Abstract
OBJECTIVE
The barrier function of the glomerular filter has been studied for decades. Albuminuria reflects a malfunction of this barrier, and in animals dysfunctional endothelial nitric-oxide (NO) synthase results in albuminuria. We aimed to analyze the importance of NO for the glomerular barrier function in humans.
RESEARCH DESIGN AND METHODS
To assess the effect of endothelial dysfunction on albuminuria, we measured the urine albumin-to-creatinine ratio (UACR) both before and after the blockade of NO synthases (NOSs) with systemic infusion of NG-monomethyl-l-arginine (l-NMMA) in two distinct study populations. In population A, 62 hypertensive patients with type 2 diabetes and, in population B, 22 patients with hypercholesterolemia but without hypertension or type 2 diabetes were examined. All subjects had normal renal function.
RESULTS
There was a significant increase in the UACR in response to NOS inhibition with l-NMMA in hypertensive patients with type 2 diabetes (study population A) and in patients with hypercholesterolemia (study population B). Linear regression analyses revealed that the change in mean arterial presssure in response to l-NMMA was not related to the increase in the UACR in response to l-NMMA in either population, even after adjusting for filtration fraction.
CONCLUSIONS
NOS inhibition provokes albuminuria that is unrelated to changes in blood pressure. It is noteworthy that this finding was evident in patient groups prone to endothelial dysfunction and albuminuria. Thus, acute deterioration of endothelial function by reducing NO activity causes an increase in albuminuria.
Albuminuria predicts renal disease progression and cardiovascular risk (1–3). There is increasing evidence for a continuous relation between albuminuria and cardiovascular risk, even at levels of albuminuria within the normal range (4). In diabetes, the prevalence of microalbuminuria varies from 20 to 40% (5–7), and prevention of microalbuminuria was investigated in large-scale prospective studies (e.g., the BErgamo NEphrologic DIabetes Complications Trial) (8). In the general population, microalbuminuria also seems to be a common phenomenon. The Prevention of Renal and Vascular Endstage Disease Study (9) reported a prevalence of microalbuminuria of 6.6%, after excluding diabetes and hypertension, which is in accordance with other findings of several cross-sectional studies (10–12) that reported a prevalence of 5–8% of microalbuminuria in general “healthy” populations.
Albuminuria is considered to be the result of a malfunction of the glomerular filtration barrier, although failure of tubular reuptake of albumin also is discussed as a contributor. The glomerular filtration barrier consists of three layers that separate the intravascular from the urine space. These layers are the glomerular endothelium, which is fenestrated; the glomerular basement membrane; and the podocytes with its foot processes. Although the glomerular filtration barrier was the object of intensive studies for decades, the role and effects of the individual layers on albuminuria remain controversial (13–15). However, currently there is increasing evidence that the glomerular endothelium also may play a significant role in glomerular permselectivity and, hence, genesis of albuminuria (14,16).
One of the most important mediators released by the endothelium is nitric oxide (NO), which is generated by a variety of NO synthases (NOSs), including endothelial NOS (eNOS) (17). Endothelium-derived NO has been widely used as general marker of endothelial function (18). Altered production and release of NO (i.e., reduced basal NO activity) (19) can be directly assessed by systemic infusion of NG-monomethyl-l-arginine (l-NMMA) in humans and have been found to be decreased in renal disease (20).
A diabetic mouse model knockout of eNOS expression resulted in significantly increased albuminuria compared with that in control mice (21). Genetic association studies in humans have shown that eNOS polymorphisms that potentially impair eNOS gene transcription and activity are associated with an increased risk of diabetic nephropathy (22,23).
Although the meaning of endothelial dysfunction in relation to albuminuria has been discussed repeatedly, until now no analysis on the change in albuminuria in response to inhibition of NO, resulting in a reduction of basal NO activity, has been carried out in humans. Therefore, as a proof of concept, we tested in two study populations the hypothesis that reduced NO activity causes albuminuria.
RESEARCH DESIGN AND METHODS
Subjects were recruited by advertising in local newspapers in the area of Erlangen-Nürnberg, Germany, and eligible subjects were enrolled consecutively in one of two subpopulations, according to their clinical characteristics. Written informed consent was obtained prior to study inclusion. Study protocols were approved by the local ethics committee (University of Erlangen-Nürnberg), and the study was conducted in accordance with the Declaration of Helsinki and the principles of good clinical practice guidelines.
Study population A
The main inclusion criteria of study population A were having type 2 diabetes and arterial hypertension defined as mean seated systolic blood pressure (SBP) 130–179 mmHg and/or diastolic blood pressure (DBP) 80–109 mmHg or being on antihypertensive medications. Other inclusion criteria were normoalbuminuria or microalbuminuria and glomerular filtration rate (GFR) >1.34 mL/s. The main exclusion criteria were having poor diabetes control (A1C >9%); receiving thiazolidinediones and/or the initiation of statins for at least 4 weeks prior to the commencement of the study; having hypertension-related end-organ damage, such as proliferative retinopathy and symptomatic cardiovascular disease; and being a smoker. Antihypertensive medication was paused for at least 2 weeks.
Study population B
Study population B was composed of patients with hypercholesterolemia defined as having a fasting LDL cholesterol level of >4.14 mmol/L and <6.58 mmol/L and fasting triglycerides <4.0 mmol/L, while not receiving statins for at least 4 weeks before study inclusion. The main exclusion criteria included having diabetes, micro- or macroalbuminuria, arterial hypertension, homozygous familial hypercholesterolemia, and hyperlipoproteinamia type III; showing evidence of having cardiovascular or renal disease; and being a smoker. All had an estimated GFR of >1.34 mL/s and were free of any cardiovascular medications.
Experimental protocol
All subjects were studied in the supine position at the same time in the morning in a quiet and temperature-controlled room. Before the experimental protocol was started, patients were advised to empty their bladder. This urine was discarded. A constant infusion (250 mL/h) of saline was administered throughout the protocol. After 115–120 min of rest, patients were asked to urinate and basal urine was obtained. Thereafter, the NOS inhibitor l-NMMA (Clinalfa, Läufelingen, Switzerland) was administered intravenously as a bolus infusion (3 mg/kg body wt) over 5 min, followed by constant infusion over 25 min with a rate of 0.05 mg/kg/min. Therefore, the total dose was 4.25 mg/kg body wt. At the end of the l-NMMA phase, urine was collected again (l-NMMA sample). For safety reasons, an infusion of 100 mg/kg body wt of l-arginine (6% l-arginine hydrochloride; University Hospital Pharmacy, Erlangen, Germany) was infused to counteract l-NMMA–induced vasoconstriction.
In parallel, renal hemodynamic parameters were determined by the constant-infusion input-clearence technique with inulin (Inutest; Fresenius, Linz, Austria) and sodium p-aminohippurate (Clinalpha, Basel, Switzerland) for GFR and renal plasma flow (RPF), respectively, as previously outlined in detail (24). In brief, after a bolus infusion of inulin and p-aminohippurate over a 15-min period and a subsequent constant infusion over 105 min, a steady state between the input and renal excretion of the tracer substances was reached, and, in addition, the administration of the above-mentioned experimental substance (l-NMMA) was started. Renal vascular resistance was calculated as the product of mean arterial pressure (MAP) and 1-hematocrit divided by RPF.
Throughout the infusion protocol, blood pressure (SBP, DBP, and MAP) and heart rate were monitored at fixed intervals (every 5 min) by means of an oscillometric device (Dinamap; Criticon, Norderstedt, Germany).
Measurement of urinary albumin and creatinine
All samples were centrally measured by the laboratory of the University of Erlangen-Nürnberg, according to established methods. In brief, urine albumin concentration was measured by a turbidimetric method. The interassay coefficient of variation was 3.44%. Creatinine concentration in urine was measured photometrically by the Jaffe method. The interassay coefficient of variation was 2.03%. The urine albumin-to-creatinine ratio (UACR) was calculated by dividing urinary albumin by urinary creatinine.
Statistical analysis
Normal distribution of data was confirmed by a Kolmogorov-Smirnow test before further analysis. Normally distributed data were compared by paired Student t tests and are expressed as means ± SD. Data of UACR were not normally distributed; therefore, medians and interquartile ranges are reported. UACR values were log transformed before statistical analysis. Where indicated, a multiple stepwise regression analysis with significance levels of 0.05 for entry and 0.10 for removal of a variable at each forward step was conducted. Two-tailed values of P < 0.05 were considered statistically significant. All analyses were performed using SPSS version 15.0 (SPSS, Chicago, IL).
RESULTS
The baseline clinical characteristics of the participants in population A (n = 62) are given in Table 1. This population comprised middle-aged patients with diabetes, hypertension, and partly elevated cholesterol levels, but all had normal renal function. Of 62 subjects, 25 had low-grade albuminuria (male subjects: >10 mg/g creatinine; female subjects >15 mg/g creatinine) and 6 had microalbuminuria (30–299 mg/g creatinine), but none had macroalbuminuria.
TABLE 1.
Baseline characteristics of the participants of study population A
| Characteristics | |
|---|---|
| Age (years) | 59.6 ± 8 |
| Sex (male/female) | 43/19 |
| Weight (kg) | 85.6 ± 16 |
| Height (cm) | 171 ± 10 |
| BMI (kg/m2) | 29.2 ± 4.9 |
| Casual SBP (mmHg) | 146 ± 15 |
| Casual DBP (mmHg) | 84 ± 11 |
| Blood glucose (mmol/L) | 8.55 ± 2.5 |
| Triglycerides (mmol/L) | 1.85 ± 1.2 |
| Total cholesterol (mmol/L) | 4.87 ± 0.7 |
| LDL cholesterol (mmol/L) | 3.21 ± 0.9 |
| HDL cholesterol (mmol/L) | 1.32 ± 0.4 |
| Serum creatinine (μmol/L) | 69.8 ± 8.8 |
| GFR (mL/s) | 2.37 ± 0.4 |
Data are means ± SD.
The baseline clinical characteristics of the subjects in study populations B (n = 22) are given in Table 2. In this group, patients had elevated LDL cholesterol levels, whereas blood pressure, fasting glucose, and other baseline parameters were in the normal range. All subjects had a normal kidney function. Only three patients had low-grade albuminuria, but none had micro- or macroalbuminuria.
TABLE 2.
Baseline characteristics of the participants of study population B
| Characteristics | |
|---|---|
| Age (years) | 52.7 ± 14 |
| Sex (male/female) | 15/7 |
| Weight (kg) | 80.5 ± 16 |
| Height (cm) | 174 ± 10 |
| BMI (kg/m2) | 26.6 ± 3.7 |
| Casual SBP (mmHg) | 128 ± 15 |
| Casual DBP (mmHg) | 77 ± 7 |
| Blood glucose (mmol/L) | 5.38 ± 0.6 |
| Triglycerides (mmol/L) | 1.98 ± 0.7 |
| Total cholesterol (mmol/L) | 7.20 ± 0.9 |
| LDL cholesterol (mmol/L) | 4.66 ± 0.6 |
| HDL cholesterol (mmol/L) | 1.55 ± 0.4 |
| Serum creatinine (μmol/L) | 77.8 ± 8.8 |
| GFR (mL/s) | 2.34 ± 0.3 |
Data are means ± SD.
Systemic hemodynamics in response to l-NMMA
Infusion of l-NMMA led to an increase in SBP (population A: from 142 ± 14 to 155 ± 17 mmHg; population B: from 127 ± 17 to 141 ± 20 mmHg; both P < 0.001) and DBP (population A: from 78 ± 10 to 85 ± 11 mmHg; population B: from 75 ± 9 to 81 ± 10 mmHg; both P < 0.001) and to a decrease in heart rate (population A: from 66 ± 10 to 62 ± 10 bpm; population B: from 58 ± 7 to 54 ± 7 bpm; both P < 0.001). MAP, which is considered to be a parameter of renal perfusion pressure, increased in population A (from 100 ± 10 to 108 ± 11 mmHg; P < 0.001) and in population B (from 94 ± 10 to 103 ± 13 mmHg; P < 0.001).
Change in UACR in response to l-NMMA
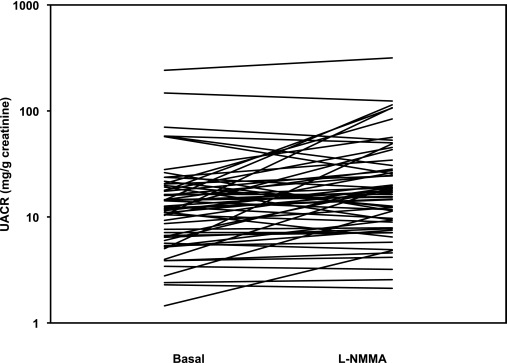
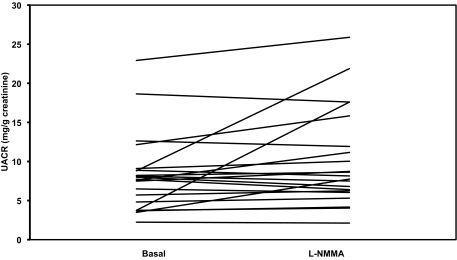
There was a significant increase in the UACR in response to the blockade of eNOS with l-NMMA in the hypertensive patients with type 2 diabetes (baseline: 12.3 mg/g creatinine [6.4–19.1] vs. l-NMMA: 16.9 mg/g creatinine [8.9–28.3]; P = 0.001) (Fig. 1) and in patients with hypercholesterolemia (baseline: 7.7 mg/g creatinine [4.0–8.9] vs. l-NMMA: 7.9 mg/g creatinine [6.1–14.7]; P = 0.044) (Fig. 2).
FIG. 1.
UACR before and after systemic infusion of the NO inhibitor l-NMMA in study population A on a log-scaled axis.
FIG. 2.
UACR before and after systemic infusion of the NO inhibitor l-NMMA in study population B.
Because increased blood pressure attributed to l-NMMA infusion also may led to an increased renal perfusion pressure and thereby to elevated albumin excretion, we performed additional analyses of our data. To assess the influence of MAP changes attributed to l-NMMA infusion as a potential confounding factor as well as altered renal hemodynamics, multiple linear regression analyses were performed. MAP change in response to l-NMMA infusion was not related to the increase in log-transformed UACR attributed to l-NMMA infusion in both study populations (population A: β = 0.235, P = 0.304, and population B: β = 0.024, P = 0.949). Similarly, changes of SBP and DBP also were not related to changes of log-transformed UACR after l-NMMA infusion (P > 0.20, data not shown). Furthermore, in both populations there was no relation between the change in RPF (population A: β = −0.006, P = 0.975, and population B: β = −0.278, P = 0.522), change in GFR (population A: β = −0.124, P = 0.698, and population B: β = −0.122, P = 0.606), change in filtration fraction (GFR/RPF) (population A: β = −0.165, P = 0.237, and population B: β = 0.054, P = 0.832), and change in renal vascular resistance (population A: β = 0.119, P = 0.772, and population B: β = 0.182, P = 0.363) and the increase in log-transformed UACR in response to l-NMMA infusion. Although not fully determined, metabolic factors such as hyperglycemia, A1C, and hyperlipidemia may influence endothelial permeability. However, neither fasting blood glucose and A1C, respectively, nor elevated LDL cholesterol were related with either baseline UACR or the change of log-transformed UACR in response to l-NMMA (P > 0.20, data not shown).
DISCUSSION
Almost two decades ago, Deckert et al. (25) proposed what usually has been cited as the Steno hypothesis, which states that microalbuminuria reflects generalized vascular damage. This hypothesis links impaired vascular endothelial function to vascular leakage of albumin that, in terms of the kidney, easily can be detected by measuring urinary albumin excretion. Microalbuminuria may simply reflect the specific renal manifestations of generalized vascular dysfunction. Indeed, albuminuria was associated with an increased systemic permeability of albumin in both type 1 diabetes (26) and type 2 diabetes in some (27) but not in all (28) studies as well as in clinically healthy subjects (29). However, the underlying mechanisms of this increased permeability remain to be fully elucidated. The importance of NO for intact vascular function and endothelial integrity has been thoroughly documented. Because decreased bioavailability of NO is a major contributor to endothelial function, we have investigated whether an acute reduction of basal NO activity provokes albuminuria.
By measuring albuminuria both before and after systemic infusion of l-NMMA, we could demonstrate that impaired renal endothelial function leads to a significant increase of albuminuria in response to NOS inhibition with l-NMMA. Our direct methodological approach proves, for the first time in humans, the previously proposed concept that impaired basal NO activity, and thus endothelial dysfunction, is a major pathogenic mechanism that increases the permeability of the vascular wall and hence the genesis of albuminuria. Using a before-and-after design without a control group may limit the assessment of the magnitude of the observed effect. Nevertheless, our data suggest that the change of UACR in response to inhibition of NO, resulting in a reduction of basal NO activity, may serve as a marker of endothelial function in the kidney, which can be easily measured.
It is noteworthy that these findings were evident in both hypertensive patients with type 2 diabetes and patients with hypercholesterolemia but without hypertension and type 2 diabetes. The change of MAP according to l-NMMA was not related to the change of UACR according to l-NMMA, even after adjustment for filtration fraction (GFR/RPF), ruling out an impact of changes of blood pressure and renal perfusion pressure on increased albumin excretion. We are aware that multiple regression analysis is an indirect statistical approach to assess potential influence of systemic and renal hemodynamic changes on the change of log-transformed UACR attributed to l-NMMA infusion, which, however, is one of the most accepted methods to account for potential confounding factors in vivo in humans.
Our findings are in line with animal models, genetic studies, and large studies with prospective character. It has been shown in a diabetic mouse model (leprdb/db) that genetic eradication of eNOS expression (eNOS−/−) resulted in an increase of albuminuria compared with control, leprdb/db, or eNOS−/− mice (21). Moreover, induction of diabetes in an inbred eNOS knockout animal model on the CF57/BL6 background, which is known to be resistant to nephropathy, has caused glomerular endothelial injury and resulted in overt albuminuria (30). Sharma et al. (31) have shown, using an in vitro approach and thereby ruling out the effects of altered hemodynamics or circulating cells of factors, that inhibition of NOS activity resulted in an increased glomerular capillary permeability to albumin. It has been suggested that inhibition of NOS could enhance O2−-mediated oxidative injury, which can be attenuated by exogenous NO and/or by O2− scavengers. These findings were supported by a similar approach, and it has been concluded that NOS-catalyzed NO production is an important mechanism in regulating glomerular permeability to albumin, which likely involves control of the bioavailability of superoxide (32). In accordance, Giner et al. (33) have reported elevated levels of markers of oxidative stress in hypertensive patients with microalbuminuria compared with those without microalbuminuria, and similar results were reported from type 2 diabetic patients (34). Genetic studies in humans have shown that eNOS polymorphisms that potentially impair eNOS gene transcription and activity are associated with an increased risk of diabetic nephropathy (22,23).
In a small Japanese study (35), ~200 diabetic patients with normo-, micro-, and macroalbuminuria were investigated. Percentage change of flow-mediated dilation of the brachial artery, to be in part related to endothelial function and endogenous NO production, was significantly decreased in micro- and macroalbuminuric compared with normoalbuminuric patients. Furthermore, flow-mediated vasodilation was significantly correlated with the degree of albuminuria. In the Hoorn Study (36), even after adjustment for age, sex, baseline arterial diameter, and other potential confounding factors, flow-mediated vasodilation was lower in the presence of microalbuminuria.
There are many large, prospective trials evaluating the impact of therapeutic approaches to reduce microalbuminuria in diverse populations. These trials are focused on cardiovascular events or on the progression of renal disease and not on an improvement of endothelial function. Interestingly, it was shown that the therapeutic approaches associated with lowering albuminuria (e.g., rev. in 37) also are known to improve endothelial function.
In the two study populations, we found that NOS inhibition, as assessed by measuring UACR both before and after the blockade of NOS with systemic infusion of l-NMMA provokes albuminuria that is unrelated to changes in the systemic circulation. Interestingly, this finding was evident in patient groups prone to endothelial dysfunction and albuminuria, and, therefore, findings cannot be extrapolated to the general population. Thus, additional studies are necessary to evaluate whether acute deterioration of endothelial function by reducing NO activity causes an increase of albuminuria also in the general population.
ACKNOWLEDGMENTS
The study was supported by German Research Foundation Grant SFB 423 (“Kidney Injury— Pathogenesis and Regenerative Mechanisms;” SFB 423 TP B5) to R.E.S. and M.P. Schneider.
No potential conflicts of interest relevant to this article were reported.
C.O. researched data and wrote the manuscript. M.P.Schneider researched data, contributed to the discussion, and reviewed/edited the manuscript. C.D. researched data, contributed to the discussion, and reviewed/edited the manuscript. M.P. Schlaich researched data, contributed to the data, and reviewed/edited the manuscript. R.E.S. reviewed/edited the manuscript.
Parts of this article were presented at the World Congress of Nephrology 2009, Milan, Italy, 22–26 May 2009, as well as at the 19th European Meeting on Hypertension of the European Society of Hypertension 2009, Milan, Italy, 12–16 June 2009.
The authors are grateful for the expert technical assistance of research nurses Ingrid Fleischmann, Dorothea Bader-Schmieder, Simone Pejkovic, Susanne Avendano, and Ulrike Heinritz and medical technical assistant Ortrun Alter (Department of Nephrology and Hypertension, University Hospital Erlangen, Erlangen, Germany).
Footnotes
Clinical trial reg no. NCT00136188, clinicaltrials.gov.
REFERENCES
- 1.Kannel WB, Stampfer MJ, Castelli WP, Verter J. The prognostic significance of proteinuria: the Framingham study. Am Heart J 1984;108:1347–1352 [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson O, Wilhelmsen L, Elmfeldt D, Pennert K, Wedel H, Wikstrand J, Berglund G. Predictors of cardiovascular morbidity in treated hypertension: results from the primary preventive trial in Göteborg, Sweden. J Hypertens 1985;3:167–176 [DOI] [PubMed] [Google Scholar]
- 3.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, de Jong PE, de Zeeuw D, Shahinfar S, Ruggenenti P, Remuzzi G, Levey AS, the AIPRD Study Group. Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001;60:1131–1140 [DOI] [PubMed] [Google Scholar]
- 4.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE, the Prevention of Renal and Vascular End Stage Disease (PREVEND) Study Group Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002;106:1777–1782 [DOI] [PubMed] [Google Scholar]
- 5.Schmitz A, Vaeth M. Microalbuminuria: a major risk factor in non-insulin-dependent diabetes. A 10-year follow-up study of 503 patients. Diabet Med 1988;5:126–134 [DOI] [PubMed] [Google Scholar]
- 6.Savage S, Nagel NJ, Estacio RO, Lukken N, Schrier RW. Clinical factors associated with urinary albumin excretion in type II diabetes. Am J Kidney Dis 1995;25:836–844 [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Mann JF, Pogue J, Dinneen SF, Hallé JP, Hoogwerf B, Joyce C, Rashkow A, Young J, Zinman B, Yusuf S. Prevalence and determinants of microalbuminuria in high-risk diabetic and nondiabetic patients in the Heart Outcomes Prevention Evaluation Study: the HOPE Study Investigators. Diabetes Care 2000;23(Suppl. 2):B35–B39 [PubMed] [Google Scholar]
- 8.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G, the Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators Preventing microalbuminuria in type 2 diabetes. N Engl J Med 2004;351:1941–1951 [DOI] [PubMed] [Google Scholar]
- 9.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, Van Gilst WH, De Zeeuw D, De Jong PE, the Prevend Study Group Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 2001;249:519–526 [DOI] [PubMed] [Google Scholar]
- 10.Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int 2002;61:2165–2175 [DOI] [PubMed] [Google Scholar]
- 11.Romundstad S, Holmen J, Kvenild K, Aakervik O, Hallan H. Clinical relevance of microalbuminuria screening in self-reported non-diabetic/non-hypertensive persons identified in a large health screening: the Nord-Trøndelag Health Study (HUNT), Norway. Clin Nephrol 2003;59:241–251 [DOI] [PubMed] [Google Scholar]
- 12.Atkins RC, Polkinghorne KR, Briganti EM, Shaw JE, Zimmet PZ, Chadban SJ. Prevalence of albuminuria in Australia: the AusDiab Kidney Study. Kidney Int Suppl 2004;66(Suppl. 92s):S22–S24 [DOI] [PubMed] [Google Scholar]
- 13.Smithies O. Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci USA 2003;100:4108–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballermann BJ, Stan RV. Resolved: capillary endothelium is a major contributor to the glomerular filtration barrier. J Am Soc Nephrol 2007;18:2432–2438 [DOI] [PubMed] [Google Scholar]
- 15.Comper WD, Haraldsson B, Deen WM. Resolved: normal glomeruli filter nephrotic levels of albumin. J Am Soc Nephrol 2008;19:427–432 [DOI] [PubMed] [Google Scholar]
- 16.Ballermann BJ. Contribution of the endothelium to the glomerular permselectivity barrier in health and disease. Nephron Physiol 2007;106:19–25 [DOI] [PubMed] [Google Scholar]
- 17.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 2001;280:F193–F206 [DOI] [PubMed] [Google Scholar]
- 18.Wolfrum S, Jensen KS, Liao JK. Endothelium-dependent effects of statins. Arterioscler Thromb Vasc Biol 2003;23:729–736 [DOI] [PubMed] [Google Scholar]
- 19.Stehouwer CD. Endothelial dysfunction in diabetic nephropathy: state of the art and potential significance for non-diabetic renal disease. Nephrol Dial Transplant 2004;19:778–781 [DOI] [PubMed] [Google Scholar]
- 20.Schäufele TG, Schlaich MP, Delles C, Klingbeil AU, Fleischmann EH, Schmieder RE. Impaired basal NO activity in patients with glomerular disease and the influence of oxidative stress. Kidney Int 2006;70:1177–1181 [DOI] [PubMed] [Google Scholar]
- 21.Mohan S, Reddick RL, Musi N, Horn DA, Yan B, Prihoda TJ, Natarajan M, Abboud-Werner SL. Diabetic eNOS knockout mice develop distinct macro- and microvascular complications. Lab Invest 2008;88:515–528 [DOI] [PubMed] [Google Scholar]
- 22.Zanchi A, Moczulski DK, Hanna LS, Wantman M, Warram JH, Krolewski AS. Risk of advanced diabetic nephropathy in type 1 diabetes is associated with endothelial nitric oxide synthase gene polymorphism. Kidney Int 2000;57:405–413 [DOI] [PubMed] [Google Scholar]
- 23.Shin Shin Y, Baek SH, Chang KY, Park CW, Yang CW, Jin DC, Kim YS, Chang YS, Bang BK. Relations between eNOS Glu298Asp polymorphism and progression of diabetic nephropathy. Diabetes Res Clin Pract 2004;65:257–265 [DOI] [PubMed] [Google Scholar]
- 24.Schmieder RE, Veelken R, Schobel H, Dominiak P, Mann JF, Luft FC. Glomerular hyperfiltration during sympathetic nervous system activation in early essential hypertension. J Am Soc Nephrol 1997;8:893–900 [DOI] [PubMed] [Google Scholar]
- 25.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage: the Steno hypothesis. Diabetologia 1989;32:219–226 [DOI] [PubMed] [Google Scholar]
- 26.Feldt-Rasmussen B. Increased transcapillary escape rate of albumin in type 1 (insulin-dependent) diabetic patients with microalbuminuria. Diabetologia 1986;29:282–286 [DOI] [PubMed] [Google Scholar]
- 27.Nannipieri M, Rizzo L, Rapuano A, Pilo A, Penno G, Navalesi R. Increased transcapillary escape rate of albumin in microalbuminuric type II diabetic patients. Diabetes Care 1995;18:1–9 [DOI] [PubMed] [Google Scholar]
- 28.Nosadini R, Velussi M, Brocco E, Abaterusso C, Piarulli F, Morgia G, Satta A, Faedda R, Abhyankar A, Luthman H, Tonolo G. Altered transcapillary escape of albumin and microalbuminuria reflects two different pathogenetic mechanisms. Diabetes 2005;54:228–233 [DOI] [PubMed] [Google Scholar]
- 29.Jensen JS, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Microalbuminuria reflects a generalized transvascular albumin leakiness in clinically healthy subjects. Clin Sci (Lond) 1995;88:629–633 [DOI] [PubMed] [Google Scholar]
- 30.Kanetsuna Y, Takahashi K, Nagata M, Gannon MA, Breyer MD, Harris RC, Takahashi T. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol 2007;170:1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma M, McCarthy ET, Savin VJ, Lianos EA. Nitric oxide preserves the glomerular protein permeability barrier by antagonizing superoxide. Kidney Int 2005;68:2735–2744 [DOI] [PubMed] [Google Scholar]
- 32.Datta PK, Sharma M, Duann P, Lianos EA. Effect of nitric oxide synthase inhibition on proteinuria in glomerular immune injury. Exp Biol Med (Maywood) 2006;231:576–584 [DOI] [PubMed] [Google Scholar]
- 33.Giner V, Tormos C, Chaves FJ, Sáez G, Redón J. Microalbuminuria and oxidative stress in essential hypertension. J Intern Med 2004;255:588–594 [DOI] [PubMed] [Google Scholar]
- 34.Ozdemir G, Ozden M, Maral H, Kuskay S, Cetinalp P, Tarkun I. Malondialdehyde, glutathione, glutathione peroxidase and homocysteine levels in type 2 diabetic patients with and without microalbuminuria. Ann Clin Biochem 2005;42:99–104 [DOI] [PubMed] [Google Scholar]
- 35.Makino H, Doi K, Hiuge A, Nagumo A, Okada S, Miyamoto Y, Suzuki M, Yoshimasa Y. Impaired flow-mediated vasodilatation and insulin resistance in type 2 diabetic patients with albuminuria. Diabetes Res Clin Pract 2008;79:177–182 [DOI] [PubMed] [Google Scholar]
- 36.Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction: the Hoorn Study. Kidney Int Suppl 2004;66(Suppl. 92s):S42–S44 [DOI] [PubMed] [Google Scholar]
- 37.Schmieder RE, Schrader J, Zidek W, Tebbe U, Paar WD, Bramlage P, Pittrow D, Böhm M. Low-grade albuminuria and cardiovascular risk: what is the evidence? Clin Res Cardiol 2007;96:247–257 [DOI] [PubMed] [Google Scholar]