Abstract
Several filamentous oomycete species of the genus Halophytophthora have recently been described from marine environments, mostly from subtropical and tropical ecosystems. During a survey of oomycetes from leaf litter of Spartina alterniflora in salt marshes of southeastern Georgia, isolates of four taxa were recovered that bore similarity to some members of Halophytophthora but were highly divergent from isolates of Halophytophthora s.str. based on a combined sequence analysis of two nuclear loci. In phylogenetic analyses, these isolates were placed basal to a monophyletic group comprised of Pythium of the Pythiaceae and the Peronosporaceae. Sequence and morphology of these taxa diverged from the type species Halophytophthora vesicula, which was placed within the Peronosporaceae with maximum support. As a consequence a new family, the Salisapiliaceae, and a new genus, Salisapilia, are described to accommodate the newly discovered species, along with one species previously classified within Halophytophthora. Morphological features that separate these taxa from Halophytophthora are a smaller hyphal diameter, oospore production, lack of vesicle formation during sporulation, and a plug of hyaline material at the sporangial apex that is displaced during zoospore release. Our findings offer a first glance at the presumably much higher diversity of oomycetes in estuarine environments, of which ecological significance requires further exploration.
Keywords: internal transcribed spacer, nuclear ribosomal large subunit (nrLSU), Peronosporales, phylogeny, Pythiaceae
Introduction
The described species of marine oomycetes are diverse and include pathogens of algae, marine nematodes and crustaceans, as well as decomposers of leaf litter (Dick 2001, Sekimoto et al. 2007, Beakes & Sekimoto 2009). Species of the genera Pythium and Halophytophthora (commonly placed in the Pythiaceae) are among the few oomycetes reported from marine leaf litter from all over the world (Newell 1992, Nakagiri et al. 2001), with an assumed centre of diversity in the subtropics and tropics. The genus Halophytophthora was originally erected to accommodate pythiaceous taxa that were formerly referred to as Phytophthora but which all originated from marine leaf litter (Ho & Jong 1990), thus representing a heterogeneous genus defined by its ecological preference. Members of the genus exhibit zoospore release with or without the presence of a vesicle or with a semi persistent vesicle. These features, along with other asexual characters, were used to initially segregate the first nine species of the genus from Phytophthora and for delineation among them. Subsequently, additional members of this genus have also been described on the basis of morphological characters, with the most recent in 2003 (Ho et al. 2003). However, there is so far no comprehensive phylogeny for this genus, and although some conference abstracts report some phylogenetic investigation in this group (Nakagiri & Okane 2005, Nakagiri et al. 2008) only a single species of Halophytophthora s.str. has been included in multigene phylogenetic investigations in the Peronosporales (Göker et al. 2007). Given the range of zoospore release patterns exhibited, many of which are present in either Phytophthora or Pythium, it is reasonable to question whether the genus is indeed a monophyletic group, or whether the inclusion of all marine oomycetes from leaf litter in a single genus is synthetic and not reflecting evolutionary relationships. Phylogenetic analyses in several groups of oomycetes have revealed morphological characters suitable for the delineation of phylogenetic groups that had not been previously considered valuable for taxonomic studies, e.g. in Albuginales (white blister rusts, Voglmayr & Riethmüller 2006, Choi et al. 2007, 2008, Thines et al. 2009a, Ploch et al. 2010), Peronosporaceae (downy mildews and Phytophthora, Göker et al. 2003, Voglmayr et al. 2004, Thines et al. 2006, 2007, Voglmayr & Constantinescu 2008), and water moulds (Saprolegniales, Riethmüller et al. 1999, Hulvey et al. 2007, Sekimoto et al. 2009). It was thus the aim of this study to evaluate with molecular phylogenetic tools, whether the morphologically divergent isolates recently sampled from salt marshes in southeastern North America and the type species of Halophytophthora, form a monophyletic assemblage or are polyphyletic in origin.
MATERIALS AND METHODS
Isolates
Marsh grass (Spartina alterniflora) leaf litter was collected from three salt marsh sites on Sapelo Island and adjacent islands (Georgia, USA) during the summer of 2009. Leaf litter and leaf fragments from the mud surface were collected, rinsed in ambient brackish water, and plated onto dilute V8 seawater agar (40 ml V8 juice, 3 g CaCO3, 16 g Bacto agar and 960 ml seawater) amended with 25 ppm pimaricin, 100 ppm ampicillin, 25 ppm rifampicin, 25 ppm pentachloronitrobenzene (PARP). Mycelium was observed growing from leaf material into agar after 1–3 d and was aseptically transferred to water agar plates, resulting in diffuse colonies. Single hyphal tips were transferred to dilute V8 PARP agar Petri dishes and these cultures were utilised for genetic and morphological characterisation. The oomycete isolates used in this study are listed in Table 1 and 2.
Table 1.
Collection and strain details for the oomycete isolates investigated in this study.
| Taxa recovered | Collector | Location | GPS coordinates |
|---|---|---|---|
| Salisapilia sapeloensis (LT6440) | J. Hulvey | USA, GA, Sapelo Island, Cabretta Island | N31.43888, W81.23908 |
| Salisapilia nakagirii (LT6456) | J. Hulvey | USA, GA, Sapelo Island, Cabretta Island | N31.43888, W81.23908 |
| Salisapilia sp. (LT6466) | J. Hulvey | USA, GA, Sapelo Island, Cabretta Island | N31.43888, W81.23908 |
| Salisapilia sp. (LT6460) | J. Hulvey | USA, GA, Sapelo Island, Teal Boardwalk | N31.39509, W81.27936 |
| Salisapilia sp. (LT6471) | J. Hulvey | USA, GA, Sapelo Island, Teal Boardwalk | N31.39509, W81.27936 |
| Halophytophthora sp. 2 (LT6465) | J. Hulvey | USA, GA, Sapelo Island, Teal Boardwalk | N31.39509, W81.27936 |
| Halophytophthora sp. 1 (LT6430) | J. Hulvey | USA, GA, Saint Simon’s Island | N31.15288, W81.41602 |
Table 2.
Summary of some morphological features for species of Salisapilia and Halophytophthora s.str. – NA = not available.
| Species (strain number) | Culture collection no. | Plugged discharge tube | Zoospores discharged into a vesicle | Oogonial diam (μm) | Antheridial origin | Hyphal diam (μm) | GenBank accession no. |
|
|---|---|---|---|---|---|---|---|---|
| ITS | nrLSU | |||||||
| Salisapilia tartareaa | CBS 208.95 | Yes | No | 33–66 | diclinous | 1–3 | HQ232472 | HQ232464 |
| Salisapilia sapeloensis (LT6440) | CBS 127946 | Yes | No | 35–60 | paragynous | 1–3 | HQ232466 | HQ232457 |
| Salisapilia nakagirii (LT6456) | CBS 127947 | NA | NA | 33–48 | diclinous | 1–3 | HQ232467 | HQ232458 |
| Salisapilia sp. (LT6460) | CBS 127948 | NA | NA | NA | NA | 1–3 | HQ232468 | HQ232459 |
| Salisapilia sp. (LT6466) | NA | NA | NA | NA | NA | 1–3 | HQ232470 | HQ232461 |
| Salisapilia sp. (LT6471) | CBS 127949 | NA | NA | NA | NA | 1–3 | HQ232471 | HQ232462 |
| Halophytophthora vesicula | CBS 152.96 | No | Yes | NA | NA | 1–6 | HQ232473 | HQ232463 |
| Halophytophthora sp. 1 (LT6430) | NA | No | Yes | NA | NA | 1–5 | HQ232465 | HQ232456 |
| Halophytophthora sp. 2 (LT6465) | NA | No | Yes | NA | NA | 1–6 | HQ232469 | HQ232460 |
a syn. Halophytophthora tartarea.
Morphology
Colony morphology was documented from cultures growing on V8 PARP plates. For light microscopy of sporangia and gametangia, agar cubes were aseptically removed from the leading edge of agar colonies and incubated in 15 ml of half-strength seawater (13 ‰ salinity) or distilled water in Petri dishes for 5–10 d. Sporangia and oospores were photographed and measured using a Nikon Eclipse 80i microscope, and the Nikon NIS-Elements v2.2 digital imaging software. One hundred measurements were taken for all morphological features, from which mean values were calculated.
Gene amplification and sequencing
Genomic DNA was extracted from cultures of the isolates and two of culture collection specimens using methods described previously (Lamour & Finley 2006). Subsequent PCR amplification of the rDNA ITS region (comprising partial ITS1, 5.8S, and partial ITS2 sequences), and partial nrLSU, from the nuclear genome was done for phylogenetic analyses. Amplification of the ITS gene was done using the primers ITS4 and IT5 (White et al. 1990). Primers for amplification of the D1 and D2 regions of the rDNA large subunit were LR0R (Moncalvo et al. 1995) and LR6-O (Riethmüller et al. 2002). The PCR temperature regime is as follows for all loci amplified: an initial denaturation at 96 °C for 2 min was followed by 35 cycles consisting of denaturation at 96 °C for 1 min, annealing at 55 °C for 1 min, and extension at 72 °C for 2 min. A final extension step at 72 °C for 10 min was added for the completion of only partially amplified strands (Lee & Taylor 1992). All PCR reactions were done on a thermal cycler (Mastercycler, Eppendorf, Hamburg, Germany). The amplicons were sent to the University of Tennessee Knoxville Molecular Biology Resource Center for sequencing, with the primers used in PCR. Forward and reverse sequence electropherograms were manually trimmed of poor sequence data, and assembled using the CodonCode Aligner v3.0.3 sequence alignment software (CodonCode, Dedham, MA). The sequences obtained were submitted to GenBank (for accession numbers see Table 2).
Phylogenetic analyses
The set of sequences used in this analysis was combined from the dataset of Lévesque & de Cock (2004) and the sequences of the species Aphanomyces euteiches ATCC 201684 (AY683887.1, AF235939.1), Phytophthora infestans (genome, WGS-AATU-cont1-5907, WGS-AATU-cont1-5932) and P. sojae (genome, AAQY01000172-1, AAQY01000172) with the addition of LT6440, LT6456, LT6460, LT6465, LT6466, and LT6471, which were isolated from Sapelo Island and LT6430 isolated from Saint Simon’s Island (Table 1). Also included in the analysis were H. vesicula (CBS 152.96) and H. tartarea (CBS 208.95). Sequences were aligned using MAFFT (Katoh et al. 2005), v6 (Katoh & Toh 2008a) using the Q-INS-i option (Katoh & Toh 2008b). Afterwards sequences were concatenated for phylogenetic inference. To ensure reproducibility and to avoid subjective biases, no manual editing except for the removal of leading and trailing gaps was done. Minimum evolution trees were computed using MEGA v4.0 phylogenetic analysis software (Tamura et al. 2007), with factory settings, except for using the Tamura-Nei model of nucleotide substitution. For inferring the robustness of the ME analysis, 1 000 bootstrap replicates (Felsenstein 1985) were performed. Maximum Likelihood Analysis was done using RAxML (Stamatakis 2006), on the webserver (Stamatakis et al. 2008) at http://phylobench.vital-it.ch/raxml-bb/index.php with the gamma model of rate heterogeneity in effect and maximum likelihood search. Five runs with 100 bootstrap replicates using the rapid bootstrapping algorithm implemented on the RAxML webservers (Stamatakis et al. 2008) were combined and a consensus tree was computed using MEGA for assessing bootstrap support. Bayesian analysis was done with MrBayes (Huelsenbeck & Ronquist 2003) using a webserver (Parallel MrBayes @ BioHPC v3.1.2, http://cbsuapps.tc.cornell.edu/mrbayes.aspx) with MrBayes block form generated by GUMP at Auburn University (http://131.204.120.103/srsantos/mrbayes_form/index.html) with four incrementally heated chains, which were run for 6 million generations, with every 1 000th tree sampled. The first 33 % of these trees were discarded and the remaining trees were used for computing a majority rule consensus tree and for inferring posterior probabilities.
For the comparison of sequence divergence of partial nrLSU of LT6440 to selected members of other oomycete families, the blastn algorithm was used, with standard parameters except for settings in match/mismatch scores (4, -5) and gape costs (existence: 5, extension: 5). These parameters were altered for maximizing the query coverage to 100 % in the queries. The sequence divergence of LT6440 to the type species of selected genera are listed in Table 3.
Table 3.
Homology of Homology of Salisapilia sapeloensis nrLSU (HQ232457) compared to selected oomycetes.
| Family | Species (GenBank accession no.) | Maximum identity |
|---|---|---|
| Peronosporaceae | Phytophthora infestans FJ869987.1 | 76 % |
| Peronosporaceae | Bremia lactucae EF553478.1 | 74 % |
| Peronosporaceae | Halophytophthora vesicula HQ232463.1 | 76 % |
| Peronosporaceae | Pythopythium oedochilum AY598664.1 | 78 % |
| Phytiaceae | Pythium monospermum AY598621.1 | 76 % |
| Phytiaceae | Lagenidium chthamalophilum AF235946.1 | 77 % |
| Albuginaceae | Albugo candida AF235938.1 | 76 % |
| Rhipidiaceae | Sapromyces elongatus AF235950.1 | 74 %a |
| Saprolegniaceae | Saprolegnia ferax AF235953.1 | 77 % |
| Leptolegniaceae | Aphanomyces piscicida AF235941.1 | 77 % |
a Query coverage 99 %.
RESULTS
Morphological analyses
The isolate LT6440 as well as H. tartarea exhibited an absence of a vesicle during spore discharge, and the presence of a protruding plug of material at the discharge tube apex that is displaced during zoospore release (see also Nakagiri et al. 1994). The isolates LT6430 and LT6465 showed a sporangial morphology similar to the type of H. vesicula and were exhibiting a vesicle during spore discharge and did not form a plug at the discharge tube apex which is pushed outward during zoospore release. But both specimens could not unambiguously be assigned to a known species of Halophytophthora. Halophytophthora tartarea most closely resembles LT6440 with regards to size of vegetative hyphae (1–3 μm in both species), zoospore and sporangium morphology (Fig. 3b), as well as homothallic reproduction, but differs by antheridial origin. The isolate LT6440 was homothallic and formed oospores in oogonia with paragynous (sensu Nakagiri et al. 1994) antheridium (Fig. 3c). Also the isolate LT6456 formed oospores, in this case, however, the antheridium was diclinous (Fig. 3f), similar to the situation observed in H. tartarea. The isolates LT6460, LT6466, and LT6471 (Fig. 3e) did form neither oospores nor sporangia under culture conditions. Oospores exhibited a uniformly refractile ooplast vacuole, surrounded by cytoplasm with uniformly dispersed, small lipid droplets. The wall of the oospores was thin and smooth. Colony morphology for LT6430, LT6465, and H. vesicula was predominantly regular and downy, with extensive aerial mycelium (Fig. 2a–c), while it was irregular floccose or stellate in H. tartarea, LT6440, and LT6456 (Fig. 2d–f). Further morphological characteristics are outlined in the Taxonomy section and a summary of the main characteristics is given in Table 2.
Fig. 3.
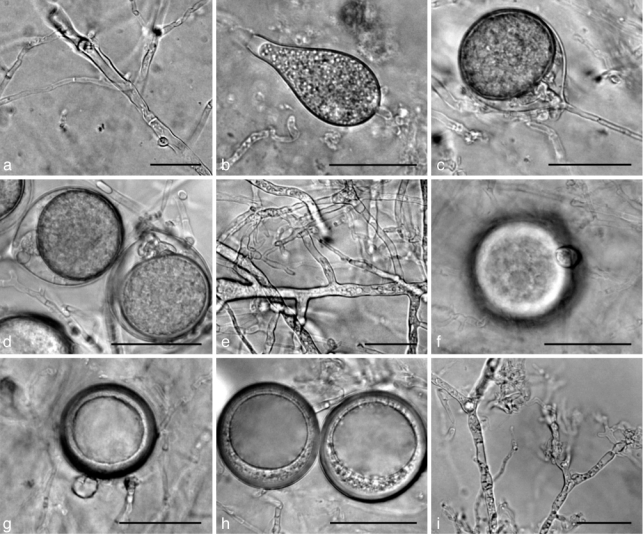
Micrographs of Salisapilia. a–d. Micrographs of Salisapilia sapeloensis LT6440. a. Branching hyphae with septae; b. ripe sporangium, note plug of material at tip of discharge tube; c. maturing oospore with simple paragynous antheridum; d. two fertilised oospores (on the left, a lobed paragynous antheridium is seen, and on the right, a branching paragynous antheridium is present). — e–h. Micrographs of Salisapilia nakagirii LT6456. e. Branching hyphae with septations; f. oogonium with antheridial cell attached; g. maturing oospore with diclinous antheridum; h. two fertilised oospores. — i. Micrographs of hyphae of Salisapilia sp. LT6471. — Scale bars: a, e, i = 10 μm; b–d, f–h = 40 μm.
Fig. 2.
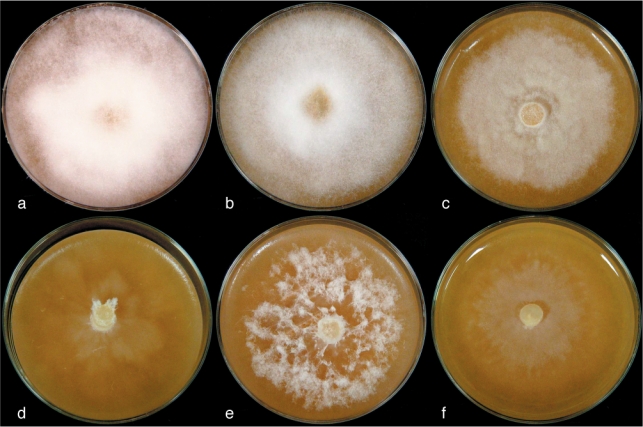
Photographs of colonies of: a–c. Halophytophthora s.str. and d–f. Salisapilia spp. isolates. a. LT6430; b. LT6465; c. H. vesicula CBS 152.96; d. S. tartarea CBS 208.95; e. S. sapeloensis LT6440; f. S. nakagirii LT6456.
Phylogenetic analyses
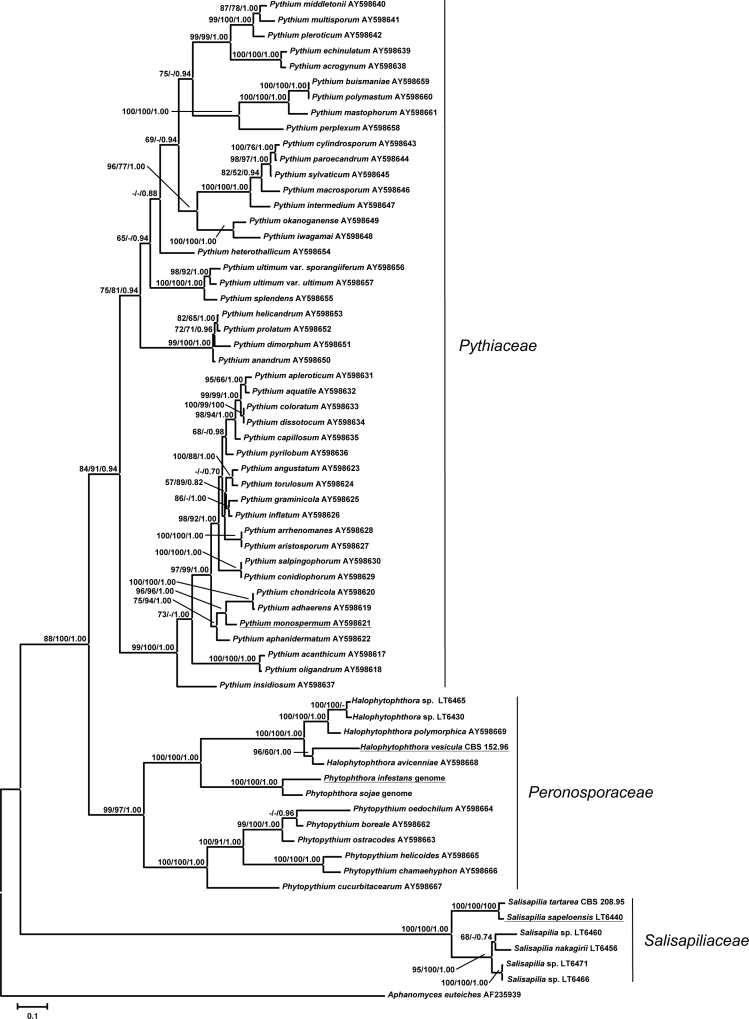
Several oomycete isolates from marsh grass litter (LT6440, LT6456, LT6460, LT6466, LT6471), and H. tartarea formed a monophyletic clade with maximum support values in all analyses (Fig. 1). This clade contained five phylogenetically distinct lineages and is sister to a monophyletic subtree that contains all members of the genus Pythium, as well as Phytophthora species, which was moderately (88 % bs) supported in Maximum Likelihood, but received maximum support in both Minimum Evolution and Bayesian analyses. Within this subtree, Pythium was placed sister to the Peronosporaceae, which contain Phytophthora and Phytopythium, but also Halophytophthora s.str. (the type of the genus, H. vesicula, as well as H. polymorphica, and H. avicenniae). The isolates LT6430 and LT6465 also cluster together with Halophytophthora s.str., which was a monophyletic assemblage that received maximum support in all analyses. The sequence divergence of the oomycetes from marsh grass litter was similar to other oomycete families (Table 3), and ranged from 74 to 78 % homology, highlighting the isolated position of this group.
Fig. 1.
Best tree from the Maximum Likelihood Analysis based on concatenated ITS and nrLSU sequences with bootstrap support values in Maximum Likelihood and Minimum Evolution analyses and Bayesian posterior probabilities in the respective order on the branches. Type species are underlined.
Taxonomy
The fact that the newly discovered phylogenetic lineage is the sister group to all other Peronosporales (Peronosporaceae s.l. and Pythiaceae) included in this study requires the recognition of the new family Salisapiliaceae, as the Pythiaceae would become paraphyletic through the inclusion of the phylogenetic lineage revealed here. This necessitates both the description of a new genus and a new family for accommodating the species of the new phylogenetic lineage.
Salisapiliaceae Hulvey, Nigrelli, Telle, Lamour & Thines, fam. nov. — MycoBank MB517464
Straminipila, Oomycota, Peronosporales. Mycelium saepe ramosum, hyphae regulariis 1–3 μm in diametro, nonnumquam inflatae et septatae. Zoosporangia hyalina, obpyriformia, ovata vel obovata, cum materia lentiformi in aqua marina semisalina vel tota salina, materia lentiformis absens in aqua destillata, tubus emittens perspicuus, cum materia hyalina lentiformi eminente ex apice qui inter egressionem zoosporarum absolutus est. Vesicula emittens perpetua non adest.
Type. Salisapilia Hulvey, Nigrelli, Telle, Lamour & Thines, gen. nov.
Straminipila, Oomycota, Peronosporales. Mycelium frequently branched, regular vegetative hyphae 1–3 μm diam, with occasional septations and hyphal swellings. Zoosporangia hyaline, obpyriform, ovoid to obovate, with plugs in half strength or full strength seawater, absent in distilled water, discharge tube conspicuous, with hyaline plug protruding from apex, which is displaced during zoospore discharge. No persistent discharge vesicle present.
Salisapilia Hulvey, Nigrelli, Telle, Lamour & Thines, gen. nov. — MycoBank MB517465; Fig. 2
Coloniae in agaro V8 stellatae vel non-stellatae vel petalatae, nonnumquam cum hyphis aeriis. Hyphae regulariis 1–3 μm in diametro, glabrae vel irregulares, interdum septatae, saepe ramosae. Hyphae nonnumquam cum tumoribus. Zoosporangia hyalina abundantia in fragmentis agari V8 in aqua marina semisalina vel tota salina, in aqua destillata absunt. Zoosporangia obpyriformia, ovata vel obovata. Tubus emittens perspicuus, 33–97 μm in longitudine, cum materia hyalina lentiformi eminente ex apice. Materia hyalina lentiformis 1–9 μm. Liberatio zoosporarum detractione materiae lentiformis et exitu zoosporarum mobilium per foramen emittens. Species oosporas facentes homothallicae sunt. Oosporae 33–66 μm in diametro, globosae vel ovatae, crescentes terminales vel intercalares inter hyphas. Antheridia paragyna vel diclina. Cellula antheridii glabra et clavata in speciebus diclinis vel simplex, lobula vel ramosa in speciebus paragynis.
Type. Salisapilia sapeloensis Hulvey, Nigrelli, Telle, Lamour & Thines, sp. nov.
Etymology. From Latin sal = salt and -sapilis = of muck or detritus.
Colonies on V8 agar stellate, or non-stellate, or petalate with occasional aerial hyphae (Fig. 2d–h). Regular vegetative hyphae 1–3 μm diam, smooth to irregular, with occasional septations, frequently branching. Hyphal swellings occasional. Abundant hyaline zoosporangia produced on V8 agar plugs in half strength or full strength seawater, absent in distilled water. Zoosporangia obpyriform, ovoid, to obovate. Discharge tube conspicuous, 33–97 μm in length, with hyaline plug protruding from apex. Hyaline plug 1–9 μm. Zoospore release occurs first by displacement of the plug, followed by exit of motile zoospores from the discharge pore. Species with known sexual reproduction homothallic. Oospores 33–66 μm diam, spherical to ovoid, arising terminal or intercalary along hyphae. Antheridia paragynous or diclinous. Antheridial cell smooth and club-like in diclinous species, or simple, lobed or branching in paragynous species.
Salisapilia nakagirii Hulvey, Nigrelli, Telle, Lamour & Thines, sp. nov. — MycoBank MB517466; Fig. 2f, 3e–h
Coloniae in agaro V8 stellatae. Hyphae glabrae vel irregulares, ramosae nonnumquamque septatae. Zoosporangia in aqua marina semisalina vel tota salina vel destillata absentia. Oogonia hyalina, globosa, 33–48 μm (medio 39 μm), pariete glabro, 2–7 μm in crassitudine. Antheridia diclina. Cellula antheridii forma clavae, 3–10 μm in longitudine. Oosporae hyalinae, 28–44 μm in diametro (medio 36 μm), pariete glabro 1–7 μm in crassitudine.
Etymology. Dedicated to Dr. Akira Nakagiri, who characterised several marine filamentous oomycetes.
Colony on V8 agar stellate (Fig. 2f). Hyphae smooth to irregular, branching and occasionally septate (Fig. 3e). Zoosporangia absent in half or full strength seawater, or distilled water. Oogonia hyaline, spherical, 33–48 μm (mean = 39 μm), with a smooth wall, 2–7 μm thick. Antheridia diclinous. Antheridial cell club shaped, 3–10 μm in length (Fig. 3f, g). Oospores hyaline, with a uniformly refractile ooplast vacuole, surrounded by cytoplasm with uniformly dispersed small lipid droplets, 28–44 μm diam (mean = 36 μm), with a smooth wall 1–7 μm thick (Fig. 3h).
Substratum — Decaying litter of Spartina alterniflora.
Known distribution — Southeastern North America.
Specimens examined. USA, Georgia, Sapelo Island, isolated from leaf litter of Spartina alterniflora at Sapelo Island, July 2009, Jon Hulvey, holotypus CBS H-20478, culture ex-type CBS 127947 = LT6456.
Salisapilia sapeloensis Hulvey, Nigrelli, Telle, Lamour & Thines, sp. nov. — MycoBank MB517467; Fig. 2e, 3a–d
Coloniae in agaro V8 irregulares, plerumque hyphis coloniarum submersis in agaro, nonnumquam cum hyphis aeriis. Hyphae glabrae vel irregulares, ramosae et nonnumquam septatae. Zoosporangia abundantia in aqua marina semisalina vel tota salina. Sporangiophori ramosi vel non ramosi, 1–2 μm in latitudine. Zoosporangia hyalina, ovata vel obpyriformia et papillata. 34–97 μm in longitudine (medio 59 μm) sine tubo emittente. Zoosporangia non aucta. Materia lentiformis sporangii in sporangiis maturis 3–8 μm. Tubus emittens oblongus, 6–18 μm in longitudine, pauxillule ab basi ad apicem angustior. In maturitate sporangiorum materia lentiformis secedit ab pariete sporangii et elongat, ergo eminens ex tubo emittente. Zoosporis emissis materia lentiformis liberatur et crebro comprimit et extendit in longitudine. Zoosporae ovatae vel reniformes, latere biflagellatae, 5–6 μm in diametro, 12–20 zoosporae in sporangio singulari (medio 15). Zoosporae digressione flagellarum stadium quietis intrant. Zoosporae in stadio quietis 5–7 μm in diametro. Oogonia hyalina, globosa vel ovata, 35–60 μm (medio 49 μm). Oosporae hyalinae, 28–56 μm (medio 48 μm), pariete glabro, 2–9 μm in crassitudine. Antheridia paragyna. Cellula antheridii simplex, lobula vel ramosa, pariete glabro, 2–9 μm in longitudine.
Etymology. Sapeloensis = of Sapelo Island, the location where the species was first isolated from.
Colony on V8 agar irregular, with colony hyphae mostly submerged in agar, with aerial hyphae (Fig. 2e). Hyphae smooth to irregular, branching and occasionally septate (Fig. 3a). Zoosporangia abundant in half or full strength seawater (Fig. 3a). Sporangiophores branched or unbranched, 1–2 μm wide. Zoosporangia hyaline, ovoid to obpyriform, and papillate. 34– 97 μm in length (mean = 59 μm), excluding discharge tube. Zoosporangia non-proliferating. Sporangial plug 3–8 μm in mature sporangia (Fig. 3b). Discharge tube elongate, 6–18 μm in length, slightly tapering from base to tip (Fig. 3b). During ripening of sporangia, the plug appears to become separate from the sporangial wall and elongates so that it is partially protruding form the discharge tube (Fig. 3b). At zoospore discharge, the plug is released, at which time it decompresses and expands several times its initial length. Zoospores ovoid to reniform, laterally biflagellate, 5–6 μm diam, each sporangium containing 12–20 zoospores (mean = 15). Zoospores encyst by withdrawal of flagella. Encysted zoospores 5–7 μm diam. Oogonia hyaline, spherical to ovoid, 35–60 μm (mean = 49). Oospores hyaline, with a uniformly refractile ooplast vacuole, surrounded by cytoplasm with uniformly dispersed small lipid droplets, 28–56 μm (mean = 48 μm), with a smooth wall, 2–9 μm thick. Antheridia paragynous. Antheridial cell may be simple, lobed or branched (Fig. 3c, d) with a smooth wall, 2–9 μm in length.
Substratum — Decaying litter of Spartina alterniflora.
Known distribution — Southeastern North America.
Specimens examined. USA, Georgia, Sapelo Island, isolated from leaf litter of Spartina alterniflora at Sapelo Island, July 2009, Jon Hulvey, holotype CBS H-20477, culture ex-type CBS 127946 = LT6440.
Salisapilia tartarea (Nakagiri & S.Y. Newell) Hulvey, Nigrelli, Telle, Lamour & Thines, comb. nov. — MycoBank MB517468
Basionym. Halophytophthora tartarea Nakagiri & S.Y. Newell, Mycoscience 35: 224.
DISCUSSION
Originally, all species of Halophytophthora were united by their lack of production of oospores, until the description of H. tartarea from leaf litter from Florida (Ho & Jong 1990, Nakagiri et al. 1994). Here we show that H. tartarea is highly divergent from Halophytophthora s.str. (the monophyletic group which includes the type species), and belongs to the newly described genus Salisapilia. Species of Salisapilia are united by the absence of a vesicle during spore discharge, and the presence of a protruding plug of material at the discharge tube apex that is displaced during zoospore release. Salisapilia tartarea is closely related to S. sapeloensis and is morphologically similar to this species with regards to size of hyphae, zoospores, sporangia, as well as homothallic reproduction, but differs markedly from it by antheridial origin, which is mostly diclinous in S. tartarea and S. nakagirii, but paragynous (sensu Nakagiri et al. 1994) in S. sapeloensis. The exact mode of oospore production in Salisapilia will require future detailed studies, as the antheridial origin might be variable (Nakagiri et al. 1994). Several other species of Halophytophthora, H. bahamensis, H. epistomium, H. exoprolifera, and H. operculata exhibit zoospore release without the presence of a vesicle (Nakagiri 2000). These species also possess a plug of material at the discharge pore apex which is displaced during zoospore release, which is considered typical for Salisapilia. None of these species seems to be conspecific with either S. nakagirii or S. sapeloensis, based on morphological and biological characteristics. However, in the absence of phylogenetic data, we refrain from transferring these species to Salisapilia, because of their partly deviating morphology, and because it cannot be ruled out at present that the operculate sporangia represent an ancestral trait of the Peronosporales s.l.
Subtleties in the zoospore release, including the presence of a persistent vs a semi persistent vesicle during zoospore release (H. masteri), or the presence of an operculum that is displaced during zoospore release (H. operculata) are features that may be phylogenetically informative, and future investigations will reveal if species sharing these features may subsequently deserve separate generic status from Halophytophthora or Salisapilia (Ho et al. 1990, 1991, 1992, Pegg & Alcorn 1982, Nakagiri et al. 1994). This seems possible, since subtleties in zoospore release have been revealed to be phylogenetically informative for other oomycete genera, such as the genera Saprolegnia, Protoachyla, and Pythiopsis of the Saprolegniales (Riethmüller et al. 1999). For H. spinosa, a phylogenetic position outside Halophytophthora s.str. has been reported in conference abstracts (Nakagiri & Okane 2005, Nakagiri et al. 2008), but it is unclear if this species belongs to the Salisapiliaceae, because of its divergent oospore morphology.
Oospores of Salisapilia exhibit a uniformly refractile, centric to subcentric ooplast vacuole, surrounded by cytoplasm with uniformly dispersed small lipid droplets. This is similar to other oomycetes in the peronosporalean lineage. The rather thin, smooth, and uniform oospore wall is similar to many species of Pythium and Phytopythium, but is much different from the more complex, multilayered resting spore walls reported from members of the Rhipidiales and the Albuginales. Also mycelium growth and sporangium formation provide further evidence for the inclusion of the Salisapiliaceae within the Peronosporales, rather than the description of a new order, which might be justified on the basis of the basal phylogenetic position, but seems superfluous at the moment.
It has been suggested that oomycetes originated from marine environments and migrated to land with host organisms early in the evolution of eukaryotes (Beakes & Sekimoto 2009). However, as no pathogenic growth could be associated with Salisapilia, it could well be possible, that several marine lineages were either non pathogenic or have reverted to a saprophytic lifestyle. Whether the plant pathogenic Peronosporaceae arose from a saprophytic ancestor, which gradually evolved to becoming pathogens in an intertidal environment, which is one plausible evolutionary scenario revealed by this study, or not, has to be revealed by future studies encompassing a broader sampling of oomycetes from marshes and mangroves. It is not finally resolved, whether several lineages of the Peronosporales independently managed the transition from marine to terrestrial and limnic environments, or whether multiple reversal events to a marine lifestyle have taken place. Considering the results from Thines et al. (2009b), who found that the Rhipidiales and Albuginales were the most basal groups of the Peronosporomycetes, a reversal to adaptation to the marine environment is the more parsimonious explanation over the theory that Halophytophthora, and possibly also Salisapilia, represent phylogenetic lineages that are originally marine and have not made the transition to a terrestrial or limnic environment (Nakagiri 2000). The fact that Halophytophthora is the sister group of Phytophthora points at the possibility that Phytophthora might have directly arisen from a marine Halophytophthora-like ancestor. However, as the genus Phytopythium is the sister group to Halophytophthora and Phytophthora, and is represented by terrestrial plant pathogens, it is equally parsimonious to assume that Halophytophthora species have colonised marsh and mangrove habitats from terrestrial or limnic environments.
The Salisapiliaceae appear to represent an ancient lineage of the Peronosporales, which is sister to a monophyletic group containing both the Pythiaceae, represented by Pythium, and the Peronosporaceae, represented by the genera Phytophthora, Phytopythium, and Halophytophthora. This phylogenetic placement not only favours the broad circumscription of the Peronosporaceae to include the closely related groups of Phytophthora and the downy mildews (Göker et al. 2007, Thines 2009) as supposed in Thines et al. (2009b), but also the inclusion of the genera Halophytophthora s.str. and Phytopythium, which are morphologically similar to Phytophthora, for avoiding an inflationary introduction of family names within the Peronosporales. All species of Pythium included in the analysis were placed in a monophyletic group sister to the Peronosporaceae, thus demonstrating that the family status of the Pythiaceae is well-deserved. Whether additional genera now classified within the Pythiaceae will associate with this group or occupy distinct phylogenetic positions will have to be clarified by future studies. The current study represents a first step towards a taxonomy of the Peronosporomycetes mirroring the evolutionary relationships of these organisms. However, phylogenetic data for several rarely sampled pythiaceous genera is lacking, and it seems likely that additional taxonomic revision will be necessary in the Peronosporales.
Acknowledgments
LN, MT and ST have been supported by the research funding programme ‘LOEWE – Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz’ of Hesse’s Ministry of Higher Education, Research, and the Arts. Mark Windham (University of Tennessee) is gratefully acknowledged for allowing access to microscopic facilities. JH acknowledges the Genome Science and Technology Graduate Program (University of Tennessee) for financial support. Thanks are due to affiliates of the University of Georgia Marine Institute (UGAMI) of Sapelo Island, GA, including Research Scientist Dr. Melissa Booth, for use of facilities, and Christoph Rost, University of Hohenheim, for help with Latin descriptions. This work is dedicated to Professor Emeritus Dr. David Porter of the University of Georgia and retired Senior Research Scientist Dr. Steve Newell from UGAMI for supporting JHs interest in the biodiversity of marine zoosporic fungi during his undergraduate studies.
Author contributions – JH, KL, MT study design; JH strain isolation, microscopy, and morphological analyses; JH, ST initial sequence analysis; ST phylogenetic analyses; JH, LN, MT taxonomic analyses; JH, KL, LN , MT, ST manuscript preparation.
REFERENCES
- Beakes GW, Sekimoto S.2009. The evolutionary phylogeny of oomycetes-insights gained from studies of holocarpic parasites of algae and invertebrates. In: Lamour K, Kamoun S. (eds), Oomycete genetics and genomics: 165–177. Wiley-Blackwell, New Jersey, USA: [Google Scholar]
- Choi Y-J, Shin H-D, Hong S-B, Thines M.2007. Morphological and molecular discrimination among Albugo candida materials infecting Capsella bursa-pastoris worldwide. Fungal Diversity 27: 11 – 34 [Google Scholar]
- Choi Y-J, Shin H-D, Ploch S, Thines M.2008. Evidence for uncharted biodiversity in the Albugo candida complex, with description of a new species. Mycological Research 112: 1327 – 1334 [DOI] [PubMed] [Google Scholar]
- Dick MW.2001. Straminipilous fungi. Kluwer Academic Publishers, Dordrecht, Netherlands: [Google Scholar]
- Felsenstein J.1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783 – 791 [DOI] [PubMed] [Google Scholar]
- Göker M, Voglmayr H, Riethmüller A, Oberwinkler F.2007. How do obligate parasites evolve? A multi-gene phylogenetic analysis of downy mildews. Fungal Genetics and Biology 44: 105 – 122 [DOI] [PubMed] [Google Scholar]
- Göker M, Voglmayr H, Riethmüller A, Weiß M, Oberwinkler F.2003. Taxonomic aspects of Peronosporaceae inferred from Bayesian molecular phylogenetics. Canadian Journal of Botany 81: 672 – 683 [Google Scholar]
- Ho HH, Chang HS, Hsieh SY.1991. Halophytophthora kandeliae: a new marine fungus from Taiwan. Mycologia 83: 419 – 424 [Google Scholar]
- Ho HH, Chang HS, Huang SH.2003. Halophytophthora elongata: a new marine species from Taiwan. Mycotaxon 85: 417 – 422 [Google Scholar]
- Ho HH, Hsieh SY, Chang HS.1990. Halophytophthora epistomium from mangrove habitats in Taiwan. Mycologia 82: 659 – 662 [Google Scholar]
- Ho HH, Jong SC.1990. Halophytophthora gen. nov., a new member of the family Pythiaceae. Mycotaxon 36: 377 – 382 [Google Scholar]
- Ho HH, Nakagiri A, Newell SY.1992. A new species of Halophytophthora from Atlantic and Pacific subtropical islands. Mycologia 84: 548 – 554 [Google Scholar]
- Huelsenbeck JP, Ronquist F.2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572 – 1574 [DOI] [PubMed] [Google Scholar]
- Hulvey JP, Padgett DE, Bailey JC.2007. Species boundaries within Saprolegnia (Saprolegniales, Oomycota) based on morphological and DNA sequence data. Mycologia 99: 421 – 429 [DOI] [PubMed] [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T.2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33: 511 – 518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Toh H.2008a. Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics 9: 286 – 298 [DOI] [PubMed] [Google Scholar]
- Katoh K, Toh H.2008b. Improved accuracy of multiple ncRNA alignment by incorporating structural information into a MAFFT-based framework. BMC Bioinformatics 9: 212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour KH, Finley SL.2006. A strategy for recovering high quality genomic DNA from a large number of Phytophthora isolates. Mycologia 98: 514 – 517 [DOI] [PubMed] [Google Scholar]
- Lee SB, Taylor JW.1992. Phylogeny of five fungus-like protoctistan Phytophthora species, inferred from the internal transcribed spacers of ribosomal DNA. Molecular Biology and Evolution 94: 636 – 653 [DOI] [PubMed] [Google Scholar]
- Lévesque CA, Cock WAM de.2004. Molecular phylogeny and taxonomy of genus Pythium. Mycological Research 108: 1363 – 1383 [DOI] [PubMed] [Google Scholar]
- Moncalvo J, Wang H, Hseu R.1995. Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacer and 25S ribosomal DNA sequences. Mycologia 87: 223 – 238 [Google Scholar]
- Nakagiri A.2000. Ecology and biodiversity of Halophytophthora species. Fungal Diversity 5: 153 – 164 [Google Scholar]
- Nakagiri A, Inaba S, Toyama K, Hsieh S-Y.2008. Diversity of marine oomycetes, Halophytophthora. Abstracts of papers presented at the meeting of the Mycological Society of Japan 52: 11 [Google Scholar]
- Nakagiri A, Newell SY, Ito T.1994. Two new Halophytophthora species, H. tartarea and H. masteri, from intertidal decomposing leaves in saltmarsh and mangrove regions. Mycoscience 35: 223 – 232 [Google Scholar]
- Nakagiri A, Okane I.2005. Phylogeny, taxonomy and ecology of Halophytophthora spinosa (marine Oomycetes). Proceedings of the annual meeting of the Mycological Society of Japan, abstracts of submitted papers 49: 169 [Google Scholar]
- Nakagiri A, Tadayoshi I, Manoch L, Tanticharoen M.2001. A new Halophytophthora species, H. porrigovesica. Mycoscience 42: 33 – 41 [Google Scholar]
- Newell SY.1992. Autumn distribution of marine Pythiaceae across a mangrove-saltmarsh boundary. Canadian Journal of Botany 70: 1912 – 1916 [Google Scholar]
- Pegg KG, Alcorn JL.1982. Phytophthora operculata sp. nov., a new marine fungus. Mycotaxon 16: 99 – 102 [Google Scholar]
- Ploch S, Choi Y-J, Rost C, Shin H-D, Schilling E, Thines M.2010. Evolution of diversity in Albugo is driven by high host specificity and multiple speciation events on closely related Brassicaceae. Molecular Biology and Evolution 57: 812 – 820 [DOI] [PubMed] [Google Scholar]
- Riethmüller A, Voglmayr H, Göker M, Weiß M, Oberwinkler F.2002. Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia 94: 834 – 849 [DOI] [PubMed] [Google Scholar]
- Riethmüller A, Weiß M, Oberwinkler F.1999. Phylogenetic studies of Saprolegniomycetidae and related groups based on nuclear large subunit ribosomal DNA sequences. Canadian Journal of Botany 77: 1790 – 1800 [Google Scholar]
- Sekimoto S, Hatai K, Honda D.2007. Molecular phylogeny of an unidentified Haliphthoros-like marine oomycete and Haliphthoros milfordensis inferred from nuclear-encoded small- and large-subunit rRNA genes and mitochondrial-encoded cox2 gene. Mycoscience 48: 212 – 221 [Google Scholar]
- Sekimoto S, Klochkova TA, West JA, Beakes GW, Honda D.2009. Olpidiopsis bostrychiae sp. nov.: an endoparasitic oomycete that infects Bostrychia and other red algae (Rhodophyta). Phycologia 48: 460 – 472 [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J.2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758 – 771 [DOI] [PubMed] [Google Scholar]
- Stamatakis S.2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688 – 2690 [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S.2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596 – 1599 [DOI] [PubMed] [Google Scholar]
- Thines M.2009. Bridging the gulf: Phytophthora and downy mildews are connected by rare grass parasites. PLoS ONE 4: e4790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines M, Choi Y-J, Kemen E, Ploch S, Holub EB, Shin H-D, Jones JDG.2009a. A new species of Albugo parasitic to Arabidopsis thaliana reveals new evolutionary patterns in white blister rusts (Albuginaceae). Persoonia 22: 123 – 128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines M, Göker M, Oberwinkler F, Spring O.2007. A revision of Plasmopara penniseti, with implications for the host range of the downy mildews with pyriform haustoria. Mycological Research 111: 1377 – 1385 [DOI] [PubMed] [Google Scholar]
- Thines M, Göker M, Spring O, Oberwinkler F.2006. A revision of Bremia graminicola. Mycological Research 110: 646 – 656 [DOI] [PubMed] [Google Scholar]
- Thines M, Voglmayer H, Göker M.2009b. Taxonomy and phylogeny of the downy mildews (Peronosporales). In: Lamour K, Kamoun S. (eds), Oomycete genetics and genomics: 165–177. Wiley-Blackwell, New Jersey, USA: [Google Scholar]
- Voglmayr H, Constantinescu O.2008. Revision and reclassification of three Plasmopara species based on morphological and molecular phylogenetic data. Mycological Research 112: 487 – 501 [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Riethmüller A.2006. Phylogenetic relationships of Albugo species (white blister rusts) based on LSU rDNA sequence and oospore data. Mycological Research 110: 75 – 85 [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Riethmüller A, Göker M, Weiss M, Oberwinkler F.2004. Phylogenetic relationships of Plasmopara, Bremia and other genera of downy mildew pathogens with pyriform haustoria based on Bayesian analysis of partial LSU rDNA sequence data. Mycological Research 108: 1011 – 1024 [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns SL, Taylor JW.1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR protocols: a guide to methods: 315–322. Academic Press Inc., New York, USA: [Google Scholar]