Abstract
OBJECTIVE
The metabolism of hepatic- and intestinally derived lipoproteins is regulated in a complex fashion by nutrients, hormones, and neurologic and other factors. Recent studies in animal models suggest an important role for glucagon acting via the glucagon receptor in regulating hepatic triglyceride (TG) secretion. Here we examined the direct effects of glucagon on regulation of hepatic and intestinal lipoprotein metabolism in humans.
RESEARCH DESIGN AND METHODS
Eight healthy men underwent two studies each, in random order, 4–6 weeks apart in which de novo lipogenesis, kinetics of larger VLDL1 TG, and kinetics of VLDL1 and smaller VLDL2 apolipoprotein (apo)B100 and B48 were studied using established stable isotope enrichment methods. Subjects were studied in the constant fed state under conditions of a pancreatic clamp (with infusion of somatostatin, insulin, and growth hormone) at either basal glucagon (BG study, 64.5 ± 2.1 pg/mL) or hyperglucagonemia (high glucagon [HG] study, 183.2 ± 5.1 pg/mL).
RESULTS
There were no significant differences in plasma concentration of VLDL1 or VLDL2 TG, apoB100 or apoB48 between BG and HG studies. There was, however, lower (P < 0.05) VLDL1 apoB100 fractional catabolic rate (−39%) and production rate (−30%) in HG versus BG, but no difference in de novo lipogenesis or TG turnover, and glucagon had no effect on intestinal (B48-containing) lipoprotein metabolism.
CONCLUSIONS
Glucagon acutely regulates hepatic but not intestinal lipoprotein particle metabolism in humans both by decreasing hepatic lipoprotein particle production as well as by inhibiting particle clearance, with no net effect on particle concentration.
Dyslipidemia is a well-recognized characteristic of insulin-resistant states and type 2 diabetes and contributes to the development of atherogenic cardiovascular disease (1,2). Hypertriglyceridemia, low HDL cholesterol, and increased small, dense LDL particles are typical features of dyslipidemia in insulin resistance and type 2 diabetes (1). Both hepatic (apolipoprotein [apo]B100-containing) and intestinal (apoB48-containing) lipoproteins are increased in insulin resistance and type 2 diabetes (1–5). Increased production of both hepatic and intestinal lipoproteins as well as impairment in particle clearance contributes to the elevated plasma concentrations (1,6–10).
The mechanisms whereby hepatic and intestinal lipoprotein production is regulated are not clearly understood. In animal models (rev. in [6]) and humans (9–14), production of these particles is subject to substrate supply and hormonal regulation; thus free fatty acids (FFAs) stimulate and acute hyperinsulinemia suppresses hepatic and intestinal lipoprotein production (9,10,12–15). Whereas defects of insulin secretion and action and elevation of plasma FFAs are well described abnormalities of type 2 diabetes, less appreciated is the dysregulation of glucagon secretion that is also present in diabetes (16,17). Although fasting concentrations of glucagon in diabetic patients are usually similar to those in nondiabetic subjects, suppression of glucagon after glucose ingestion is impaired in type 2 diabetic patients (16). Postprandial hyperglucagonemia may play an important role in the dysregulation of carbohydrate and lipid metabolism in type 2 diabetes, and suppression of glucagon action has been proposed as a therapeutic approach to the treatment of type 2 diabetes (18,19). Recently, glucagon signaling through the glucagon receptor (Gcgr) has been shown to be essential for control of hepatic lipid homeostasis in mice (20). Although several studies in animals have indicated that chronic exogenous glucagon treatment may exert hypolipidemic effects (20–22), modulation of the Gcgr has yielded conflicting results (20,23). Because of potential confounding factors in chronic studies, the direct role of glucagon in regulating hepatic and intestinal lipoprotein metabolism is not clear, nor has this been directly examined in humans.
The objective of the current study, therefore, was to investigate the effect of acute hyperglucagonemia on hepatic and intestinal lipoprotein metabolism in healthy humans. Because infusion of glucagon affects the secretion of other pancreatic hormones such as insulin, which is known to exert independent effects on lipoprotein metabolism, studies were performed under conditions of a pancreatic clamp. Because we were interested in examining both intestinal as well as hepatic lipoprotein metabolism and it is technically difficult to measure intestinal lipoprotein production rates in fasted humans, studies were conducted in the constant fed state.
RESEARCH DESIGN AND METHODS
Subjects
Eight healthy, normolipidemic men participated in this study. Their demographic characteristics and fasting biochemical profiles are shown in Table 1. None of the participants had any previous history of cardiovascular disease, gastrointestinal or systemic illness, or surgical intervention within 6 months before the studies. No subject was taking medications, and all had normal oral glucose tolerance tests performed immediately before enrollment in the study. The Research Ethics Board of the University Health Network, University of Toronto, approved the study, and all subjects gave written informed consent before their participation.
TABLE 1.
Demographic characteristics and fasting biochemical parameters of subjects (n = 8)
| Characteristic | Mean ± SE | Range |
|---|---|---|
| Age (years) | 41.3 ± 3.0 | 30–55 |
| Weight (kg) | 70.9 ± 3.5 | 59.0–87.0 |
| BMI (kg/m2) | 22.5 ± 0.6 | 20.0–25.6 |
| Glucose (mmol/L) | 5.4 ± 0.2 | 4.8–6.3 |
| Insulin (pmol/L) | 47.5 ± 6.8 | 17.4–81.0 |
| Glucagon (pg/mL) | 69.1 ± 6.5 | 47.1–100.5 |
| Plasma (mmol/L) | ||
| FFA | 0.23 ± 0.04 | 0.11–0.44 |
| TG | 1.03 ± 0.11 | 0.63–1.60 |
| Total cholesterol | 4.02 ± 0.17 | 3.50–4.76 |
| VLDL | ||
| 1 ApoB48 (mg/L) | 0.65 ± 0.17 | 0.14–1.72 |
| 1 ApoB100 (mg/dL) | 2.15 ± 0.36 | 1.08–3.75 |
| 2 ApoB48 (mg/L) | 0.85 ± 0.15 | 0.20–1.35 |
| 2 ApoB100 (mg/dL) | 2.67 ± 0.40 | 1.41–5.06 |
Experimental protocol for lipoprotein kinetic studies
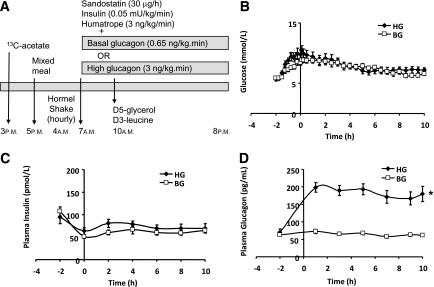
Each subject underwent two separate lipoprotein kinetic studies in random order, 4 to 6 weeks apart (Fig. 1A). In each study, two intravenous catheters were inserted, one into a superficial vein in each forearm, for infusion and for blood sampling, respectively. At 3 p.m., a constant infusion of sodium 1-13C-acetate (15 gm/L at 32 mL/h; Cambridge Isotope Laboratories, Andover, MA) was started for assessment of de novo lipogenesis (24,25). Subjects were fasted after a mixed meal at 5 p.m.. Starting at 4 a.m. the next day, a liquid formula (Hormel Great Shake Plus, Hormel Health Laboratories, Savannah, GA; total fat 10% by weight, saturated fat 1.5%, trans fat 0%, monounsaturated fat 2.6%, polyunsaturated fat 5.6%, cholesterol 0%; 49% calories from fat, 38% from carbohydrates, 13% from proteins) was ingested hourly for the first 3 h and every half hour thereafter to achieve a constant fed state. Each aliquot was calculated to evenly spread the total daily caloric requirement across the course of the study. The Harris-Benedict equation was used to estimate the total daily energy requirement for each subject. Kinetic studies were performed in the constant fed state because apoB48 levels are too low in the fasted state to allow accurate assessment of isotopic enrichments.
FIG. 1.
Study protocol (A) and plasma concentrations of glucose (B), insulin (C), and glucagon (D) over the time course of the study. A constant infusion of sodium 1-13C-acetate was started at 3 p.m. on the day before the kinetics study. A mixed meal was provided that day at 5 p.m., after which the subject fasted. At 4 a.m. the next day subjects started to ingest identical hourly, then half hourly, volumes of a liquid high fat nutritional supplement to maintain a constant fed state. At 7 a.m., i.e., 3 h after starting to ingest the formula, a pancreatic clamp was started with infusion of somatostatin, insulin, growth hormone, and glucagon, the latter to achieve either BG (□) or HG (♦) plasma concentrations. At 10 a.m. (referred to as time 0 for the lipoprotein kinetic study), i.e., 3 h after starting the pancreatic clamp, a bolus of [1,1,2,3,3-2H5]-glycerol (d5-glycerol) was administered and a primed, constant infusion of l-[5,5,5-2H3]-leucine (d3-leucine) was started and continued for 10 h (A). Plasma glucose (B) and insulin (C) concentrations were similar in BG and HG studies, whereas glucagon (D) was approximately threefold higher in HG vs. BG. *P < 0.0001 HG vs. BG.
At 7 a.m., i.e., 3 h after starting the liquid formula, a pancreatic clamp was started with the following infusion rates: 30 μg/h somatostatin (Sandostatin, Norvatis Pharmaceuticals Canada, Dorval, QC, Canada), 0.05 mU/kg/min insulin (Humulin R, Eli Lilly Canada, Toronto, ON, Canada), 3 ng/kg/min human recombinant growth hormone (Humatrope, Eli Lilly), and 20% dextrose as required in a few subjects towards the end of the study at a variable rate to maintain euglycemia. Glucagon (Eli Lilly Canada) was infused at different rates in each of the two separate studies to achieve either basal glucagon (BG; 0.65 ng/kg/min) or high glucagon (HG; 3 ng/kg/min) circulating glucagon levels. All hormones were diluted in 1 L of half-strength normal saline and infused with a syringe pump (B. Braun Medical, Bethlehem, PA). Autologous serum (5 mL), freshly prepared from the subject’s blood, was added to the saline as carrier before hormone dilution.
Six hours after starting the liquid formula ingestion and 3 h after starting the pancreatic clamp, subjects received a bolus of [1,1,2,3,3-2H5]-glycerol (d5-glycerol, 75 μmol/kg; Cambridge Isotope Laboratories) and a primed, constant infusion (10 µmol/kg bolus followed by 10 μmol/kg/h for 10 h) of l-[5,5,5-2H3]-leucine (d3-leucine; Cambridge Isotope Laboratories) for assessment of triglyceride (TG) and lipoprotein kinetics, respectively, as previously described (26–28). After the start of the d3-leucine infusion, blood samples were collected at 1, 3, 5, 7, 9, and 10 h for isolation of lipoproteins. Blood samples for insulin, FFA and TG analysis were collected at regular intervals as previously described (9). At the end of the kinetic study, a bolus of heparin sodium (60 i.u./kg, Baxter Pharmaceuticals, Mississauga, ON, Canada) was injected. Blood samples (5 mL) were collected after 10 min into EDTA tubes on ice, and plasma was separated immediately and stored at −80°C until performance of lipase assays.
Laboratory methods
VLDL1 and VLDL2 fractions were isolated from fresh plasma using cumulative flotation gradient ultracentrifugation (29). In brief, plasma was adjusted to d = 1.10 g/mL with NaCl. A discontinuous density gradient consisting of 4 mL of d = 1.10 g/mL of plasma, 3 mL of d = 1.063 g/mL, 3 mL of d = 1.019 g/mL, and 2.8 mL of d = 1.006 g/mL NaCl solution was created. Samples were then centrifuged at 40,000 revolutions per min at 4°C in a Ti40 SW rotor (Beckman, Palo Alto, CA). Consecutive runs were performed to separate fractions that correspond to chylomicron (Sf > 400, 38 min), VLDL1 (Sf 60–400, 3 h 28 min), and VLDL2 (Sf 20–60, 17 h). After each step 1 mL of the gradient containing specific lipoprotein fraction was aspirated and 1 mL of d = 1.006 g/mL was used to refill the tubes before the next run.
Aliquots of VLDL fractions (∼1 mg protein) were delipidated and separated by preparative 3.3% SDS-PAGE. Gel bands corresponding to apoB48 and apoB100 were excised and hydrolyzed, and amino acids were derivatized to allow for the determination of plasma leucine isotopic enrichment as described (8). Briefly, gel bands were incubated at 110°C with 6N HCl for 24 h, dried under vacuum before being derivatized with 100 µL mixture (1:1) of acetonitrile: N-tert-butyldimethyl-N-methyltrifluoracetamide (Sigma-Aldrich). Plasma free amino acids were recovered from 0.25 mL plasma after precipitation of proteins with acetone and extraction of the aqueous phase with hexane (30). The aqueous phase was dried under vacuum, amino acids were derivatized, and enrichments were determined as above. Derivatized samples were analyzed by electron impact ionization gas chromatography–mass spectrometry (GCMS; Agilent 5975/6890N, Agilent Technologies Canada, Mississauga, ON, Canada) using helium as the carrier gas. Selective ion monitoring at m/z = 200 and 203 was performed, and tracer-to-tracee ratios were calculated from isotopic ratios for each sample according to a standard curve of isotopic enrichment.
VLDL1 fractions during the time course of the lipoprotein kinetic study (0–10 h) were processed for estimation of VLDL-TG and glycerol turnover, as previously described (26). Briefly, deproteinized VLDL1 fractions were separated on thin layer chromatography (TLC) plates and TLC scraping corresponding to TGs were collected. Lipids were extracted and dried, and fatty acid methyl esters (FAMEs) were prepared. FAMEs were dissolved in heptane, and the molecular ions of palmitate methyl ester were monitored on GCMS at m/z = 270 and 272. Glycerol was derivatized with 100 μL 33% heptafluorobutyric anhydride (HFB) anhydrate in ethyl acetate (HFBA: ethyl acetate = 1:2, vol/vol). Ions of HFB-glycerol were monitored at m/z = 467 and 472.
Commercial kits were used to measure cholesterol (Roche Diagnostics, Mannheim, Germany), TG (Roche Diagnostics), FFA (Wako Industrials, Osaka, Japan), insulin (Millipore, Billerica, MA), and glucagon (Millipore). ApoB100 was separated by 3–8% SDS-PAGE and quantified using an LDL apoB100 standard as previously described (31). ApoB48 mass was measured using a human apoB48 ELISA kit (Shibayagi, Shibukawa, Gunma, Japan). Lipoprotein lipase and hepatic lipase activities in postheparin plasma were measured using a triolein emulsion containing radiolabeled triolein as previously described (32).
Kinetic analysis
Stable isotope enrichment curves for apoB48 and apoB100 were fitted to a multicompartmental model using SAAM II software (version 1.2, University of Washington, Seattle, WA) to estimate the fractional catabolic rates (FCR) of VLDL1 and VLDL2 apoB48 or VLDL1 and VLDL2 apoB100, as previously described (10). The model consisted of synthesis of both VLDL1 and VLDL2 apoB from the precursor pool via a delay compartment, as well as conversion from VLDL1 to VLDL2. Individual enrichment (tracer to tracee ratios) and apoB masses were used to derive kinetic rate constants, which were independent for the two subsystems. Plasma leucine enrichment was used as a forcing function. FCR of VLDL1 was the sum of the conversion from VLDL1 to VLDL2 and direct loss from the VLDL1 compartment. Production rates (PR) of each apolipoprotein were calculated as the FCR multiplied by pool size, where pool size equaled average plasma concentration (in mg/L) between 1 and 10 h of the kinetic study × plasma volume (estimated as 0.045 L/kg body wt).
The monoexponential slopes of VLDL1-TG glycerol stable isotope enrichment curves were determined from the peak of the isotopic enrichment to the end of the kinetic study (usually between 2 and 10 h), on the log-linear portion of the curve (26). This approach, when compared with compartmental modeling, might slightly underestimate the FCR of VLDL-TG. However, any potential bias due to the choice of method should be similar to each treatment arm and not affect the conclusion of the study. De novo synthesis of VLDL1-TG palmitate was computed using mass isotopomer distribution analysis (24,25). VLDL1-TG palmitate pool was calculated as average VLDL1-TG concentration during the lipoprotein kinetic study × plasma volume (0.045 L/kg body wt), and percentage of newly synthesized palmitate in VLDL1-TG was used to illustrate de novo lipogenesis.
Statistics
Results are presented as mean ± SE. Repeated-measures ANOVA was used to compare the time course of parameters during the kinetic experiments. Paired t test was used to compare FCR and PR of lipoprotein and TG and de novo lipogenesis between the two treatments. All statistics were performed with SAS (version 8, Cary, NC). A P value <0.05 was considered significant.
RESULTS
Pancreatic clamp
During the pancreatic clamp, levels of plasma glucose transiently increased approximately twofold but returned toward baseline after 4 h and remained ∼1.5-fold elevated for the duration for the study. Glucose was well matched between treatment arms (Fig. 1B). Plasma insulin levels were maintained at basal after an initial increase and were similar in both treatment arms of the study (Fig. 1C). Plasma growth hormone levels (not illustrated) rose from basal but were closely matched between treatment arms (0.40 ± 0.10 μg/L in BG and 0.49 ± 0.15 μg/L in HG at basal, 1.15 ± 0.12 μg/L in BG and 1.17 ± 0.10 μg/L in HG in clamp). Plasma glucagon levels were maintained at basal in BG (Fig. 1D). In contrast, in the HG arm there was a significant threefold elevation of circulating glucagon concentration (183.2 ± 5.1 pg/mL in HG vs. 64.5 ± 2.1 pg/mL in BG, P < 0.0001), which was maintained for 13 h.
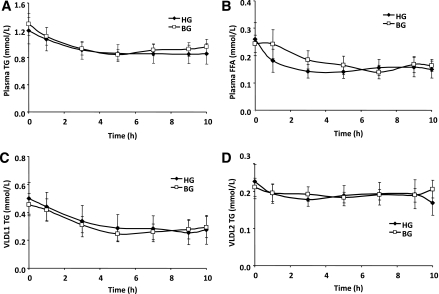
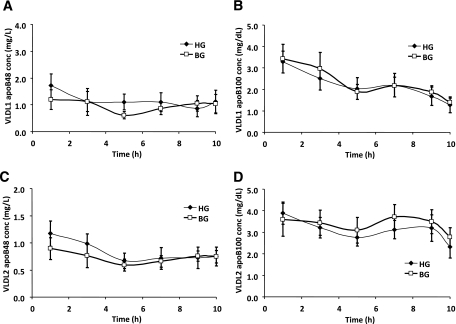
Effects of acute hyperglucagonemia on plasma TG, FFA, and VLDL-TG, and apoB48 and apoB100 concentrations
Plasma TG (Fig. 2A) and FFA (Fig. 2B) did not differ between BG and HG studies throughout the study. In both VLDL1 (Fig. 2C) and VLDL2 (Fig. 2D) fractions, TG concentrations were similar between BG and HG studies. ApoB48 and apoB100 in both VLDL fractions remained constant for the duration of the lipoprotein kinetics study and did not differ between BG and HG studies (Fig. 3, A–D). The levels of TG, apoB48, and apoB100 in plasma and VLDL fractions and FFAs in plasma during the lipoprotein kinetic study were not significantly different from the basal levels (before the pancreatic clamp).
FIG. 2.
Plasma TG (A), plasma FFA (B), VLDL1-TG (C), and VLDL2-TG (D) over the time course of the lipoprotein kinetic study in subjects receiving either BG (□) or HG (♦) infusion rate during pancreatic clamp. N = 8. P = NS HG vs. BG for all parameters.
FIG. 3.
VLDL1 apoB48 (A), VLDL1 apoB100 (B), VLDL2 apoB48 (C), and VLDL2 apoB100 (D) over the time course of the lipoprotein kinetic study in subjects receiving either BG (□) or HG (♦) infusion rate during pancreatic clamp. Conc, concentration. N = 8. P = NS HG vs. BG for all parameters.
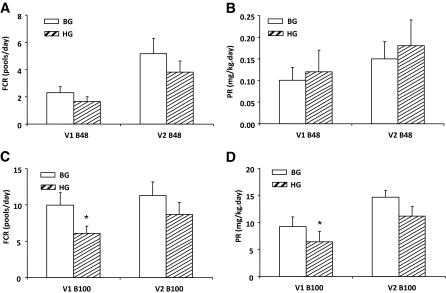
Effects of acute hyperglucagonemia on VLDL apoB48 and apoB100 fractional catabolic rates and production rates
Fractional catabolic rates of VLDL1 and VLDL2 apoB48 were not significantly different between BG and HG studies (Fig. 4A). Similarly, PRs of apoB48 in both VLDL fractions were similar in BG and HG (Fig. 4B). In contrast, the FCR of VLDL1 apoB100 was 39% lower in HG (6.1 ± 1.0 pools/day) vs. BG (10.0 ± 1.7 pool/day) (P < 0.01) (Fig. 4C). A similar magnitude of reduction in PR was observed for VLDL1 apoB100 in HG vs. BG (6.4 ± 1.9 vs. 9.2 ± 1.8 mg/kg/day, P < 0.05) (Fig. 4D). The trend was similar for apoB100 FCR and PR in VLDL2, although these parameters did not reach statistical significance (FCR = 8.7 ± 1.7 vs. 11.3 ± 1.8 pool/day, P = 0.08; PR = 11.1 ± 1.8 vs. 14.9 ± 1.2 mg/kg/day, P = 0.1). The similar VLDL apoB100 concentrations in Fig. 3B and D were thus explained by the simultaneous reduction in both FCR and PR in HG vs. BG, with no net effect on plasma pool size. A further analysis of the source of VLDL2 apoB100 production indicates that the differences were mainly due to production via VLDL1 (HG 6.4 ± 1.9 vs. BG 9.2 ± 1.8 mg/kg/day, P < 0.05) but not direct production (HG 4.7 ± 1.1 vs. 5.5 ± 1.3 mg/kg/day, P = NS).
FIG. 4.
Effect of acute hyperglucagonemia on VLDL apoB FCR (A and C) and PR (B and D) in subjects receiving either BG (white bar) or HG (hatched bar) infusion rate during pancreatic clamp. N = 8. The only significant differences were a lower FCR and PR for VLDL1-apoB100 in HG vs. BG. *P < 0.05 HG vs. BG.
Effects of acute hyperglucagonemia on de novo lipogenesis, VLDL1-TG turnover, and postheparin lipase activities
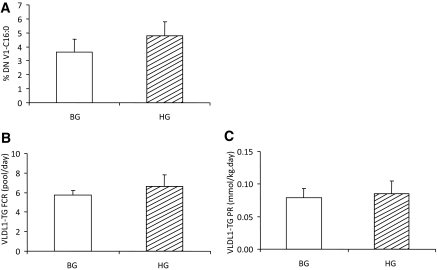
As indicated in Fig. 5A, newly synthesized palmitate from acetate in VLDL1-TG was not different between BG and HG study arms. Fractional catabolic rates and PR of VLDL1-TG also did not differ between BG vs. HG (Fig. 5, B and C, respectively). Postheparin lipase activities (not illustrated) were not significantly different between BG (20 ± 12 and 35 ± 8 nmol/min/μg for lipoprotein lipase and hepatic lipase, respectively) and HG (34 ± 15 and 41 ± 10 nmol/min/μg for lipoprotein lipase and hepatic lipase, respectively).
FIG. 5.
Effect of acute hyperglucagonemia on de novo lipogenesis (A), VLDL1-TG FCR (B), and PR (C) in subjects receiving either BG (white bar) or HG (hatched bar) infusion rate during pancreatic clamp. VLDL apoB FCR and PR are shown. %DN V1-C16:0, percentage of newly synthesized palmitate in VLDL1-TG. There were no significant differences for any of the parameters illustrated.
DISCUSSION
Because glucagon affects numerous aspects of metabolism that could in turn have direct or indirect effects on lipoprotein metabolism, the direct role of glucagon in lipid and lipoprotein metabolism remains unclear. The current study is the first to examine the direct role of short-term elevation of glucagon, under the condition of pancreatic clamp and in the fed state, on hepatic and intestinal lipoprotein metabolism in humans. We have shown in this study that acute hyperglucagonemia, in a controlled experimental setting in which other hormone levels and circulating metabolites are well matched between studies, does not affect plasma concentrations of apoB-containing intestinal and hepatic lipoproteins and does not affect the rate of de novo lipogenesis or TG metabolism but does impair both the clearance and PR of larger (VLDL1) hepatically derived lipoproteins.
Glucagon plays a key role in carbohydrate (33–35) and lipid homeostasis (20), and postprandial hyperglucagonemia likely contributes to many of the metabolic defects of type 2 diabetes, with suppression of glucagon action being proposed as a therapeutic approach to the treatment of type 2 diabetes (18,19). Chronic administration of exogenous glucagon in animals generated hypolipemidemic effects, with reduced plasma TG, FFA, and cholesterol; decreased VLDL secretion from the liver; increased hepatic fatty acid (FA) oxidation; and decreased hepatic accumulation of lipid (21,36–38). Glucagon decreased plasma FFAs and VLDL-TG and prevented fatty liver in dairy cows receiving daily injections of glucagon for 2 weeks (21,39,40). In rats receiving daily injections of glucagon for 8 or 21 days, TG in chylomicron and VLDL (22,37,41) and plasma cholesterol concentrations were decreased (36,37). On the other hand, genetic ablation of the Gcgr in mice increased circulating TG and FFAs after fasting, whereas administration of glucagon for 24 h produced the opposite effects (20). Gcgr−/− mice had increased plasma and hepatic TG (20) and were resistant to hepatic accumulation of lipid (42). However, reduced Gcgr expression with antisense oligonucleotide in db/db mice for 3 weeks decreased circulating TG and FFAs (23). Longuet et al. (20) recently showed that Gcgr signaling is essential for regulation of hepatic lipid homeostasis. When compared with wild-type (WT) littermates, Gcgr−/− mice had increased plasma TG and hepatic TG secretion after fasting for 16 h. Subcutaneous injection of glucagon over 24 h decreased plasma TG and FFAs in WT mice. Acute, single dose injection of glucagon also decreased TG secretion in WT mice. During fasting, glucagon, acting via the Gcgr, stimulated peroxisome proliferator–activated receptor (PPAR-α) activity in a p38 mitogen-activated protein kinase (MAPK)- and AMP-activated protein kinase (AMPK)-dependent manner, subsequently diverting more FA for β-oxidation instead of TG synthesis, thus reducing hepatic TG accumulation. Glucagon also decreased hepatic TG secretion independent of PPAR-α activation and FA β-oxidation (20). It is important to be cognizant of the fact that in chronic studies potential confounding effects due to compensatory secretion of other hormones, such as insulin, and changes in plasma FFA and glucose concentrations, cannot be ruled out. Indeed, concomitant increase of insulin was detectable in dairy cows after glucagon injection, at least transiently (39).
In the current study we administered glucagon under pancreatic clamp conditions to generate a threefold elevation of circulating glucagon above basal. Fasting glucagon concentrations in healthy subjects are ∼100 pg/mL (16). In obese nondiabetic subjects, plasma glucagon concentration was reported as ∼70 pg/mL in the postabsorptive state and increased to 100–144 pg/mL during prolonged fasting (43). The level of hyperglucagonemia achieved in the current study is within the reported range of various studies with glucagon infusion (17,34,44). This degree of hyperglucagonemia was maintained for 12 h with the levels of insulin, glucose, FFAs, and growth hormone well matched between the two experimental arms of the study. Thus the above-mentioned potential confounding factors were excluded. To be able to assess apoB48 stable isotope enrichment with a high degree of precision, subjects were maintained in a constant fed state to stimulate intestinal lipoprotein production. Under these conditions, we found that hyperglucagonemia decreased the production of large, hepatically derived, VLDL1 lipoprotein particle production (with a nonsignificant similar trend in smaller VLDL2). The reduction in apoB100-containing lipoprotein production was accompanied by a reduction in particle clearance of similar magnitude, leading to unchanged circulating VLDL apoB100 concentration (i.e., particle pool size). No significant effects of hyperglucagonemia were observed on intestinal lipoprotein metabolism, de novo lipogenesis, or TG turnover.
Very limited information is available regarding glucagon effects on hepatic lipoprotein production. Guettet et al. (22) reported that exogenous glucagon (twice daily injection for 21 days) increased clearance of TG-rich lipoprotein in high-sucrose fed rats. In an acute study with glucagon in dogs, 2-h glucagon infusion decreased removal of Intralipid (45). The mechanism whereby hyperglucagonemia decreases FCR of hepatic lipoprotein particles is not known. Although glucagon might affect lipase activities (46), postheparin lipoprotein lipase and hepatic lipase activities were assessed at the end of our study and were not significantly affected by HG infusion. Therefore, the reduction in FCR could not be explained by reduction in lipase activities. In cultured rat hepatocytes, 24-h treatment with glucagon increased LDL binding to its receptor and promoted degradation (47). Although glucagon has been shown to activate PPAR-α in rodent hepatocytes (20), PPAR-α agonist fibrates reduce VLDL-apoB100 through enhancing clearance and decreasing production in hyperlipemic patients (48). The observed effects of hyperglucagonemia on hepatic lipoprotein particle production in the current study thus may not be attributed to its effects on PPAR-α. The impact of reduced hepatic lipoprotein particle turnover is not clear and needs further study. Although the glucagon receptor is expressed in the intestine (49), its physiological role in the intestine is largely unknown. In the current study, intestinal lipoprotein production was not affected by acute hyperglucagonemia, suggesting lack of a direct role of glucagon in regulating intestinal lipoprotein production in healthy humans.
The mechanism whereby glucagon differentially regulates the clearance of lipoprotein particles from hepatic and intestinal origins remains unclear. Clearance of apoB-containing particle involves both receptor-dependent and -independent pathways. Because postheparin lipase activities were not significantly affected by glucagon levels in this study and it is generally accepted that intravascular lipolysis of apoB100- and apoB48-containing lipoprotein particles share common pathways, it is more likely that the regulation points reside in the pathways involving interaction of lipoprotein particles with cell surface receptors. LDL receptor is known to mediate apoB-containing lipoprotein metabolism (50) and is subject to regulation by glucagon in rats (47,51). Whether glucagon, under the current study conditions, differentially affects binding, uptake, and degradation of apoB100- versus apoB48- containing lipoprotein particles via the LDL receptor pathway warrants further study.
In conclusion, the current study does not support a role for glucagon in regulating intestinal lipoprotein production, in contrast with our previous studies, which have shown that insulin and FFAs are important regulators of both intestinal and hepatic lipoprotein production (9,10,14). Similarly, acute hyperglucagonemia does not affect de novo lipogenesis or lipoprotein TG metabolism. In contrast, there is a very consistent effect of acute hyperglucagonemia in suppressing the production of large, hepatically derived, apoB100-containing lipoproteins in humans, but this effect is offset by a reduction of similar magnitude in the clearance of the particles. Because hyperglucagonemia does not affect plasma concentrations of apoB100-containing lipoproteins, glucagon appears to have a less significant role than either insulin or FFAs in regulating hepatic lipoprotein production. The mechanism of its effect on apoB100-containing particle production and clearance remains to be determined. Because relative postprandial hyperglucagonemia occurs in diabetic and insulin-resistant individuals who usually also exhibit hyperinsulinemia, future studies in this population may provide more physiological and clinical relevance.
ACKNOWLEDGMENTS
This work was supported by funding from the Canadian Institutes of Health Research (MOP-43839). G.F.L. holds a Canada Research Chair in Diabetes and is a Career Investigator of the Heart and Stroke Foundation of Canada. M.P. was the recipient of a Postdoctoral Fellowship Award from the Banting and Best Diabetes Centre. B.W.P. was supported by National Institutes of Health P30 DK56341 (Clinical Nutrition Research Unit).
No potential conflicts of interest relevant to this article were reported.
C.X. and M.P. performed the studies, analyzed the data, and wrote the article. L.S. performed laboratory analyses and contributed intellectually to study design and interpretation of data. B.W.P. assisted with kinetics analyses and contributed intellectually to data interpretation and reviewed and edited the article. G.F.L. obtained grant funding to support the research, designed the study, analyzed data, interpreted data, and edited the article.
Parts of this study were presented in abstract form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors are indebted to Patricia Harley and Kristine Puzeris for assistance with subject recruitment and conducting the clinical protocol. The authors would like to thank Dr. John Hill and Eugene Chu (University of British Columbia and St. Paul’s Hospital, Vancouver, BC, Canada) for lipase activity assays.
Footnotes
Clinical trial reg. no. NCT01155206, clinicaltrials.gov.
REFERENCES
- 1.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis GF, Steiner G. Hypertriglyceridemia and its metabolic consequences as a risk factor for atherosclerotic cardiovascular disease in non-insulin-dependent diabetes mellitus. Diabetes Metab Rev 1996;12:37–56 [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi A, Fukushima M, Sakai M, et al. Remnant-like particle cholesterol, triglycerides, and insulin resistance in nonobese Japanese type 2 diabetic patients. Diabetes Care 2000;23:1766–1769 [DOI] [PubMed] [Google Scholar]
- 4.Schaefer EJ, McNamara JR, Shah PK, et al. Framingham Offspring Study Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care 2002;25:989–994 [DOI] [PubMed] [Google Scholar]
- 5.Curtin A, Deegan P, Owens D, Collins P, Johnson A, Tomkin GH. Elevated triglyceride-rich lipoproteins in diabetes. A study of apolipoprotein B-48. Acta Diabetol 1996;33:205–210 [DOI] [PubMed] [Google Scholar]
- 6.Adeli K, Lewis GF. Intestinal lipoprotein overproduction in insulin-resistant states. Curr Opin Lipidol 2008;19:221–228 [DOI] [PubMed] [Google Scholar]
- 7.Chan DC, Nguyen MN, Watts GF, Barrett PH. Plasma apolipoprotein C-III transport in centrally obese men: associations with very low-density lipoprotein apolipoprotein B and high-density lipoprotein apolipoprotein A-I metabolism. J Clin Endocrinol Metab 2008;93:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B-48-containing lipoproteins in humans. Arterioscler Thromb Vasc Biol 2006;26:1357–1363 [DOI] [PubMed] [Google Scholar]
- 9.Duez H, Lamarche B, Valéro R, et al. Both intestinal and hepatic lipoprotein production are stimulated by an acute elevation of plasma free fatty acids in humans. Circulation 2008;117:2369–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlic M, Xiao C, Szeto L, Patterson BW, Lewis GF. Insulin acutely inhibits intestinal lipoprotein secretion in humans in part by suppressing plasma free fatty acids. Diabetes 2010;59:580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malmström R, Packard CJ, Caslake M, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia 1997;40:454–462 [DOI] [PubMed] [Google Scholar]
- 12.Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes 1993;42:833–842 [DOI] [PubMed] [Google Scholar]
- 13.Lewis GF, Zinman B, Uffelman KD, Szeto L, Weller B, Steiner G. VLDL production is decreased to a similar extent by acute portal vs. peripheral venous insulin. Am J Physiol 1994;267:E566–E572 [DOI] [PubMed] [Google Scholar]
- 14.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest 1995;95:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malmström R, Packard CJ, Caslake M, et al. Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes 1998;47:779–787 [DOI] [PubMed] [Google Scholar]
- 16.Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest 1970;49:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med 1978;299:433–436 [DOI] [PubMed] [Google Scholar]
- 18.Lefèbvre PJ. Glucagon and its family revisited. Diabetes Care 1995;18:715–730 [DOI] [PubMed] [Google Scholar]
- 19.Ali S, Drucker DJ. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab 2009;296:E415–E421 [DOI] [PubMed] [Google Scholar]
- 20.Longuet C, Sinclair EM, Maida A, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab 2008;8:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bobe G, Ametaj BN, Young JW, Beitz DC. Effects of exogenous glucagon on lipids in lipoproteins and liver of lactating dairy cows. J Dairy Sci 2003;86:2895–2903 [DOI] [PubMed] [Google Scholar]
- 22.Guettet C, Rostaqui N, Navarro N, Lecuyer B, Mathe D. Effect of chronic glucagon administration on the metabolism of triacylglycerol-rich lipoproteins in rats fed a high sucrose diet. J Nutr 1991;121:24–30 [DOI] [PubMed] [Google Scholar]
- 23.Liang Y, Osborne MC, Monia BP, et al. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 2004;53:410–417 [DOI] [PubMed] [Google Scholar]
- 24.Hellerstein MK, Kletke C, Kaempfer S, Wu K, Shackleton CH. Use of mass isotopomer distributions in secreted lipids to sample lipogenic acetyl-CoA pool in vivo in humans. Am J Physiol 1991;261:E479–E486 [DOI] [PubMed] [Google Scholar]
- 25.Chinkes DL, Aarsland A, Rosenblatt J, Wolfe RR. Comparison of mass isotopomer dilution methods used to compute VLDL production in vivo. Am J Physiol 1996;271:E373–E383 [DOI] [PubMed] [Google Scholar]
- 26.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res 2002;43:223–233 [PubMed] [Google Scholar]
- 27.Batal R, Tremblay M, Barrett PH, et al. Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J Lipid Res 2000;41:706–718 [PubMed] [Google Scholar]
- 28.Lemieux S, Patterson BW, Carpentier A, Lewis GF, Steiner G. A stable isotope method using a [(2)H(5)]glycerol bolus to measure very low density lipoprotein triglyceride kinetics in humans. J Lipid Res 1999;40:2111–2117 [PubMed] [Google Scholar]
- 29.Lindgren FT, Jensen LC, Hatch FT. The isolation and quantitative analysis of serum lipoproteins. In Blood Lipids and Lipoproteins: Quantitation, Composition, and Metabolism. Nelson GJ, Ed. New York, John Wiley & Sons, 1972 [Google Scholar]
- 30.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 1999;40:2118–2124 [PubMed] [Google Scholar]
- 31.Karpe F, Hamsten A, Uffelman K, Steiner G. Apolipoprotein B-48. Methods Enzymol 1996;263:95–104 [DOI] [PubMed] [Google Scholar]
- 32.Hill JS, Yang D, Nikazy J, Curtiss LK, Sparrow JT, Wong H. Subdomain chimeras of hepatic lipase and lipoprotein lipase. Localization of heparin and cofactor binding. J Biol Chem 1998;273:30979–30984 [DOI] [PubMed] [Google Scholar]
- 33.Sherwin RS, Fisher M, Hendler R, Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med 1976;294:455–461 [DOI] [PubMed] [Google Scholar]
- 34.Shah P, Basu A, Basu R, Rizza R. Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol 1999;277:E283–E290 [DOI] [PubMed] [Google Scholar]
- 35.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2000;85:4053–4059 [DOI] [PubMed] [Google Scholar]
- 36.Guettet C, Mathe D, Riottot M, Lutton C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Biochim Biophys Acta 1988;963:215–223 [DOI] [PubMed] [Google Scholar]
- 37.Guettet C, Mathé D, Navarro N, Lecuyer B. Effects of chronic glucagon administration on rat lipoprotein composition. Biochim Biophys Acta 1989;1005:233–238 [DOI] [PubMed] [Google Scholar]
- 38.Pégorier JP, Garcia-Garcia MV, Prip-Buus C, Duée PH, Kohl C, Girard J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Biochem J 1989;264:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobe G, Sonon RN, Ametaj BN, Young JW, Beitz DC. Metabolic responses of lactating dairy cows to single and multiple subcutaneous injections of glucagon. J Dairy Sci 2003;86:2072–2081 [DOI] [PubMed] [Google Scholar]
- 40.Osman MA, Allen PS, Mehyar NA, et al. Acute metabolic responses of postpartal dairy cows to subcutaneous glucagon injections, oral glycerol, or both. J Dairy Sci 2008;91:3311–3322 [DOI] [PubMed] [Google Scholar]
- 41.Guettet C, Rostaqui N, Mathé D, Lecuyer B, Navarro N, Jacotot B. Effect of chronic glucagon administration on lipoprotein composition in normally fed, fasted and cholesterol-fed rats. Lipids 1991;26:451–458 [DOI] [PubMed] [Google Scholar]
- 42.Conarello SL, Jiang G, Mu J, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 2007;50:142–150 [DOI] [PubMed] [Google Scholar]
- 43.Marliss EB, Aoki TT, Unger RH, Soeldner JS, Cahill GF., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest 1970;49:2256–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charlton MR, Nair KS. Role of hyperglucagonemia in catabolism associated with type 1 diabetes: effects on leucine metabolism and the resting metabolic rate. Diabetes 1998;47:1748–1756 [DOI] [PubMed] [Google Scholar]
- 45.Hallberg D. Insulin and glucagon in the regulation of removal rate of exogenous lipids from the blood in dogs. Acta Chir Scand 1970;136:291–297 [PubMed] [Google Scholar]
- 46.Caren R, Corbo L. Glucagon and plasma lipoprotein lipase. Proc Soc Exp Biol Med 1974;146:1106–1110 [DOI] [PubMed] [Google Scholar]
- 47.Brown NF, Salter AM, Fears R, Brindley DN. Glucagon, cyclic AMP and adrenaline stimulate the degradation of low-density lipoprotein by cultured rat hepatocytes. Biochem J 1989;262:425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis 2010;210:35–40 [DOI] [PubMed] [Google Scholar]
- 49.Hansen LH, Abrahamsen N, Nishimura E. Glucagon receptor mRNA distribution in rat tissues. Peptides 1995;16:1163–1166 [DOI] [PubMed] [Google Scholar]
- 50.Young SG. Recent progress in understanding apolipoprotein B. Circulation 1990;82:1574–1594 [DOI] [PubMed] [Google Scholar]
- 51.Rudling M, Angelin B. Stimulation of rat hepatic low density lipoprotein receptors by glucagon. Evidence of a novel regulatory mechanism in vivo. J Clin Invest 1993;91:2796–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]