Abstract
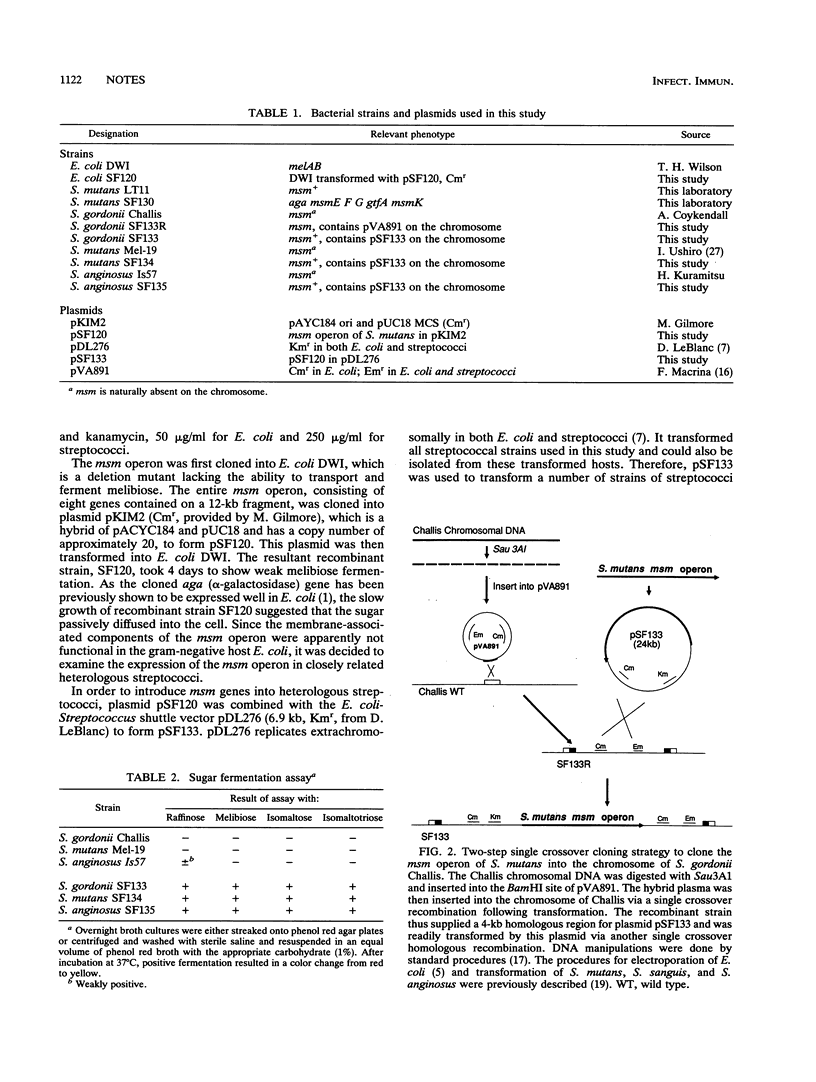
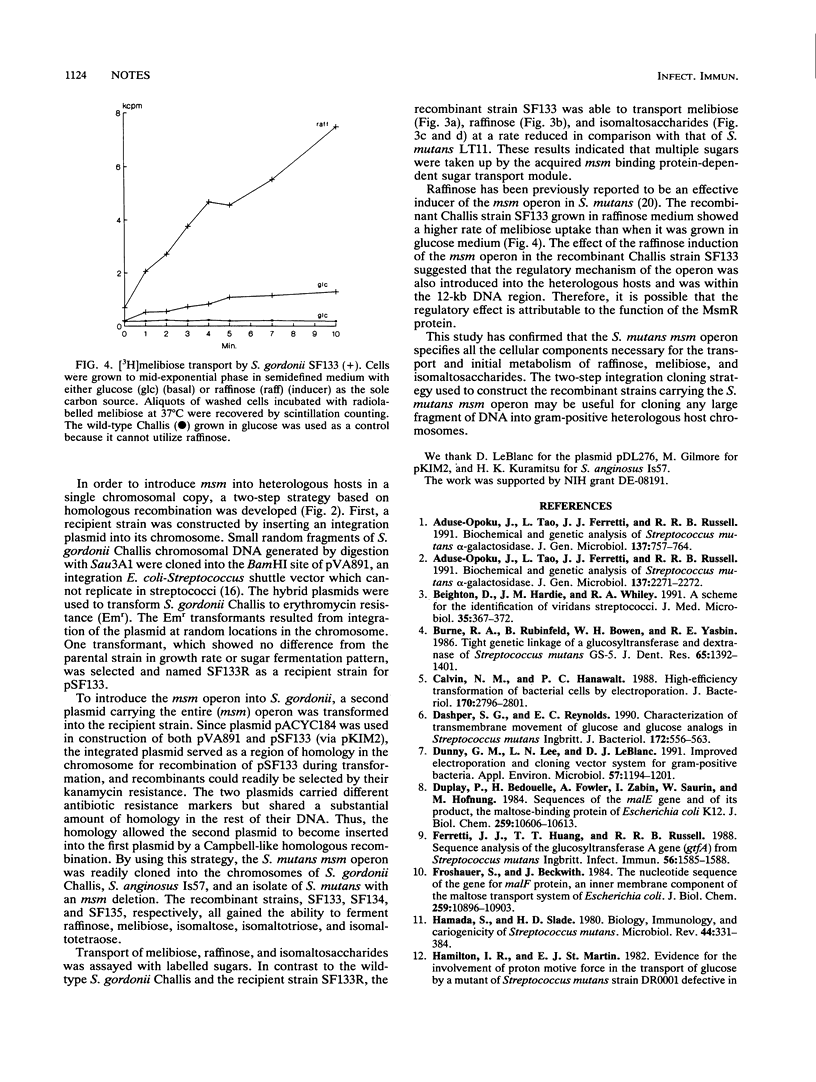
The multiple sugar metabolism (msm) operon of Streptococcus mutans is responsible for the uptake and metabolism of a variety of sugars. In order to further characterize the substrate specificities of the transport system, a 12-kb region of DNA containing the entire msm operon was cloned, via a novel two-step integration strategy, into the chromosomes of two heterologous streptococcal strains, Streptococcus gordonii Challis and Streptococcus anginosus Is57, as well as the chromosome of a natural isolate of S. mutans with a deletion of the msm region. These strains are unable to transport or ferment melibiose, raffinose, or isomaltosaccharides, but the newly constructed recombinants gained the ability to ferment all of these sugars. The S. gordonii Challis construct containing msm was shown to transport radiolabelled melibiose, raffinose, isomaltotriose, and isomaltotetraose, and the transport function was also subjected to induction by raffinose, an inducer of the msm operon in S. mutans. The results confirm the role of the msm operon in the transport and metabolism of melibiose, raffinose, and isomaltosaccharides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aduse-Opoku J., Tao L., Ferretti J. J., Russell R. R. Biochemical and genetic analysis of Streptococcus mutans alpha-galactosidase. J Gen Microbiol. 1991 Apr;137(4):757–764. doi: 10.1099/00221287-137-4-757. [DOI] [PubMed] [Google Scholar]
- Aduse-Opoku J., Tao L., Ferretti J. J., Russell R. R. Biochemical and genetic analysis of Streptococcus mutans alpha-galactosidase. J Gen Microbiol. 1991 Sep;137(9):2271–2272. doi: 10.1099/00221287-137-9-2271. [DOI] [PubMed] [Google Scholar]
- Beighton D., Hardie J. M., Whiley R. A. A scheme for the identification of viridans streptococci. J Med Microbiol. 1991 Dec;35(6):367–372. doi: 10.1099/00222615-35-6-367. [DOI] [PubMed] [Google Scholar]
- Burne R. A., Rubinfeld B., Bowen W. H., Yasbin R. E. Tight genetic linkage of a glucosyltransferase and dextranase of Streptococcus mutans GS-5. J Dent Res. 1986 Dec;65(12):1392–1401. doi: 10.1177/00220345860650120301. [DOI] [PubMed] [Google Scholar]
- Calvin N. M., Hanawalt P. C. High-efficiency transformation of bacterial cells by electroporation. J Bacteriol. 1988 Jun;170(6):2796–2801. doi: 10.1128/jb.170.6.2796-2801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashper S. G., Reynolds E. C. Characterization of transmembrane movement of glucose and glucose analogs in Streptococcus mutants Ingbritt. J Bacteriol. 1990 Feb;172(2):556–563. doi: 10.1128/jb.172.2.556-563.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Lee L. N., LeBlanc D. J. Improved electroporation and cloning vector system for gram-positive bacteria. Appl Environ Microbiol. 1991 Apr;57(4):1194–1201. doi: 10.1128/aem.57.4.1194-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplay P., Bedouelle H., Fowler A., Zabin I., Saurin W., Hofnung M. Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10606–10613. [PubMed] [Google Scholar]
- Ferretti J. J., Huang T. T., Russell R. R. Sequence analysis of the glucosyltransferase A gene (gtfA) from Streptococcus mutans Ingbritt. Infect Immun. 1988 Jun;56(6):1585–1588. doi: 10.1128/iai.56.6.1585-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froshauer S., Beckwith J. The nucleotide sequence of the gene for malF protein, an inner membrane component of the maltose transport system of Escherichia coli. Repeated DNA sequences are found in the malE-malF intercistronic region. J Biol Chem. 1984 Sep 10;259(17):10896–10903. [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Lodge J., Poy F. Carbohydrate uptake in the oral pathogen Streptococcus mutans: mechanisms and regulation by protein phosphorylation. Biochimie. 1989 Sep-Oct;71(9-10):997–1004. doi: 10.1016/0300-9084(89)90103-x. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Williamson M. I., Marsh P. D., Ellwood D. C. Evidence that glucose and sucrose uptake in oral streptococcal bacteria involves independent phosphotransferase and proton-motive force-mediated mechanisms. Arch Oral Biol. 1984;29(11):871–878. doi: 10.1016/0003-9969(84)90085-2. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- Martin B., Alloing G., Boucraut C., Claverys J. P. The difficulty of cloning Streptococcus pneumoniae mal and ami loci in Escherichia coli: toxicity of malX and amiA gene products. Gene. 1989 Aug 15;80(2):227–238. doi: 10.1016/0378-1119(89)90287-4. [DOI] [PubMed] [Google Scholar]
- Perry D., Kuramitsu H. K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981 Jun;32(3):1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Aduse-Opoku J., Sutcliffe I. C., Tao L., Ferretti J. J. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J Biol Chem. 1992 Mar 5;267(7):4631–4637. [PubMed] [Google Scholar]
- Russell R. R., Donald A. C., Douglas C. W. Fructosyltransferase activity of a glucan-binding protein from Streptococcus mutans. J Gen Microbiol. 1983 Oct;129(10):3243–3250. doi: 10.1099/00221287-129-10-3243. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Ferretti J. J. Nucleotide sequence of the dextran glucosidase (dexB) gene of Streptococcus mutans. J Gen Microbiol. 1990 May;136(5):803–810. doi: 10.1099/00221287-136-5-803. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Mukasa H., Shimamura A., Ferretti J. J. Streptococcus mutans gtfA gene specifies sucrose phosphorylase. Infect Immun. 1988 Oct;56(10):2763–2765. doi: 10.1128/iai.56.10.2763-2765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Effect of growth conditions on sucrose phosphotransferase activity of Streptococcus mutans. Infect Immun. 1980 Mar;27(3):922–927. doi: 10.1128/iai.27.3.922-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Sucrose transport by Streptococcus mutans. Evidence for multiple transport systems. Biochim Biophys Acta. 1982 Nov 22;692(3):415–424. doi: 10.1016/0005-2736(82)90392-3. [DOI] [PubMed] [Google Scholar]
- Ushiro I., Lumb S. M., Aduse-Opoku J., Ferretti J. J., Russell R. R. Chromosomal deletions in melibiose-negative isolates of Streptococcus mutans. J Dent Res. 1991 Nov;70(11):1422–1426. doi: 10.1177/00220345910700110501. [DOI] [PubMed] [Google Scholar]


