Abstract
A large panel of hybridomas, secreting monoclonal antibodies (MAbs) specific for diphtheria toxin (DT) and prepared by immunization with either intact DT or its A or B fragment (DTA or DTB), have been isolated and characterized. The 213 MAbs were initially screened for reactivity to DT by enzyme-linked immunosorbent assay analyses and then were classified for their reactivity with DT, DTB, or DTA by solid-phase Western blot (immunoblot) analyses; 129 DTB-specific, 51 DTA-specific, and 33 non-fragment-assignable MAbs were obtained. Of the DTB MAbs, 118 recognize epitopes between residues 194 and 453, 10 recognize epitopes between residues 454 and 481, and 1 recognizes an epitope present in denatured toxin but not present in native DT located within the carboxyl-terminal receptor-binding region of DT (residues 482 to 535). Those MAbs that were the most protective in a cytotoxicity assay recognized native toxin in solution and inhibited binding of radiolabeled toxin to Vero cells to the greatest extent. A number of MAbs were able to detect epitopes that became more or less accessible when the toxin was preincubated at acidic (endosomal-mimicking) pH, suggesting that the epitopes they recognize may be important in the low-pH-induced insertion and/or translocation of DT across the endosomal membrane.
Full text
PDF

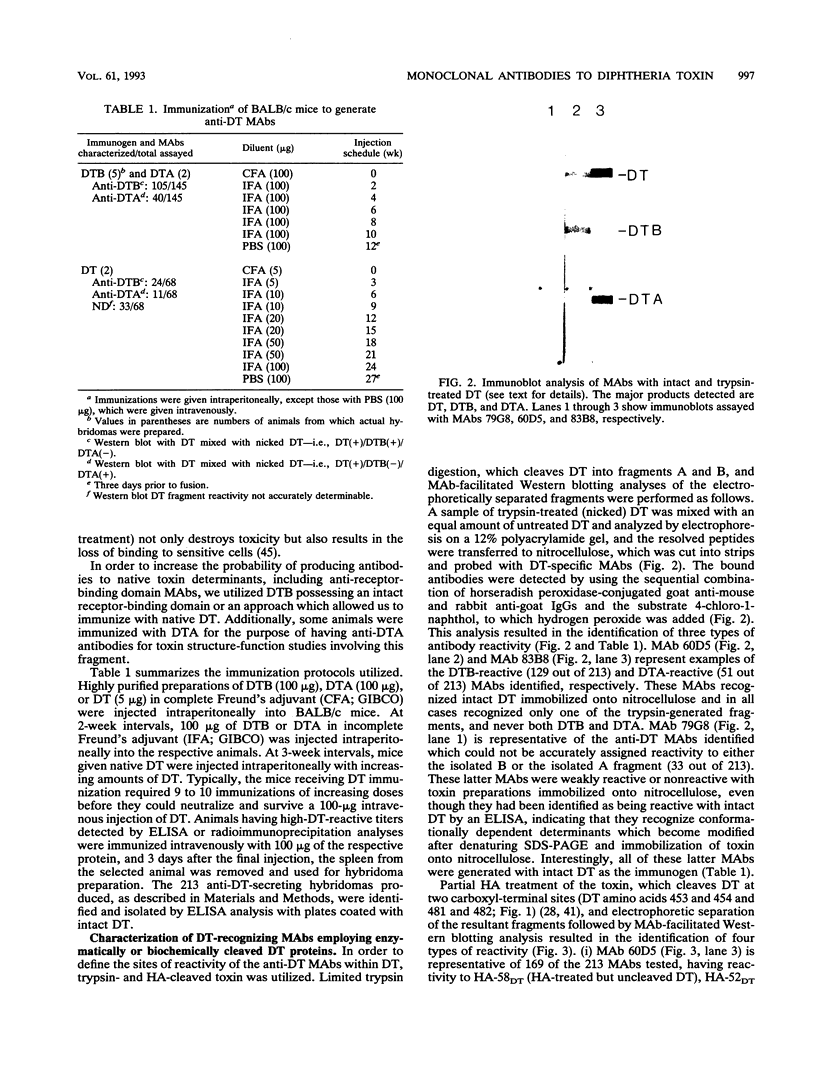
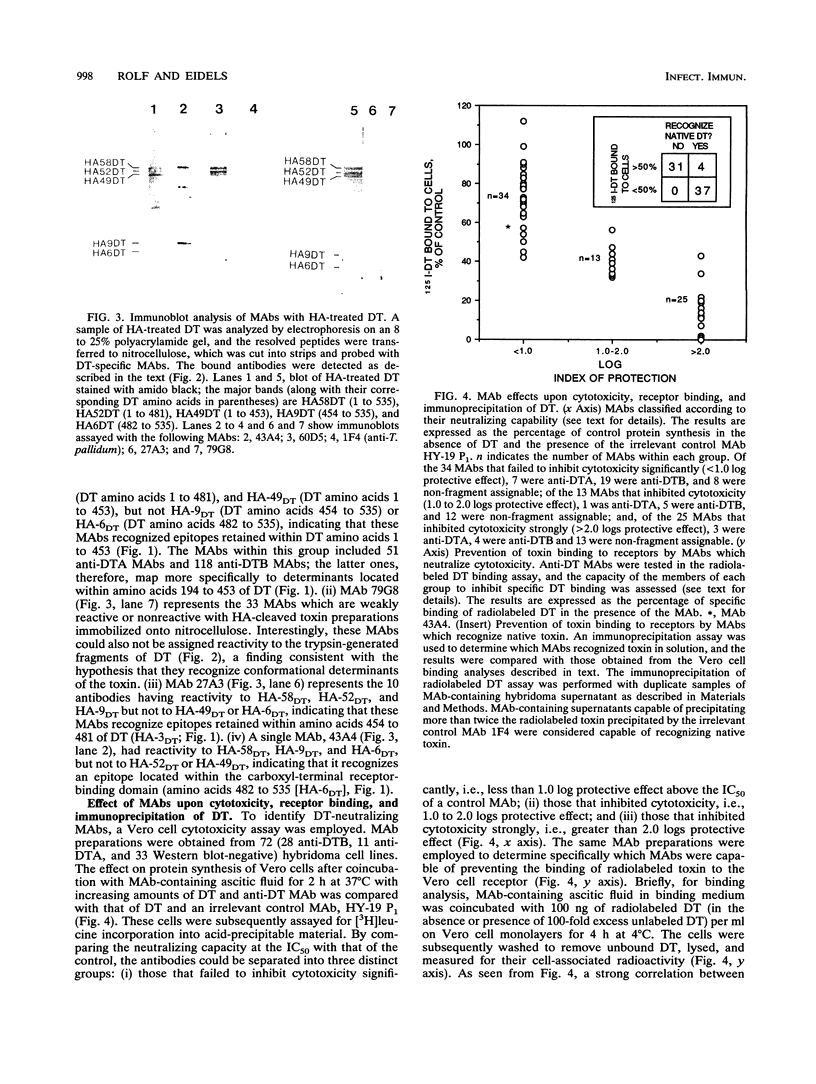




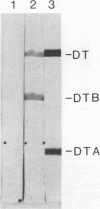
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autran B., Triebel F., Viguier M., Jolivet M., Falmagne P., Debre P. Monoclonal B-cell response to diphtheria toxoid: evidence for cross-reactive epitopes. Immunology. 1987 Apr;60(4):531–538. [PMC free article] [PubMed] [Google Scholar]
- Bacha P., Murphy J. R., Reichlin S. Thyrotropin-releasing hormone-diphtheria toxin-related polypeptide conjugates. Potential role of the hydrophobic domain in toxin entry. J Biol Chem. 1983 Feb 10;258(3):1565–1570. [PubMed] [Google Scholar]
- Bigio M., Rossi R., Nucci D., Antoni G., Rappuoli R., Ratti G. Conformational changes in diphtheria toxoids. Analysis with monoclonal antibodies. FEBS Lett. 1987 Jun 29;218(2):271–276. doi: 10.1016/0014-5793(87)81060-8. [DOI] [PubMed] [Google Scholar]
- Blewitt M. G., Chung L. A., London E. Effect of pH on the conformation of diphtheria toxin and its implications for membrane penetration. Biochemistry. 1985 Sep 24;24(20):5458–5464. doi: 10.1021/bi00341a027. [DOI] [PubMed] [Google Scholar]
- Brasseur R., Cabiaux V., Falmagne P., Ruysschaert J. M. pH dependent insertion of a diphtheria toxin B fragment peptide into the lipid membrane: a conformational analysis. Biochem Biophys Res Commun. 1986 Apr 14;136(1):160–168. doi: 10.1016/0006-291x(86)90890-9. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Barbieri J. T., Collier R. J. Dimeric form of diphtheria toxin: purification and characterization. Biochemistry. 1986 May 6;25(9):2425–2430. doi: 10.1021/bi00357a019. [DOI] [PubMed] [Google Scholar]
- Choe S., Bennett M. J., Fujii G., Curmi P. M., Kantardjieff K. A., Collier R. J., Eisenberg D. The crystal structure of diphtheria toxin. Nature. 1992 May 21;357(6375):216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Structure-activity relationships in diphtheria toxin and Pseudomonas aeruginosa exotoxin A. Cancer Treat Res. 1988;37:25–35. doi: 10.1007/978-1-4613-1083-9_3. [DOI] [PubMed] [Google Scholar]
- Colombatti M., Greenfield L., Youle R. J. Cloned fragment of diphtheria toxin linked to T cell-specific antibody identifies regions of B chain active in cell entry. J Biol Chem. 1986 Mar 5;261(7):3030–3035. [PubMed] [Google Scholar]
- Cryz S. J., Welkos S. L., Holmes R. K. Immunochemical studies of diphtherial toxin and related nontoxic mutant proteins. Infect Immun. 1980 Dec;30(3):835–846. doi: 10.1128/iai.30.3.835-846.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Ross L. L., Hart D. A. Diphtheria toxin-receptor interaction: a polyphosphate-insensitive diphtheria toxin-binding domain. Biochem Biophys Res Commun. 1982 Nov 30;109(2):493–499. doi: 10.1016/0006-291x(82)91748-x. [DOI] [PubMed] [Google Scholar]
- Falmagne P., Capiau C., Lambotte P., Zanen J., Cabiaux V., Ruysschaert J. M. The complete amino acid sequence of diphtheria toxin fragment B. Correlation with its lipid-binding properties. Biochim Biophys Acta. 1985 Jan 21;827(1):45–50. doi: 10.1016/0167-4838(85)90099-8. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa S., Uchida T., Mekada E., Moynihan M. R., Okada Y. Monoclonal antibody against diphtheria toxin. Effect on toxin binding and entry into cells. J Biol Chem. 1983 Apr 10;258(7):4311–4317. [PubMed] [Google Scholar]
- Jiang J. X., Abrams F. S., London E. Folding changes in membrane-inserted diphtheria toxin that may play important roles in its translocation. Biochemistry. 1991 Apr 23;30(16):3857–3864. doi: 10.1021/bi00230a008. [DOI] [PubMed] [Google Scholar]
- Jiang J. X., Chung L. A., London E. Self-translocation of diphtheria toxin across model membranes. J Biol Chem. 1991 Dec 15;266(35):24003–24010. [PubMed] [Google Scholar]
- Leppla S., Dorland R. B., Middlebrook J. L. Inhibition of diphtheria toxin degradation and cytotoxic action by chloroquine. J Biol Chem. 1980 Mar 25;255(6):2247–2250. [PubMed] [Google Scholar]
- Lory S., Collier R. J. Diphtheria toxin: nucleotide binding and toxin heterogeneity. Proc Natl Acad Sci U S A. 1980 Jan;77(1):267–271. doi: 10.1073/pnas.77.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekada E., Uchida T., Okada Y. Methylamine stimulates the action of ricin toxin but inhibits that of diphtheria toxin. J Biol Chem. 1981 Feb 10;256(3):1225–1228. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Differential chemical protection of mammalian cells from the exotoxins of Corynebacterium diphtheriae and Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):232–239. doi: 10.1128/iai.16.1.232-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D. A., Villemez C. L. Specific chemical cleavage of diphtheria toxin with hydroxylamine. Purification and characterization of the modified proteins. J Biol Chem. 1988 Nov 15;263(32):17122–17127. [PubMed] [Google Scholar]
- Naglich J. G., Eidels L. Isolation of diphtheria toxin-sensitive mouse cells from a toxin-resistant population transfected with monkey DNA. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7250–7254. doi: 10.1073/pnas.87.18.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglich J. G., Metherall J. E., Russell D. W., Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992 Jun 12;69(6):1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- Naglich J. G., Rolf J. M., Eidels L. Expression of functional diphtheria toxin receptors on highly toxin-sensitive mouse cells that specifically bind radioiodinated toxin. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2170–2174. doi: 10.1073/pnas.89.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Pastan I., FitzGerald D. Analysis of Pseudomonas exotoxin activation and conformational changes by using monoclonal antibodies as probes. Infect Immun. 1991 Jan;59(1):407–414. doi: 10.1128/iai.59.1.407-414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- Proia R. L., Eidels L., Hart D. A. Diphtheria toxin:receptor interaction. Characterization of the receptor interaction with the nucleotide-free toxin, the nucleotide-bound toxin, and the B-fragment of the toxin. J Biol Chem. 1981 May 25;256(10):4991–4997. [PubMed] [Google Scholar]
- Proia R. L., Wray S. K., Hart D. A., Eidels L. Characterization and affinity labeling of the cationic phosphate-binding (nucleotide-binding) peptide located in the receptor-binding region of the B-fragment of diphtheria toxin. J Biol Chem. 1980 Dec 25;255(24):12025–12033. [PubMed] [Google Scholar]
- Rittenberg M. B., Pinney C. T., Jr, Iglewski B. H. Antigenic relationships on the diphtheria toxin molecule: antitoxin versus antitoxoid. Infect Immun. 1976 Jul;14(1):122–128. doi: 10.1128/iai.14.1.122-128.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf J. M., Gaudin H. M., Eidels L. Localization of the diphtheria toxin receptor-binding domain to the carboxyl-terminal Mr approximately 6000 region of the toxin. J Biol Chem. 1990 May 5;265(13):7331–7337. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Rapid entry of nicked diphtheria toxin into cells at low pH. Characterization of the entry process and effects of low pH on the toxin molecule. J Biol Chem. 1981 Sep 10;256(17):9068–9076. [PubMed] [Google Scholar]
- Schmidt W., Schmidt M. A. Mapping of linear B-cell epitopes of the S2 subunit of pertussis toxin. Infect Immun. 1989 Feb;57(2):438–445. doi: 10.1128/iai.57.2.438-445.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T., Yamada M., Sugawa H., Mekada E., Uchida T., Okada Y. Monoclonal antibodies against diphtheria toxin fragment A. Characterization and introduction into living cells. Exp Cell Res. 1984 Apr;151(2):344–353. doi: 10.1016/0014-4827(84)90385-9. [DOI] [PubMed] [Google Scholar]
- Zanen J., Muyldermans G., Beugnier N. Competitive antagonists of the action of diphtheria toxin in HeLa cells. FEBS Lett. 1976 Jul 15;66(2):261–263. doi: 10.1016/0014-5793(76)80518-2. [DOI] [PubMed] [Google Scholar]
- Zucker D. R., Murphy J. R. Monoclonal antibody analysis of diphtheria toxin--I. Localization of epitopes and neutralization of cytotoxicity. Mol Immunol. 1984 Sep;21(9):785–793. doi: 10.1016/0161-5890(84)90165-2. [DOI] [PubMed] [Google Scholar]
- Zucker D. R., Murphy J. R., Pappenheimer A. M., Jr Monoclonal antibody analysis of diphtheria toxin--II. Inhibition of ADP-ribosyl-transferase activity. Mol Immunol. 1984 Sep;21(9):795–800. doi: 10.1016/0161-5890(84)90166-4. [DOI] [PubMed] [Google Scholar]