Abstract
Expression of the ctx and tcp genes, which encode cholera toxin and the toxin coregulated pilus, the Vibrio cholerae O1 virulence determinants having the largest contribution to cholera disease, is repressed by the nucleoid-associated protein H-NS and activated by the AraC-like transcriptional regulator ToxT. To elucidate the molecular mechanism by which H-NS controls transcription of the ctxAB operon, H-NS repression and binding were characterized by using a promoter truncation series, gel mobility shift assays, and DNase I footprinting. Promoter regions found to be important for H-NS repression correlated with in vitro binding. Four main H-NS binding regions are present at ctx. One region overlaps the high-affinity ToxT binding site and extends upstream, another overlaps the ToxT low-affinity binding site around the −35 element, and the remaining two are located adjacent to one another downstream of the transcriptional start site. Competition for binding to the overlapping H-NS/ToxT binding sites was observed in gel mobility shift assays, where ToxT was found to displace H-NS from the ctx promoter region. In addition, regulatory differences between the ctx and tcpA promoters were examined. H-NS was found to have a higher relative binding affinity for the ctx promoter than for the tcpA promoter in vitro. In contrast to ToxT-dependent activation of the tcpA promoter, ToxT activation of ctx did not require the C-terminal domain of the α-subunit of RNA polymerase. These findings demonstrate that transcriptional regulation of ctx and tcpA by H-NS and ToxT is mechanistically distinct, and this may lead to important differences in the expression of these coregulated genes.
Vibrio cholerae is the etiological agent of the human diarrheal disease cholera. Two virulence factors that are produced by V. cholerae and are essential for disease are toxin-coregulated pilus (TCP) (62) and cholera toxin (CT) (28). TCP is a type IV pilus that is assembled by polymerization of the pilin subunit, TcpA, and forms long filaments that laterally associate into bundles. Expression of TCP in vitro results in autoagglutination of the bacterium, and TCP-mediated bacterium-bacterium interactions in vivo facilitate microcolony formation on the intestinal epithelium. Pilus biogenesis requires at least 9 other proteins in addition to TcpA, which are encoded in an operon located on the Vibrio pathogenicity island (VPI). The pilus biogenesis apparatus plays a second role in colonization by secreting the cotranscribed, soluble colonization factor TcpF, which is also essential for colonization but for which a mechanism remains unknown (29, 30). The second main virulence factor, CT, is a potent A1B5 subunit, ADP-ribosylating toxin that is responsible for the severe watery diarrhea that is associated with cholera. CT is encoded by the ctxAB operon, which is located on the lysogenic bacteriophage CTXφ. The VPI and CTXφ were both acquired by horizontal gene transfer. TCP serves as the high-affinity receptor for CTXφ that links TCP production to ctx acquisition (64).
Expression of the genes encoding TCP and CT in V. cholerae is controlled by a complex regulatory cascade that is influenced by both specific regulators, such as ToxR/S, TcpP/H, and ToxT, and global regulators, such as cyclic AMP (cAMP)-cAMP receptor protein (CRP), H-NS, and IHF (for reviews, see references 7 and 58). The cascade is initiated by two proteins encoded within the ancestral chromosome, AphA and AphB, functioning synergistically to activate transcription of tcpPH (31, 59). TcpP and TcpH (4, 18) are transmembrane proteins encoded on the VPI that, along with a second pair of transmembrane proteins that are encoded on the ancestral chromosome, ToxR and ToxS (42, 43), activate transcription at the toxT promoter (20, 34). ToxT directly activates expression of many genes on the VPI, including the biosynthetic genes for TCP (5, 9, 24, 72) and the accessory colonization factor (ACF) genes acfA and acfD (52, 68) and aldA (67). ToxT autoregulates its own expression through activation of the upstream tcpA promoter and readthrough into toxT (71). Additionally, ToxT regulates some genes that are not encoded on the VPI, including the ctxAB operon, which encodes the subunits of CT (9). ToxT therefore functions to coordinately regulate virulence gene expression in V. cholerae by activating genes encoded on both the VPI and the CTX element in response to environmental cues.
ToxT is a member of the AraC/XylS family of transcriptional regulators (21, 49). The binding sites of many AraC family members are adjacent to, or overlap, the −35 region of promoters. Interactions between AraC family members and RNA polymerase (RNAP) have been demonstrated in various systems (1, 13, 16, 22, 23, 25, 27, 40, 66). ToxT binding sites have been identified at the tcpA and ctx promoters as well as at the promoter regions between the divergently transcribed aldA-tagA and acfA-acfD genes (24, 67, 68, 72). A 13-bp consensus ToxT binding motif, or toxbox (yrTTTTwwTwAww, where r can be either A or G, w can be either A or T, and y can be either C or T), has been proposed by Withey and DiRita based on the characterized ToxT binding sites (69). The toxbox is found as a direct repeat at the ctx and tcpA promoters, but the orientation and distance of the toxbox from the −35 differ between these two promoters (Fig. 1 A and B). ToxT, like other AraC/XylS family members, is proposed to interact with the C-terminal domain (CTD) of the α-subunit of RNAP (α-CTD) at the tcpA promoter, since ToxT-dependent activation of tcpA requires the α-CTD (24). It is unknown if the α-CTD of RNAP is important at other ToxT-regulated promoters. Given the diversity of toxbox arrangements at ToxT-regulated promoters (69), it is likely that ToxT may form various promoter-specific interactions with RNAP subunits.
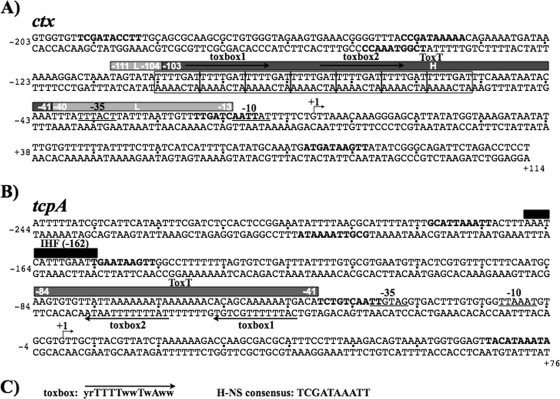
FIG. 1.
Organization of the tcpA and ctx promoters. Base pair sequences of the ctx (A) and tcpA (B) promoter fragments relative to their transcriptional start sites (+1) are shown. The core promoter −10 and −35 elements are underlined. The promoter regions protected by ToxT from DNase I (72) are denoted by solid gray bars above the sequence. At ctx, the low-affinity (L; light gray) and high-affinity (H; dark gray) ToxT binding sites are shown. The low-affinity sites are those that require higher concentrations of ToxT to show protection (72). Proposed ToxT consensus sites, toxboxes, are indicated by arrows, and the consensus toxbox sequence is shown in panel C, where r = A/G, w = A/T, and y = C/T. (C) The IHF consensus site centered at −162 of tcpA is indicated by a black bar. Putative H-NS nucleation sites with seven or more matches to the 10-bp H-NS consensus (35) are indicated in boldface letters.
Bacterial virulence gene expression is controlled by complex and overlapping regulatory systems encoded on pathogenicity islands, plasmids, and elsewhere within the genome. The nucleoid-associated protein H-NS is an abundant global regulator that influences various cellular processes (10, 11, 45), including virulence gene expression in many pathogens. Recent studies have contributed to our understanding of how this DNA binding protein with relatively low sequence specificity recognizes DNA. Whole-genome analysis of H-NS binding sites in Escherichia coli and Salmonella enterica serovar Typimurium revealed that H-NS preferentially binds intrinsically curved, AT-rich DNA often associated with horizontally acquired elements (15, 39, 46, 50). It has been suggested that H-NS may serve to silence genes located on these elements, such as virulence genes, until dedicated transcription factors or the promoters themselves can evolve to relieve H-NS repression. H-NS is thought to silence transcription by directly interfering with RNAP action or blocking specific activators at nearby promoter regions. H-NS has recently been proposed to bind to high-affinity nucleation sites with the 10-bp consensus motif TCGATAAATT and subsequently polymerize along AT-rich DNA to silence regions of the chromosome (2, 35).
H-NS has been shown to function at multiple promoters within the virulence regulon to reduce virulence gene expression under particular environmental conditions (14, 33, 47, 60, 72). H-NS is a repressor of toxT, tcpA, and ctx expression (47). A variety of data indicate that H-NS directly influences transcription at each of these promoters but that the level of H-NS repression varies. We have recently shown that V. cholerae H-NS directly binds the tcpA promoter and that H-NS binding overlaps the AT-rich ToxT binding site as well as an upstream IHF binding site (60). The overlapping H-NS/ToxT binding site at the tcpA promoter suggests that ToxT alleviates H-NS repression at tcpA in addition to interacting with the α-CTD of RNAP to activate transcription (24). Direct H-NS binding to the ctx promoter has not yet been demonstrated but is suggested by genetic studies at this promoter (47, 72).
Expression of tcpA and ctx are both activated by ToxT and repressed by H-NS. However, it seems that ToxT-dependent activation and H-NS-dependent repression are mechanistically distinct at these promoters. H-NS represses basal ctx expression to a greater extent than tcpA expression (47, 72). Yu and DiRita also showed that the ToxT binding site at ctx is of higher affinity than the ToxT site at tcpA (72). In addition, characterization of the in vivo temporal expression pattern in the El Tor biotype of V. cholerae revealed that the coordinately regulated tcpA and ctx genes are actually sequentially expressed in the infant mouse model of cholera (36). Expression of tcpA initiates 2 h prior to ctx expression in vivo.
MATERIALS AND METHODS
Bacterial strains and growth.
The V. cholerae and E. coli strains and plasmids used in this study are listed in Table 1. Strains were maintained at −70°C in Luria-Bertani (LB) medium (41) containing 30% (vol/vol) glycerol. Antibiotics were used at the following concentrations in LB medium: ampicillin (Ap), 100 μg/ml; chloramphenicol (Cm), 30 μg/ml; streptomycin (Sm), 1 mg/ml; tetracycline (Tc), 15 μg/ml. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was used in LB agar at 40 μg/ml (Sigma). Arabinose was added to the growth medium at a final concentration of 0.02%.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Reference or source |
|---|---|---|
| V. cholerae strains | ||
| O395 Sm | Classical Ogawa, Smr | 62 |
| KSK218 | CG842 ctx-lacZ | 57 |
| MBN019 | KSK218 ΔtoxT | 47 |
| MBN153 | MBN019 Δhns1 | 47 |
| E. coli strains | ||
| ER2566 | Expression strain, T7 RNA polymerase | New England Biolabs |
| BL21(DE3) | Expression strain, hsdS gal (λ imm21 nin5 lacuv5-T7 gene 1) (rB− mB−) | Lab collection |
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR, Smr | 55 |
| DL1976 | MC4100 hns651, Tcr | 65 |
| MBN425 | MC4100 (λctx-lacZYA)1, (−522/+114) | This work |
| MBN426 | MBN425 hns651, Tcr | This work |
| MBN410 | MC4100 (λctx-lacZYA)2, (−202/+114) | This work |
| MBN411 | MBN410 hns651, Tcr | This work |
| MBN392 | MC4100 (λctx-lacZYA)3, (−118/+114) | This work |
| MBN408 | MBN392 hns651, Tcr | This work |
| MBN412 | MC4100 (λctx-lacZYA)4, (−65/+114) | This work |
| MBN424 | MBN412 hns651, Tcr | This work |
| Plasmids | ||
| pRS415 | lacZYA transcriptional fusion vector | 56 |
| pMIN46 | pRS415::(ctx-lacZYA)1, Apr | 48 |
| pRHK1 | pRS415::(ctx-lacZYA)2, Apr | This work |
| pRHK2 | pRS415::(ctx-lacZYA)3, Apr | This work |
| pRHK3 | pRS415::(ctx-lacZYA)4, Apr | This work |
| pBAD22 | Expression vector, Apr | 17 |
| pBlueScript | Cloning vector, Apr | Stratagene |
| pBS-ctx | pBlueScript, 316-bp ctx promoter fragment (−202/+114) | This work |
| pTXB-1 | Expression vector for intein/chitin binding domain fusion, Apr | New England Biolabs |
| pEAS10 | pTXB-1, hns Apr | 60 |
| pRH81 | pBAD22, 6His toxT | 24 |
| pACYC184 | Expression plasmid, Cmr | 6 |
| pTSS-5 | pACYC184 toxT+, Cmr | 3 |
| pMMB66EH | Expression plasmid, Apr | 12 |
| pRH170 | Full-length rpoA in pMMB66EH | 24 |
| pRH171 | rpoAΔ235 in pMMB66EH | 24 |
Strain and plasmid construction.
The E. coli (ctx-lacZYA)1 fusion was constructed by amplifying the O395 ctx promoter region from −522 to +114, with respect to the transcriptional start site, using primers MN37 and MN42 (Table 2). The resulting fragment was cloned into pRS415 to generate pMIN46. The (ctx-lacZYA)1 fusion was recombined onto λRS45 (56) and integrated into the chromosome of MC4100 to create MBN425. Similarly, the E. coli (ctx-lacZYA)2 fusion was constructed by amplifying the ctx promoter region from −202 to +114 using primers MN34 and MN37. The MN34/MN37 fragment was cloned into pRS415, generating pRHK1. The (ctx-lacZYA)2 fusion was recombined onto λRS45 and integrated into the chromosome of MC4100 to create MBN410. The E. coli (ctx-lacZYA)3 fusion was constructed by amplifying the ctx promoter region from −118 to +114 using primers MN35 and MN37. The MN35/MN37 fragment was cloned into pRS415, generating pRHK2. The (ctx-lacZYA)3 fusion was recombined onto λRS45 and integrated into the chromosome of MC4100 to create MBN392. The E. coli (ctx-lacZYA)4 fusion was constructed by amplifying the ctx promoter region from −65 to +114 using primers MN36 and MN37. The MN36/MN37 fragment was cloned into pRS415, generating pRHK3. The (ctx-lacZYA)4 fusion was recombined onto λRS45 and integrated into the chromosome of MC4100 to create MBN412.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequencea |
|---|---|
| MN34 | GATCGGAATTCAAGTGAAACGGGGTTTACCG |
| MN35 | GATCGGAATTCGGACTAAATAGTATATTTTG |
| MN36 | GATCGGAATTCGATTTTTGATTTCAAATAATAC |
| MN37 | GATCGGGATCCAGGAGGTCTAGAATCTGCCC |
| MN42 | GATCGGAATTCTCGAGTCAGAGCAATCCGAG |
| ctx42R | CGCTGCAAAGGTATCGAAC |
| ctxE1 | GATCGGAATTCGTGGTGTTCGATACCTTTGCAGCG |
| TCP-SAL | GACTCGTCGACAATTTCGATCTCCACTCCGG |
| TCP-XHO | GTCAACTCGAGCATATTTATGTAACTCCACC |
All oligonucleotides are shown 5′ to 3′. Underlined portions indicate sequences encoding relevant restriction sites.
The hns651 mutation from DL1976 was transduced into each of the E. coli ctx-lacZYA fusion strains by using P1vir. Resultant strains were confirmed to be deficient for hns by PCR and by salicin utilization on MacConkey medium supplemented with 0.5% salicin.
β-Galactosidase assays.
β-Galactosidase activity was determined by the method of Miller (41) with the following modifications. E. coli strains harboring ctx-lacZ λlysogens were assayed for β-galactosidase activity after growth to mid-log phase in LB medium at 30°C and shaking. Due to TCP-mediated bacterial autoagglutination, the specific activity of strains in the KSK218 background (V. cholerae ctx-lacZ) was calculated using the protein concentration determined by a bicinchoninic acid procedure (Pierce) rather than by using the more standard optical density normalization.
Purification of H-NS and ToxT.
H-NS was purified from the E. coli strain ER2566 containing the V. cholerae hns expression plasmid pEAS10 by using the IMPACT-CN system as previously described (60). Briefly, the cells were grown in LB Amp at 37°C, and hns expression was induced with isopropyl-β-d-thiogalactopyranoside (IPTG) overnight at 16°C, the culture was centrifuged, sonicated, recentrifuged, and the supernatant was loaded onto a chitin column. The chitin binding domain was cleaved off H-NS by using dithiothreitol (DTT), H-NS was eluted off the column, and the protein was dialyzed overnight in 20 mM Tris (pH 8.0), 1 mM EDTA (pH 8.0), 5 mM potassium glutamate, and 0.1 mM DTT. Purified protein was stored at −70°C in 10% glycerol.
ToxT protein was purified essentially as previously described (24). Briefly, BL21 cells containing the 6His-ToxT expression plasmid pRH81 were grown at 37°C to mid-log phase, arabinose was added to a final concentration of 0.02%, and growth was continued at 12°C for an additional 12 h. Cell pellets were resuspended in extraction buffer (50 mM sodium phosphate, 300 mM NaCl; pH 7.0) and lysed by sonication. Following a clarifying spin at 12,000 rpm in a Sorvall SS34 rotor for 30 min, the supernatant was passed over Talon metal affinity resin (Clontech), washed with extraction buffer containing 20 mM imidizole, and ToxT was then eluted from the column with extraction buffer containing 200 mM imidazole. ToxT-containing fractions were pooled and dialyzed against TEN buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA [pH 8.0], 150 mM NaCl) containing 1 mM DTT. Protein was stored at −70°C in 10% glycerol.
The purity of the proteins was >90% as determined densitometrically after separation by SDS-PAGE and staining with Coomassie blue. The Bradford method was used to determine the concentration of H-NS and 6His-ToxT in relation to a standard curve for bovine serum albumin (BSA). The concentrations were not corrected for potential differences in dye reactivity between the purified proteins and BSA.
Gel mobility shift assays.
DNA fragments of the tcpA and ctx promoter regions were generated by PCR from V. cholerae O395 chromosomal DNA with the oligonucleotide pairs MN42/ctx42R (C1; −522 to −178), ctxE1/MN37 (C2; −202 to +114), MN35/MN37 (C3; −112 to +114), and MN36/MN37 (C4; −65 to +114) for ctx and TCP-SAL/TCP-XHO (tcp; −226 and +78) for tcpA. H-NS gel mobility shift assays were carried out in a volume of 20 μl containing DNA binding buffer (40 mM HEPES, 100 mM potassium glutamate, 10 mM magnesium aspartate, 0.022% NP-40, 100 μg/ml BSA, 10% glycerol), 10 ng of digoxigenin (DIG)-labeled DNA, 1 μg of calf thymus DNA, and purified H-NS. After incubation at room temperature for 20 min, protein-DNA complexes were separated by 5% nondenaturing polyacrylamide gel electrophoresis in 0.5× Tris-EDTA (TE) buffer at 4°C and transferred to nylon. DNA was visualized with an anti-DIG detection kit (Roche) followed by enhanced chemiluminescence detection.
ToxT gel mobility shift assays were carried out in a 20-μl mixture containing DNA binding buffer (10 mM Tris [pH 7.5], 100 mM KCl, 1 mM EDTA [pH 8.0], 1 mM DTT, 10% glycerol, 300 mg/ml BSA), 10 ng of DIG-labeled DNA, 1.5 μg of calf thymus DNA, and purified 6His-ToxT. After incubation at 30°C for 15 min, protein-DNA complexes were separated on 5% nondenaturing polyacrylamide gels in 0.5× TE buffer at 4°C and visualized as described above.
DNase I footprinting.
A ctx promoter fragment was amplified from O395 chromosomal DNA with primer pair ctxE1/MN37 (−202 to +114). The fragment was ligated into pBlueScript (Stratagene), generating pBS-ctx. Fragments for labeling were cut from pBS-ctx by restriction digestion with EcoRI and BamHI. Strand labeling was carried out essentially as described by Kovacikova and Skorupski (32). Binding reactions were performed in 6.5 μl with 33P-end-labeled DNA, purified H-NS, and binding buffer as described above. After incubation at 30°C for 15 min, protein-DNA complexes were digested with 1 μl of various dilutions of DNase I (Promega) for 5 min at 30°C. Reactions were stopped in 2 mM EGTA followed by heat inactivation at 65°C for 10 min and then spot dialyzed for 30 min against 10 mM Tris (pH 8.0), 0.1 mM EDTA. They were then heated to 90°C for 5 min in formamide loading buffer (Epicentre) and separated on a 6% polyacrylamide sequencing gel in 1× TBE at ambient temperature. Gels were dried and visualized by autoradiography.
RESULTS
H-NS repression utilizes heptad repeats within the ctx promoter.
Characteristics of the ctx promoter that potentially favor a direct interaction of H-NS include a high AT content over the promoter as well as regions of predicted curvature (data not shown). In addition, scanning of the ctx promoter for putative H-NS nucleation sites using the 10-bp consensus identified by Lang et al. (35) revealed five sites with greater than seven matches to the consensus binding site, TCGATAAAT (Fig. 1A, bold sequences). The ctx promoter also contains a region of notably high AT content (86%) composed of seven tandem repeats of the sequence TTTTGAT (Fig. 1A, boxes). The heptad repeat sequences have previously been shown as binding sites for the transcriptional activators of ctx, ToxR (37, 43, 53), and ToxT (5, 71). It was hypothesized that H-NS may bind to this repeat region of the ctx promoter utilized by the transcriptional activator proteins and that occupation of these sites by H-NS could prevent ctx activation.
To genetically determine the region required for H-NS regulation of this promoter, a series of ctx-promoter lacZ fusions containing various lengths of promoter DNA were constructed in the plasmid pRS415 (Fig. 2A). These fusions were integrated as single lambda lysogens in the E. coli chromosome, and the expression from each was measured based on β-galactosidase activity in the presence and absence of hns. The three longest constructs, C1, C2, and C3, had low and equivalent basal levels of β-galactosidase activity in the presence of the wild-type hns allele (Fig. 2A). The corresponding hns-deficient strains were highly derepressed, with the degree of induction ranging from 16- to 23-fold. As expected, expression from each of these three fusions could be activated by the presence of a plasmid encoding ToxT (pMT5) (data not shown). The shortest construct, C4, lacks six of the seven TTTTGAT sites (Fig. 2A). In the presence of hns, C4 had a 4- to 6-fold increase in expression compared to the three longer fusions (Fig. 2A). This increase in expression suggests that H-NS may bind to the repeats to repress ctx expression. Additional derepression of the C4 fusion was achieved in the absence of hns, suggesting that sites remaining within this short fusion (−65 to +114) also contribute to repression by H-NS. The shortest fusion was not activated in the presence of ToxT (data not shown), which is consistent with the known binding sites for each of these activators (24, 72). By truncating the promoter distal sequence, we were able to show that the region containing the TTTTGAT sites, previously implicated in ToxR and ToxT binding, also contributes to H-NS-mediated repression of ctx expression and that H-NS repression still occurs on the −65/+114 region of ctx.
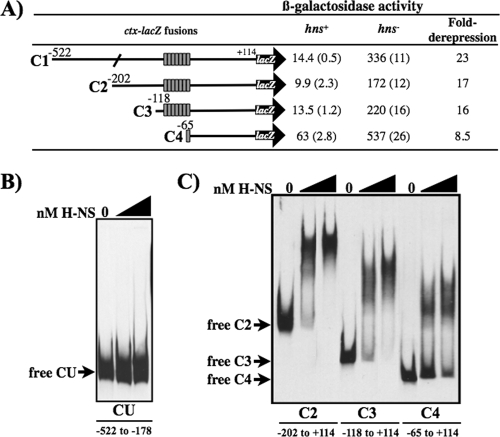
FIG. 2.
Promoter truncation analysis at ctx. (A) Schematic representations of the extent of the ctx promoter present in each fusion construct are shown to the left. Gray rectangles each represent one reiteration of the heptad repeat 5′-TTTTGAT-3′. Culture of E. coli strains that are either hns+ or hns deficient (hns651) and carry a chromosomal copy of each fusion construct were grown to mid-log phase in LB medium at 30°C with shaking and assayed for β-galactosidase production. Data are expressed in Miller units and the standard deviation. The fold derepression was calculated as the increase in β-galatosidase activity in the hns-deficient strain compared to the hns+ strain. (B and C) DIG-labeled ctx promoter fragments from −522 to −178 (CU), −202 to + 114 (C2), −118 to + 114 (C3), and −65 to + 114 (C4). Fragments were incubated with purified H-NS and analyzed on a nondenaturing polyacrylamide gel. The first lane in each set has no protein added, the second lane contains 180 nM H-NS, and the third lane contains 360 nM H-NS.
Direct binding of H-NS to the ctx promoter.
Gel mobility shift assays (Fig. 2B) were performed to examine if regions identified as important for H-NS repression (Fig. 2A) were also important for H-NS binding at the ctx promoter. Specific binding of H-NS to the ctx promoter region was examined on promoter fragments that were constructed based on the promoter truncation series in Fig. 2A. Fragment CU (upstream region) extends from −522 to −178 of the C1 promoter fusion and was used to monitor H-NS binding to this upstream region. Fragments C2, C3, and C4 correspond to the promoter fusions in Fig. 2A. Each of the DNA fragments was incubated with 180 or 360 nM purified V. cholerae H-NS, and the samples were analyzed on native polyacrylamide gels. H-NS binding was observed on the C2, C3, and C4 promoter fragments (Fig. 2C), but not on the CU fragment (Fig. 2B). H-NS binding was decreased on the C4 fragment, where only one heptad repeat remains compared to the C3 and C2 fragments. These results are consistent with expression data in Fig. 2A, which showed that H-NS has a greater repressive effect on the C2 and C3 fragments than the C4 fragment (16- versus 8.5-fold) and confirmed that the heptad repeats are involved in H-NS binding to the ctx promoter.
H-NS protects the ToxT binding site, −35, and promoter distal regions from DNase I at ctx.
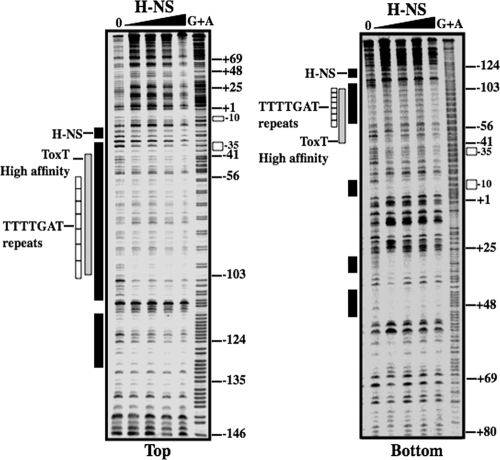
DNase I footprinting was used to determine the location of H-NS binding within the ctx promoter. Radiolabeled fragments were generated that spanned the ctx promoter region from −202 to +114 (Fig. 2A, construct C2). Fragments were incubated with increasing amounts of H-NS and digested with DNase I as described in Materials and Methods. The reactions were then subjected to electrophoresis on a denaturing polyacrylamide gel. H-NS protection was widespread over the ctx promoter region (Fig. 3). Four main regions of H-NS protection were identified: one overlapping the high-affinity ToxT binding site and extending upstream, one overlapping the ToxT low-affinity binding site and the −35 element, and two adjacent regions downstream of the transcription start (Fig. 3; see also Fig. 1). These results indicate that H-NS binding overlaps the binding sites of ToxT and RNAP at the ctx promoter. The overlapping H-NS and ToxT binding sites suggest that ToxT might displace H-NS in order to bind the ctx promoter and recruit RNAP to activate transcription.
FIG. 3.
DNase I protection by H-NS at the ctx promoter. The top and bottom strands of the ctx promoter fragments were incubated with increasing amounts of purified H-NS (0.25, 0.5, 1, and 2 μM) and treated with DNase I. Regions protected from DNase I cleavage by H-NS are shown by black bars. The ToxT high-affinity site protected from DNase I cleavage (72) is shown by a gray bar. White boxes represent the seven repeats of the heptad sequence 5′-TTTTGAT-3′. Open rectangles to the right represent the positions of the −10 and −35 hexamers. In each gel, a G+A DNA sequencing ladder is shown. The numbering is relative to the transcription start site.
Competition of ToxT with H-NS at the ctx promoter.
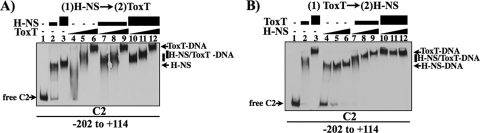
It was hypothesized that displacement of H-NS by ToxT at the ctx promoter region is essential for transcriptional activation of the toxin genes, since ToxT protein levels increase while H-NS levels do not change under the transition to inducing conditions (data not shown). Competitive gel mobility shift assays were performed between H-NS and ToxT on the ctx promoter region to examine the effect of adding ToxT to prebound H-NS-DNA complexes. The DNA was incubated in ToxT binding buffer with a low (165 nM) or high (330 nM) concentration of H-NS that gave a partial or complete shift of the free DNA, respectively. After 15 min at 30°C, increasing amounts of ToxT (0.9, 1.8, and 3.6 μM) were added to free DNA or H-NS-prebound DNA, and incubation was continued for an additional 15 min. When ToxT was incubated alone with DNA, the ToxT-DNA complexes (lanes 4 to 6) migrated as diffuse bands that tightened to a distinct band at the highest ToxT concentration. Given the large region of DNase I protection by ToxT at ctx that could accommodate two to four ToxT monomers (Fig. 1A), the diffuse banding pattern may represent various ToxT-DNA species with different sites occupied. Alternatively, ToxT may bend the ctx promoter and contribute to differences in migration in the gel. However, ToxT-induced bending of the ctx promoter was not observed when we used a circular permutation assay, which allows visualization of protein-induced DNA bending in a standard gel mobility shift assay (data not shown). A more compact migrating species was observed upon addition of 0.9 and 1.8 μM ToxT to a prebound H-NS fragment compared to these ToxT concentrations alone (Fig. 4A, compare lanes 7, 8, 10, and 11, containing H-NS, to lanes 4 and 5, containing just ToxT). These distinct H-NS/ToxT-DNA bands suggest that H-NS and ToxT can simultaneously occupy the DNA when ToxT concentrations are relatively low. The condensed banding pattern also suggests that the presence of H-NS on the DNA may facilitate a more ordered binding of ToxT to the promoter fragment. The ability of H-NS to bend the ToxT binding site or interact with ToxT was investigated in order to understand how H-NS could affect ToxT binding. A circular permutation assay with H-NS and the ctx promoter and a bacterial two-hybrid experiment investigating H-NS and ToxT protein interactions suggested that H-NS did not bend the ctx promoter fragment or directly interact with ToxT (data not shown). Addition of 3.6 μM ToxT to prebound H-NS-DNA complexes (165 or 330 nM H-NS) resulted in a shift to a predominately ToxT-DNA complex (compare lanes 9 and 12, containing H-NS and ToxT, to lane 6, containing just ToxT). These results suggest that at low levels of ToxT, H-NS and ToxT can both be bound to the DNA, while at high levels of ToxT, ToxT displaces H-NS.
FIG. 4.
Interaction of H-NS and ToxT with ctx promoter DNA. Competitive gel mobility shift assays were carried out with the ctx promoter fragment (C2; −202 to +114) by combining various amounts of H-NS and ToxT. (A) Fixed amounts of H-NS with increasing levels of ToxT. H-NS was added at 0 (lanes 1 and 4 to 6), 0.165 (lanes 2 and 7 to 9), and 0.33 μM (lanes 3 and 10 to 12), followed by addition of ToxT at 0 (lanes 1 to 3), 0.9 (lanes 4, 7, and 10), 1.8 (lanes 5, 8, and 11), and 3.6 μM (lanes 6, 9, and 12). (B) Fixed amounts of ToxT with increasing levels of H-NS. ToxT was added at 0 (lanes 1 and 4 to 6), 1.8 (lanes 2 and 7 to 9), and 3.6 μM (lanes 3 and 10 to 12), followed by addition of H-NS at 0 (lanes 1 to 3), 0.165 (lanes 4, 7, and 10), 0.33 (lanes 5, 8, and 11), and 0.66 μM (lanes 6, 9, and 12).
Competition of H-NS and ToxT at the ctx promoter.
Competition of H-NS for ToxT-prebound DNA was also investigated in competitive gel mobility shift assays. DNA was incubated with ToxT followed by addition of H-NS to the ToxT-DNA prebound complexes. ToxT at 1.8 and 3.6 μM was incubated with the ctx promoter fragment (Fig. 4B, lanes 2 and 3). Addition of increasing amounts of H-NS to the 1.8 μM ToxT-DNA complexes (lanes 7 to 9) resulted in a shift that was more retarded in migration than with 1.8 μM ToxT alone (lane 2) or H-NS alone (lanes 4 to 6). This banding pattern suggested that H-NS was able to bind the ToxT-DNA complex at low levels of ToxT but was unable to displace ToxT from the DNA. Addition of H-NS to DNA incubated with 3.6 μM ToxT (lanes 10 to 12) did not alter the migration of the ToxT-DNA species (lane 3), suggesting that at high ToxT levels H-NS is unable to displace ToxT from the DNA and may not be able to bind to the promoter fragment, even at sites downstream or upstream of the ToxT binding region.
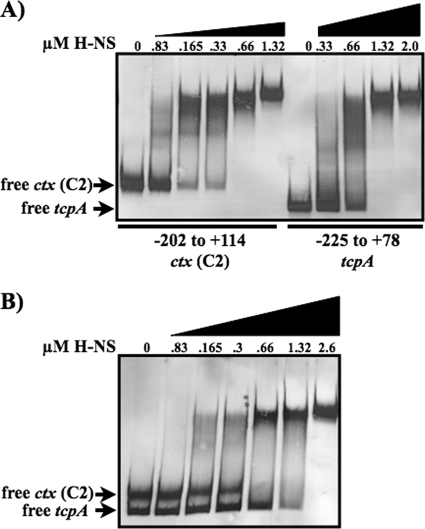
H-NS has a higher relative affinity for the ctx promoter than the tcpA promoter.
H-NS represses the transcription of both ctx and tcpA. Genetic studies have shown that H-NS repression is greater at ctx than tcpA (47, 72). We previously showed direct binding of H-NS at the tcpA promoter (60). Gel mobility shift assays were used to investigate if the differences in H-NS repression between the two virulence promoters correlated with differences in H-NS binding to the promoters. Promoter fragments from ctx (C2, −202 to +114) and tcpA (−225 to +78) were incubated with increasing amounts of H-NS to measure the relative binding affinity of H-NS for the two promoters. H-NS bound to both the ctx and tcpA promoter fragments (Fig. 5A). Comparison of the shifting of the ctx and tcpA promoter fragments in the presence of equimolar H-NS revealed that H-NS bound with higher affinity to the ctx promoter fragment than to the tcpA promoter fragment. The ctx fragment was completely shifted in the presence of 0.66 μM H-NS, whereas the tcpA fragment was not fully shifted at 0.66 μM H-NS but required 1.32 μM H-NS to completely shift the free DNA (Fig. 5A). To further examine the differential binding affinities of H-NS for these promoter regions, increasing amounts of H-NS were incubated with a mixture of equimolar ctx and tcpA promoter fragments (Fig. 5B). The ctx fragment is 13 bp longer and migrates slower in the gel than the tcpA fragment. A full shift of the free ctx fragment was observed at a lower H-NS concentration (0.66 μM) than was the free tcpA fragment (2.6 μM). These results demonstrate that H-NS has a higher relative binding affinity for the ctx promoter than for the tcpA promoter, and it is possible that this may contribute to differences in H-NS-mediated repression of these virulence genes.
FIG. 5.
H-NS binding at ctx and tcpA. Results of a gel mobility shift assay show H-NS binding to the ctx and tcpA promoter fragments. DIG-labeled ctx (−202 to +114) and tcp (−225 to +78) promoter fragments were incubated individually (A) or together (B) with the indicated amounts of purified H-NS.
ToxT does not require α-CTD of RNA polymerase to activate transcription at ctx.
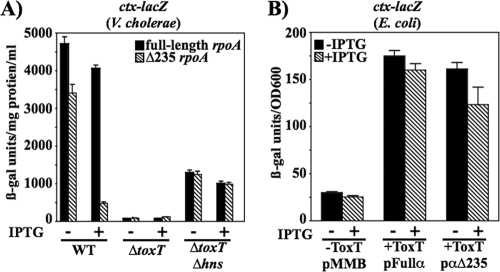
Yu and DiRita (72) determined that at ctx, ToxT has a higher affinity for its binding site than it does at tcpA. In addition, they found that ToxT stimulated RNAP transcriptional activation at both promoters, suggesting that ToxT functions not only to overcome H-NS repression from shared binding sites but also by interacting with RNAP. Many AraC family members require the α-CTD of RNA polymerase to activate transcription at their respective promoters (23, 25, 26, 40, 54). We previously reported a requirement for the α-CTD of RNA polymerase in ToxT-dependent activation at the tcpA promoter (24), where the ToxT binding site extends from −84 to −41 (Fig. 1B). In contrast, the ctx promoter may be a class II ToxT-dependent promoter due to the ToxT binding site overlapping the −35 determinant for RNAP. To determine if the α-CTD of RNA polymerase is required for ToxT-dependent activation at ctx, we performed the same genetic analysis that had been used at tcpA and that was based on a method described by Holcroft and Egan (23). Expression of ctx-lacZ was monitored upon expression of a truncated rpoA gene (Δ235 rpoA) that encodes an α-subunit that is missing the C-terminal domain. The truncated α-subunit has a dominant negative effect at promoters that require the α-CTD of RNAP for activation. This is based on previous experiments performed in E. coli (19) as well as our results for tcpA activation in V. cholerae and E. coli (24). A full-length rpoA was also expressed for comparison. The full and truncated rpoA constructs were introduced into V. cholerae strains carrying a chromosomal ctx-lacZ fusion. In the wild-type background, expression of the truncated α-subunit upon addition of IPTG resulted in a 7.5-fold decrease in ctx-lacZ expression compared to expression of the full-length α-subunit (Fig. 6A). This effect was not seen in a ΔtoxT or a ΔtoxT Δhns background, which was used to increase the basal level of promoter activity in the absence of ToxT. These results suggest that ToxT-dependent transcription, and not basal transcription of ctx-lacZ, depends on the α-CTD of RNAP. Since ToxT autoregulates its own expression from the upstream tcpA promoter, it is likely that toxT expression itself is decreased upon expression of the truncated α-subunit of RNAP. To test if the decrease in ctx-lacZ expression is due to a direct effect of the truncated α-CTD at ctx and not due to altered toxT expression, ToxT was expressed from the α-CTD-independent tetR promoter by using plasmid pTSS-5. These experiments were carried out in E. coli, since the tetR promoter is not expressed well in V. cholerae. The E. coli λctx-lacZ strain (−522/+114; MBN425) carrying IPTG-inducible full-length rpoA or Δ235 rpoA on pMMB66EH and pTSS-5 as a source of ToxT was assayed by performing β-galactosidase assays. It was found that when ToxT was expressed from an α-CTD-independent promoter, there was only a slight loss (<1.5-fold decrease) of ToxT-dependent ctx-lacZ expression upon expression of the truncated α-CTD (+ToxT; pα235). This suggests that the 7.5-fold defect in ctx-lacZ expression observed in V. cholerae when ToxT was expressed from its own promoter was most likely indirect and due to the role of α-CTD in toxT expression levels. In contrast, expression of the truncated α-CTD in E. coli caused a 4-fold defect in ToxT-dependent activation of the tcpA-lacZ fusion (24). These results indicate that ToxT interacts with the α-CTD of RNAP to activate transcription of tcpA but not ctx.
FIG. 6.
Influence of dominant negative RNA polymerase α-subunit on ctx-lacZ expression in V. cholerae (A) and E. coli (B). (A) Cultures of V. cholerae harboring ctx-lacZ that were otherwise wild type (KSK218), ΔtoxT (MBN019), or ΔtoxT Δhns (MBN153) carrying plasmids expressing either full-length rpoA (pRH170) or truncated Δ235 rpoA (pRH171) were grown overnight in LB medium with a starting pH of 6.5 at 30°C with or without 0.04 mM IPTG. (B) Strains of E. coli λctx-lacZ (MBN425) carrying plasmids expressing either full-length rpoA (pRH170) or truncated rpoA (pRH171) in the absence or presence of the toxT-expressing plasmid pTSS-5 were grown overnight in LB medium with a starting pH of 6.5 at 30°C with or without 0.04 mM IPTG. All values are averages of at least two independent experiments.
DISCUSSION
In this study, a series of in vivo and in vitro experiments were conducted to elucidate the molecular mechanism by which the H-NS protein represses transcription of the ctx operon. A promoter deletion analysis revealed regions that were important for H-NS repression and likely H-NS binding. H-NS repression was high (8.5- to 23-fold) for all ctx promoter fusions. Deleting the sequence between −118 to −65 (C3 to C4), which included six of the seven heptad repeats, caused the largest loss in H-NS repression of ctx-lacZ expression (16- versus 8.5-fold) (Fig. 2A). Since the promoter fusions all included sequence to +114 relative to the site of the start of transcription, it is possible that H-NS may bind and regulate ctx from regions downstream of the +1 site. Taken together with the fact that the region from +1 to +114 of the ctxA gene is approximately 71% AT rich, compared with the entire ctxAB locus at 56% AT, it is possible that H-NS contributes to the regulation of ctx from sequences within the gene itself. H-NS has been shown to regulate gene expression from downstream regulatory elements in proU, coo, and others, including the eltAB operon, which encodes the heat-labile enterotoxin of enterotoxigenic E. coli (44, 51, 70).
Biochemical studies were used to support this genetic analysis and to identify H-NS binding sites within the ctx promoter. Based on DNase I footprinting, H-NS binding was found to overlap the ToxT binding site, −35 element, and two regions downstream of the +1 site. Thus, we conclude that H-NS specifically binds the ctx promoter region, supporting a direct regulatory role for H-NS in ctx gene repression. Given the regions of protection in DNase I footprinting, it is likely that H-NS functions to block transcription initiation by interfering with ToxT and RNAP binding and activation. H-NS may also function downstream of the transcriptional start site to either inhibit open complex formation by polymerase or inhibit later stages of transcriptional initiation/elongation.
Given the high intracellular levels of H-NS which vary little during bacterial growth, it is likely that the tcp and ctx operons are normally repressed by H-NS when V. cholerae is not in its host environment. This effect would be overcome by V. cholerae regulatory proteins that, under appropriate environmental conditions, could act as antirepressors to alleviate the effects of H-NS on tcp and ctx expression. Indeed, several bacterial regulatory proteins are known to alleviate H-NS repression by displacing H-NS from the DNA (8, 38, 61, 63). At the tcpA promoter we have previously reported that both ToxT and IHF act as antirepressors of H-NS (60). Here we suggest that ToxT also acts as an antirepressor of H-NS at ctx by displacement of H-NS from the promoter (Fig. 4A). Interestingly, at low concentrations of ToxT, H-NS still bound the ctx promoter fragment. This suggests that H-NS repression may occur until a threshold level of ToxT is reached that is able to fully displace H-NS. In addition, it is possible that the displacement of ToxT by H-NS is important for reintroducing H-NS repression of toxin gene transcription when the toxin is no longer required (e.g., upon dissemination back into the environment). However, competition experiments suggested that H-NS could not displace ToxT from the ctx promoter (Fig. 4B). These results suggest that ctx expression is primarily dependent on ToxT levels within the cell. The ctx genes may only be repressed as toxT expression is downregulated or upon turnover of the ToxT protein, which would allow H-NS access to the promoter.
H-NS has a more moderate repressive effect at the tcpA promoter than at the ctx promoter (47, 72). Basal levels of ctx expression increase 10-fold upon deletion of hns, while tcpA expression increases 6.3-fold in V. cholerae (47). This could correlate with the in vivo temporal delay between tcpA expression and ctx expression that has been observed in the infant mouse model (36). In these studies we found that H-NS had an almost-2-fold-higher affinity for the ctx promoter than the tcpA promoter, a difference that is similar to the observed difference in H-NS repression of these promoters. The greater H-NS repression at ctx may establish a higher threshold level of ToxT required to displace H-NS and activate expression of ctx compared to tcpA. In addition, it was previously reported that ToxT also has a higher affinity for the ctx promoter than for the tcpA promoter (72). Thus, the delay in ctx expression compared to tcpA expression may involve more than differing affinities of H-NS and ToxT for these promoters. Differences in ToxT activation at these promoters, beyond antirepression of H-NS, have also been investigated. DNase I protection by ToxT at the tcpA and ctx promoters (72) (Fig. 1A and B) suggested that the ctx promoter may be a class II promoter. We found that ToxT-dependent activation at ctx did not require the α-CTD of RNAP as it does at tcpA (24). This difference in ToxT-RNAP interactions between the promoters may also contribute to differences in expression of these coregulated genes under different environmental conditions.
Virulence factors in many enteric pathogens are negatively regulated by H-NS. Many of these same factors show positive regulation by an AraC family member. Even in V. cholerae the majority of ToxT-activated genes are also repressed by H-NS (unpublished data). Understanding how these regulatory proteins function at each promoter to coordinate gene expression will expand our understanding of how proteins, like ToxT, function to overcome H-NS repression and initiate virulence gene expression.
Acknowledgments
This work was supported by grants AI039654 (to R.K.T.) and AI041558 (to K.S.) from the National Institutes of Health. E.A.S. was supported by NIH Training Grant AI007519.
We thank Gabriela Kovacikova and Wei Lin for technical support and Christine White-Ziegler for strains and helpful discussions.
Footnotes
Published ahead of print on 17 December 2010.
REFERENCES
- 1.Bhende, P. M., and S. M. Egan. 2000. Genetic evidence that transcription activation by RhaS involves specific amino acid contacts with sigma 70. J. Bacteriol. 182:4959-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouffartigues, E., M. Buckle, C. Badaut, A. Travers, and S. Rimsky. 2007. H-NS cooperative binding to high-affinity sites in a regulatory element results in transcriptional silencing. Nat. Struct. Mol. Biol. 14:441-448. [DOI] [PubMed] [Google Scholar]
- 3.Brown, R. C., and R. K. Taylor. 1995. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol. Microbiol. 16:425-439. [DOI] [PubMed] [Google Scholar]
- 4.Carroll, P. A., K. T. Tashima, M. B. Rogers, V. J. DiRita, and S. B. Calderwood. 1997. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol. Microbiol. 25:1099-1111. [DOI] [PubMed] [Google Scholar]
- 5.Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. [DOI] [PubMed] [Google Scholar]
- 6.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter, P. A., and V. J. DiRita. 2000. Bacterial virulence gene regulation: an evolutionary perspective. Annu. Rev. Microbiol. 54:519-565. [DOI] [PubMed] [Google Scholar]
- 8.De la Cruz, M. A., et al. 2007. LeuO antagonizes H-NS and StpA-dependent repression in Salmonella enterica ompS1. Mol. Microbiol. 66:727-743. [DOI] [PubMed] [Google Scholar]
- 9.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorman, C. J. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2:391-400. [DOI] [PubMed] [Google Scholar]
- 11.Dorman, C. J. 2007. H-NS, the genome sentinel. Nat. Rev. Microbiol. 5:157-161. [DOI] [PubMed] [Google Scholar]
- 12.Furste, J. P., et al. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 13.Gallegos, M. T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. Arac/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh, A., K. Paul, and R. Chowdhury. 2006. Role of the histone-like nucleoid structuring protein in colonization, motility, and bile-dependent repression of virulence gene expression in Vibrio cholerae. Infect. Immun. 74:3060-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grainger, D. C., D. Hurd, M. D. Goldberg, and S. J. Busby. 2006. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res. 34:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffith, K. L., and R. E. J. Wolf. 2002. A comprehensive alanine scanning mutagenesis of the Escherichia coli transcriptional activator SoxS: identifying amino acids important for DNA binding and transcription activation. J. Mol. Biol. 322:237-257. [DOI] [PubMed] [Google Scholar]
- 17.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hase, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayward, R. S., K. Igarashi, and A. Ishihama. 1991. Functional specialization within the alpha-subunit of Escherichia coli RNA polymerase. J. Mol. Biol. 221:23-29. [DOI] [PubMed] [Google Scholar]
- 20.Higgins, D. E., and V. J. DiRita. 1994. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol. Microbiol. 14:17-29. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, D. E., E. Nazareno, and V. J. DiRita. 1992. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J. Bacteriol. 174:6974-6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holcroft, C. C., and S. M. Egan. 2000. Interdependence of activation at rhaSR by cyclic AMP receptor protein, the RNA polymerase alpha subunit C-terminal domain, and rhaR. J. Bacteriol. 182:6774-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holcroft, C. C., and S. M. Egan. 2000. Roles of cyclic AMP receptor protein and the carboxyl-terminal domain of the alpha subunit in transcription activation of the Escherichia coli rhaBAD operon. J. Bacteriol. 182:3529-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulbert, R. R., and R. K. Taylor. 2002. Mechanism of ToxT-dependent transcriptional activation at the Vibrio cholerae tcpA promoter. J. Bacteriol. 184:5533-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jair, K. W., W. P. Fawcett, N. Fujita, A. Ishihama, and R. E. J. Wolf. 1996. Ambidextrous transcriptional activation by SoxS: requirement for the C-terminal domain of the RNA polymerase alpha subunit in a subset of Escherichia coli superoxide-inducible genes. Mol. Microbiol. 19:307-317. [DOI] [PubMed] [Google Scholar]
- 26.Jair, K. W., et al. 1995. Purification and regulatory properties of MarA protein, a transcriptional activator of Escherichia coli multiple antibiotic and superoxide resistance promoters. J. Bacteriol. 177:7100-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jair, K. W., et al. 1996. Transcriptional activation of promoters of the superoxide and multiple antibiotic resistance regulons by Rob, a binding protein of the Escherichia coli origin of chromosomal replication. J. Bacteriol. 178:2507-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaper, J. B., J. G. J. Morris, and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirn, T. J., N. Bose, and R. K. Taylor. 2003. Secretion of a soluble colonization factor by the TCP type 4 pilus biogenesis pathway in Vibrio cholerae. Mol. Microbiol. 49:81-92. [DOI] [PubMed] [Google Scholar]
- 30.Kirn, T. J., and R. K. Taylor. 2005. TcpF is a soluble colonization factor and protective antigen secreted by El Tor and classical O1 and O139 Vibrio cholerae serogroups. Infect. Immun. 73:4461-4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacikova, G., and K. Skorupski. 2001. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol. Microbiol. 41:393-407. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan, H. H., A. Ghosh, K. Paul, and R. Chowdhury. 2004. Effect of anaerobiosis on expression of virulence factors in Vibrio cholerae. Infect. Immun. 72:3961-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krukonis, E. S., R. R. Yu, and V. J. Dirita. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67-84. [DOI] [PubMed] [Google Scholar]
- 35.Lang, B., et al. 2007. High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids. Res. 35:6330-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 37.Li, C. C., J. A. Crawford, V. J. DiRita, and J. B. Kaper. 2000. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol. Microbiol. 35:189-203. [DOI] [PubMed] [Google Scholar]
- 38.Lithgow, J. K., F. Haider, I. S. Roberts, and J. Green. 2007. Alternate SlyA and H-NS nucleoprotein complexes control hlyE expression in Escherichia coli K-12. Mol. Microbiol. 66:685-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucchini, S., et al. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2:e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin, R. G., and J. L. Rosner. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132-137. [DOI] [PubMed] [Google Scholar]
- 41.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 42.Miller, V. L., V. J. DiRita, and J. J. Mekalanos. 1989. Identification of toxS, a regulatory gene whose product enhances ToxR-mediated activation of the cholera toxin promoter. J. Bacteriol. 171:1288-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, V. L., R. K. Taylor, and J. J. Mekalanos. 1987. Cholera toxin transcriptional activator ToxR is a transmembrane DNA binding protein. Cell 48:271-279. [DOI] [PubMed] [Google Scholar]
- 44.Murphree, D., B. Froehlich, and J. R. Scott. 1997. Transcriptional control of genes encoding CS1 pili: negative regulation by a silencer and positive regulation by Rns. J. Bacteriol. 179:5736-5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Navarre, W. W., M. McClelland, S. J. Libby, and F. C. Fang. 2007. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 21:1456-1471. [DOI] [PubMed] [Google Scholar]
- 46.Navarre, W. W., et al. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313:236-238. [DOI] [PubMed] [Google Scholar]
- 47.Nye, M. B., J. D. Pfau, K. Skorupski, and R. K. Taylor. 2000. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 182:4295-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nye, M. B., and R. K. Taylor. 2003. Vibrio cholerae H-NS domain structure and function with respect to transcriptional repression of ToxR regulon genes reveals differences among H-NS family members. Mol. Microbiol. 50:427-444. [DOI] [PubMed] [Google Scholar]
- 49.Ogierman, M. A., and P. A. Manning. 1992. Homology of TcpN, a putative regulatory protein of Vibrio cholerae, to the AraC family of transcriptional activators. Gene 116:93-97. [DOI] [PubMed] [Google Scholar]
- 50.Oshima, T., S. Ishikawa, K. Kurokawa, H. Aiba, and N. Ogasawara. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 13:141-153. [DOI] [PubMed] [Google Scholar]
- 51.Owen-Hughes, T. A., et al. 1992. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell 71:255-265. [DOI] [PubMed] [Google Scholar]
- 52.Peterson, K. M., and J. J. Mekalanos. 1988. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect. Immun. 56:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfau, J. D., and R. K. Taylor. 1996. Genetic footprint on the ToxR-binding site in the promoter for cholera toxin. Mol. Microbiol. 20:213-222. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz, R., J. L. Ramos, and S. M. Egan. 2001. Interactions of the XylS regulators with the C-terminal domain of the RNA polymerase alpha subunit influence the expression level from the cognate Pm promoter. FEBS Lett. 491:207-211. [DOI] [PubMed] [Google Scholar]
- 55.Silhavy, T. J., M. L. Berman, and L. W. Enquist (ed.). 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 56.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 57.Skorupski, K., and R. K. Taylor. 1997. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 94:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skorupski, K., and R. K. Taylor. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 59.Skorupski, K., and R. K. Taylor. 1999. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol. Microbiol. 31:763-771. [DOI] [PubMed] [Google Scholar]
- 60.Stonehouse, E., G. Kovacikova, R. K. Taylor, and K. Skorupski. 2008. Integration host factor positively regulates virulence gene expression in Vibrio cholerae. J. Bacteriol. 190:4736-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stratmann, T., S. Madhusudan, and K. Schnetz. 2008. Regulation of the yjjQ-bglJ operon, encoding LuxR-type transcription factors, and the divergent yjjP gene by H-NS and LeuO. J. Bacteriol. 190:926-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U. S. A. 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turner, E. C., and C. J. Dorman. 2007. H-NS antagonism in Shigella flexneri by VirB, a virulence gene transcription regulator that is closely related to plasmid partition factors. J. Bacteriol. 189:3403-3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 65.White-Ziegler, C. A., M. L. Angus Hill, B. A. Braaten, M. W. van der Woude, and D. A. Low. 1998. Thermoregulation of Escherichia coli pap transcription: H-NS is a temperature-dependent DNA methylation blocking factor. Mol. Microbiol. 28:1121-1137. [DOI] [PubMed] [Google Scholar]
- 66.Wickstrum, J. R., and S. M. Egan. 2004. Amino acid contacts between sigma 70 domain 4 and the transcription activators RhaS and RhaR. J. Bacteriol. 186:6277-6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Withey, J. H., and V. J. Dirita. 2005. Vibrio cholerae ToxT independently activates the divergently transcribed aldA and tagA genes. J. Bacteriol. 187:7890-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Withey, J. H., and V. J. DiRita. 2005. Activation of both acfA and acfD transcription by Vibrio cholerae ToxT requires binding to two centrally located DNA sites in an inverted repeat conformation. Mol. Microbiol. 56:1062-1077. [DOI] [PubMed] [Google Scholar]
- 69.Withey, J. H., and V. J. DiRita. 2006. The toxbox: specific DNA sequence requirements for activation of Vibrio cholerae virulence genes by ToxT. Mol. Microbiol. 59:1779-1789. [DOI] [PubMed] [Google Scholar]
- 70.Yang, J., M. Tauschek, R. Strugnell, and R. M. Robins-Browne. 2005. The H-NS protein represses transcription of the eltAB operon, which encodes heat-labile enterotoxin in enterotoxigenic Escherichia coli, by binding to regions downstream of the promoter. Microbiology 151:1199-1208. [DOI] [PubMed] [Google Scholar]
- 71.Yu, R. R., and V. J. DiRita. 1999. Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae. J. Bacteriol. 181:2584-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu, R. R., and V. J. DiRita. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119-134. [DOI] [PubMed] [Google Scholar]