokines does not necessarily correlate with reduced methacholine responsiveness (28). Given that the CD4+ T cells from CC10-IDO transgenic mice still secrete substantial concentrations of cytokines (albeit significantly less than do transgene-negative mice), and because the amount of cytokine necessary to induce airway hyperresponsiveness is unknown, the intact methacholine responsiveness in CC10-IDO mice is not surprising. Although our results suggest that airway epithelial IDO activity is a provocative target for the selective modulation of pathogenic CD4+ T cells in the lung, complementary approaches will be required to address the entire spectrum of pathologies presented by a patient with asthma.
Supplementary Material
This work was supported by grants from the National Institutes of Health and National Center for Research Resources, by University of Vermont College of Medicine funds, and by National Institutes of Health/National Heart, Lung and Blood Institute institutional training grants HL089177 (M.E.P.), HL076122 (S.A.P.), National Center for Research Resources Center of Biomedical Research Excellence grants P20RR15557 (L.A.W.L.), AI067897 (J.E.B.), HL068865 and HL085646 (A.v.d.V.), and HL084200 (B.T.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0167OC on January 29, 2010
Author Disclosure: A.v.d.V. received a patent for the detection of S-nitrosylated proteins from Wolf Greenfield. He also received a sponsored grant from the National Institutes of Health (NIH) and the Flight Attendant Medical Research Institute for more than $100,001 each. S.A. received lecture fees for $1,001–$5,000 and a sponsored grant for $50,001–$100,000 from Sepracor, along with a sponsored grant from the NIH for more than $100,001. J.A. received a sponsored grant from the NIH for more than $100,001. J.L.A. received lecture fees for $1,001–$5,000 and a sponsored grant for $50,001–$100,000 from Sepracor, along with a sponsored grant from the NIH for more than $100,001. M.N.B. received lecture fees for $1,001–$5,000 and a sponsored grant for $50,001–$100,000 from Sepracor, along with a sponsored grant from the NIH for more than $100,001. J.E.B. received lecture fees for $1,001–$5,000 and a sponsored grant for $50,001–$100,000 from Sepracor, along with a sponsored grant from the NIH for more than $100,001. S.R.F.H. received a sponsored grant from the NIH for more than $100,001. S.M.M. is a full-time employee of the Waters Corporation. M.E.P. received lecture fees for $1,001–$5,000 and a sponsored grant for $50,001–$100,000 from Sepracor, along with a sponsored grant from the NIH for more than $100,001. B.T.S. received a sponsored grant from the NIH for more than $100,001. S.U. received lecture fees for $1,001–$5,000 and a sponsored grant for $50,001–$100,000 from Sepracor, along with a sponsored grant from the NIH for more than $100,001. L.A.W.L. received lecture fees from Sepracor for $1,001–$5,000 and a sponsored grant from the NIH for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 2.Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med 1992;176:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JM, Rambaldi A, Biondi A, Chen ZG, Sanderson CJ, Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol 1989;19:701–705. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Hogan SP, Henry PJ, Matthaei KI, McKenzie AN, Young IG, Rothenberg ME, Foster PS. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am J Respir Cell Mol Biol 2001;25:522–530. [DOI] [PubMed] [Google Scholar]
- 5.Coffman RL, Ohara J, Bond MW, Carty J, Zlotnik A, Paul WE. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol 1986;136:4538–4541. [PubMed] [Google Scholar]
- 6.Sanderson CJ. Interleukin-5: an eosinophil growth and activation factor. Dev Biol Stand 1988;69:23–29. [PubMed] [Google Scholar]
- 7.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002;8:885–889. [DOI] [PubMed] [Google Scholar]
- 8.National Asthma Education and Prevention Program. Expert panel report: guidelines for the diagnosis and management of asthma update on selected topics—2002. J Allergy Clin Immunol 2002;110:S141–S219. [PubMed] [Google Scholar]
- 9.Bollet AJ, Black R, Bunim JJ. Major undesirable side-effects resulting from prednisolone and prednisone. J Am Med Assoc 1955;158:459–463. [DOI] [PubMed] [Google Scholar]
- 10.Carmichael J, Paterson IC, Diaz P, Crompton GK, Kay AB, Grant IW. Corticosteroid resistance in chronic asthma. Br Med J (Clin Res Ed) 1981;282:1419–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National surveillance for asthma—United States, 1980–2004. MMWR Surveill Summ 2007;56:1–54. [PubMed] [Google Scholar]
- 12.Adams RJ, Fuhlbrigge A, Guilbert T, Lozano P, Martinez F. Inadequate use of asthma medication in the United States: results of the Asthma in America National Population Survey. J Allergy Clin Immunol 2002;110:58–64. [DOI] [PubMed] [Google Scholar]
- 13.Corrigan CJ, Kay AB. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis 1990;141:970–977. [DOI] [PubMed] [Google Scholar]
- 14.Constant SL, Brogdon JL, Piggott DA, Herrick CA, Visintin I, Ruddle NH, Bottomly K. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J Clin Invest 2002;110:1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Haar C, Kool M, Hassing I, Bol M, Lambrecht BN, Pieters R. Lung dendritic cells are stimulated by ultrafine particles and play a key role in particle adjuvant activity. J Allergy Clin Immunol 2008;121:1246–1254. [DOI] [PubMed] [Google Scholar]
- 16.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, Assoufi B, Collins JV, Durham S, Kay AB. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis 1990;142:1407–1413. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DS, Bentley AM, Hartnell A, Kay AB, Durham SR. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax 1993;48:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med 1999;189:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol 2002;168:3771–3776. [DOI] [PubMed] [Google Scholar]
- 20.Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by kynurenines. Adv Exp Med Biol 2003;527:183–190. [DOI] [PubMed] [Google Scholar]
- 21.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, Opelz G. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med 2002;196:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heseler K, Spekker K, Schmidt SK, MacKenzie CR, Daubener W. Antimicrobial and immunoregulatory effects mediated by human lung cells: role of IFN-gamma-induced tryptophan degradation. FEMS Immunol Med Microbiol 2008;52:273–281. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, Broide DH, Carson DA, Raz E. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest 2004;114:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol 2003;24:242–248. [DOI] [PubMed] [Google Scholar]
- 25.Finotto S, Neurath MF, Glickman JN, Qin S, Lehr HA, Green FH, Ackerman K, Haley K, Galle PR, Szabo SJ, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 2002;295:336–338. [DOI] [PubMed] [Google Scholar]
- 26.Hogan B, Costantini F, Lacy E, eds. 1986. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
- 27.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-kappa B activation in lipopolysaccharide-induced airway inflammation. J Immunol 2003;170:6257–6265. [DOI] [PubMed] [Google Scholar]
- 28.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 2004;173:7003–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 2002;93:263–270. [DOI] [PubMed] [Google Scholar]
- 30.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 2005;171:1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, Gelfand EW. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell–deficient mice. J Exp Med 1997;186:449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohn L, Homer RJ, MacLeod H, Mohrs M, Brombacher F, Bottomly K. Th2-induced airway mucus production is dependent on IL-4Ralpha, but not on eosinophils. J Immunol 1999;162:6178–6183. [PubMed] [Google Scholar]
- 33.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol 2000;164:3596–3599. [DOI] [PubMed] [Google Scholar]
- 34.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 2002;196:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA. Cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem 1992;267:14703–14712. [PubMed] [Google Scholar]
- 36.Urade Y, Yoshida R, Kitamura H, Hayaishi O. Induction of indoleamine 2,3-dioxygenase in alveolar interstitial cells of mouse lung by bacterial lipopolysaccharide. J Biol Chem 1983;258:6621–6627. [PubMed] [Google Scholar]
- 37.Harrod KS, Jaramillo RJ. Pseudomonas aeruginosa and tumor necrosis factor-alpha attenuate Clara cell secretory protein promoter function. Am J Respir Cell Mol Biol 2002;26:216–223. [DOI] [PubMed] [Google Scholar]
- 38.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol 2002;3:1097–1101. [DOI] [PubMed] [Google Scholar]
- 39.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med 2007;13:579–586. [DOI] [PubMed] [Google Scholar]
- 40.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol 2004;16:1391–1401. [DOI] [PubMed] [Google Scholar]
- 41.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 2004;114:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 2002;17:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemanske RF Jr, Busse WW. Asthma. J Allergy Clin Immunol 2003;111:S502–S519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knutsen AP. Lymphocytes in allergic bronchopulmonary aspergillosis. Front Biosci 2003;8:d589–d602. [DOI] [PubMed] [Google Scholar]
- 45.Chauhan B, Knutsen A, Hutcheson PS, Slavin RG, Bellone CJ. T cell subsets, epitope mapping, and HLA-restriction in patients with allergic bronchopulmonary aspergillosis. J Clin Invest 1996;97:2324–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wenzel SE. Antileukotriene drugs in the management of asthma. JAMA 1998;280:2068–2069. [DOI] [PubMed] [Google Scholar]
- 47.Dockhorn RJ, Green AW, Green E. Assessing the efficacy and safety of q. d. theophylline therapy: a multicenter study. Ann Allergy 1985;55:658–664. [PubMed] [Google Scholar]
- 48.Oehling AG, Akdis CA, Schapowal A, Blaser K, Schmitz M, Simon HU. Suppression of the immune system by oral glucocorticoid therapy in bronchial asthma. Allergy 1997;52:144–154. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, Elliott GI, Kufareva I, Abagyan R, Broide DH, et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci USA 2007;104:18619–18624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci USA 1990;87:2506–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakoby WB, Bonner DM. Kynureninase from Neurospora: purification and properties. J Biol Chem 1953;205:699–707. [PubMed] [Google Scholar]
- 52.Montagnoli C, Fallarino F, Gaziano R, Bozza S, Bellocchio S, Zelante T, Kurup WP, Pitzurra L, Puccetti P, Romani L. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J Immunol 2006;176:1712–1723. [DOI] [PubMed] [Google Scholar]
- 53.Romani L, Zelante T, De Luca A, Bozza S, Bonifazi P, Moretti S, D'Angelo C, Giovannini G, Bistoni F, Fallarino F, et al. Indoleamine 2,3-dioxygenase (IDO) in inflammation and allergy to Aspergillus. Med Mycol 2009;47 Suppl 1:S154–161. [DOI] [PubMed] [Google Scholar]
- 54.Maneechotesuwan K, Wamanuttajinda V, Kasetsinsombat K, Huabprasert S, Yaikwawong M, Barnes PJ, Wongkajornsilp A. Der p 1 suppresses indoleamine 2, 3-dioxygenase in dendritic cells from house dust mite-sensitive patients with asthma. J Allergy Clin Immunol 2009;123:239–248. [DOI] [PubMed] [Google Scholar]
- 55.Maneechotesuwan K, Supawita S, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Sputum indoleamine-2, 3-dioxygenase activity is increased in asthmatic airways by using inhaled corticosteroids. J Allergy Clin Immunol 2008;121:43–50. [DOI] [PubMed] [Google Scholar]
- 56.Le AV, Broide DH. Indoleamine-2,3-dioxygenase modulation of allergic immune responses. Curr Allergy Asthma Rep 2006;6:27–31. [DOI] [PubMed] [Google Scholar]
- 57.Gordon JR, Li F, Nayyar A, Xiang J, Zhang X. CD8 alpha+, but not CD8 alpha−, dendritic cells tolerize Th2 responses via contact-dependent and -independent mechanisms, and reverse airway hyperresponsiveness, Th2, and eosinophil responses in a mouse model of asthma. J Immunol 2005;175:1516–1522. [DOI] [PubMed] [Google Scholar]
- 58.Taher, Y. A., B. J. Piavaux, R. Gras, B. C. van Esch, G. A. Hofman, N. Bloksma, P. A. Henricks, and A. J. van Oosterhout. 2008. Indoleamine 2,3-dioxygenase-dependent tryptophan metabolites contribute to tolerance induction during allergen immunotherapy in a mouse model. J Allergy Clin Immunol 121:e982–e991. [DOI] [PubMed] [Google Scholar]
- 59.Jacoby DB, Choi AM. Influenza virus induces expression of antioxidant genes in human epithelial cells. Free Radic Biol Med 1994;16:821–824. [DOI] [PubMed] [Google Scholar]
- 60.Liu H, Liu L, Fletcher BS, Visner GA. Novel action of indoleamine 2,3-dioxygenase attenuating acute lung allograft injury. Am J Respir Crit Care Med 2006;173:566–572. [DOI] [PubMed] [Google Scholar]
- 61.Xu H, Oriss TB, Fei M, Henry AC, Melgert BN, Chen L, Mellor AL, Munn DH, Irvin CG, Ray P, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci USA 2008;105:6690–6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.