Abstract
In filamentous fungi, secondary metabolism is often linked with developmental processes such as conidiation. In this study we analyzed the link between secondary metabolism and conidiation in the main industrial producer of the β-lactam antibiotic penicillin, the ascomycete Penicillium chrysogenum. Therefore, we generated mutants defective in two central regulators of conidiation, the transcription factors BrlA and StuA. Inactivation of either brlA or stuA blocked conidiation and altered hyphal morphology during growth on solid media, as shown by light and scanning electron microscopy, but did not affect biomass production during liquid-submerged growth. Genome-wide transcriptional profiling identified a complex StuA- and BrlA-dependent regulatory network, including genes previously shown to be involved in development and secondary metabolism. Remarkably, inactivation of stuA, but not brlA, drastically downregulated expression of the penicillin biosynthetic gene cluster during solid and liquid-submerged growth. In agreement, penicillin V production was wild-type-like in brlA-deficient strains but 99% decreased in stuA-deficient strains during liquid-submerged growth, as shown by high-performance liquid chromatography (HPLC) analysis. Thus, among identified regulators of penicillin V production StuA has the most severe influence. Overexpression of stuA increased the transcript levels of brlA and abaA (another developmental regulator) and derepressed conidiation during liquid-submerged growth but did not affect penicillin V productivity. Taken together, these data demonstrate an intimate but not exclusive link between regulation of development and secondary metabolism in P. chrysogenum.
The filamentous fungus Penicillium chrysogenum plays an important role in biotechnology as the main industrial producer of the β-lactam antibiotic penicillin, a secondary metabolite. A better understanding of parameters which directly or indirectly influence biosynthesis of penicillin is fundamental for optimizing industrial production of penicillin and possibly secondary metabolites in general. Previous studies revealed an association of fungal secondary metabolism with developmental processes such as asexual reproduction, termed conidiation (12). Both processes are associated with the stationary growth phase (3). In agreement, it recently has been shown that inactivation of either VelA or LaeA, both components of the velvet-like complex, decreases conidiation and penicillin production in P. chrysogenum (27). On the other hand, inactivation of the Gα subunit of a heterotrimeric G protein, Pga1, increases conidiation but decreases production of penicillin and the mycotoxin roquefortine, another secondary metabolite, in P. chrysogenum (19). Regulation of conidiation has been extensively studied in the ascomycete Aspergillus nidulans, but little is known about regulation of conidiation in P. chrysogenum. In response to nutrient sensing, A. nidulans conidia (spores) swell and form germ tubes and subsequently septated hyphae. After gaining so-called “developmental competence” and exposure to air, hyphae initiate conidiation, which starts with the growth of an aerial stalk followed by the formation of metulae and phialides. These phialides give rise to chains of uninucleate conidia (2). Notably, nutritionally sufficient submerged culture allows vegetative growth but represses conidiation in A. nidulans and P. chrysogenum.
Conidiogenesis is under complex genetic control. In A. nidulans, FluG (assumed to be associated with the production of a small diffusible molecule) and FlbA (regulator of G-protein signaling) are key upstream activators of conidiation, both acting via inhibition of the repressive Zn2C6-type zinc finger transcription factor SfgA and FadA (ortholog of P. chrysogenum Pga1), respectively (36-38, 57). Four additional downstream transcription factors, FlbB, FlbC, FlbD, and FlbE, individually are required for transcriptional activation of brlA and subsequent conidiation (20, 21, 75, 76). Furthermore, a Pcl-like cyclin (54) and the cyclin-dependent kinase encoded by nimXCdc2 of A. nidulans seem to mediate cell cycle events during developmental cell type formation (54, 55). BrlA together with AbaA and WetA forms a regulatory cascade, required for the formation of conidiophores and conidia. The transcription factors StuA and MedA are developmental modifiers required for correct conidiophore morphogenesis through spatial and temporal regulation of brlA and abaA expression (5, 39, 44). BrlA is a C2H2-type zinc finger transcription factor, which begins to accumulate shortly after induction of development (52). Two overlapping transcripts originate from the brlA locus, brlAα and brlAβ, both having essential functions for normal development (25). brlA-deficient mutants are blocked in development at an early stage in the transition from polar growth of the conidiophore stalk to the swelling of the vesicle. The stalks somehow elongate indeterminately and fail to differentiate, which gives the mutants a “bristle” appearance (14). Furthermore, the mutants fail to accumulate other developmentally regulated transcripts like abaA and wetA (9). Forced expression of brlA in submerged cultures is sufficient to transcriptionally activate abaA and wetA and to induce conidiation (1). StuA is a basic helix-loop-helix-like transcription factor with an APSES DNA-binding motif (17). Expression of stuA is upregulated with the onset of developmental competence and remains at that level during conidiation (41). Similarly to brlA expression, stuA expression results in two overlapping and differentially regulated transcripts. Conidiophores of stuA null mutants have shortened stalks and reduced vesicles and lack metulae and phialides. In contrast to brlA mutants, stuA mutants still produce few conidia, which directly bud from the vesicle as shown in A. nidulans and A. fumigatus (42, 58). In A. nidulans, deficiency in StuA, but not BrlA, also impairs sexual development (35, 78).
BrlA orthologs are present only in Aspergillus, Neosartorya, Penicillium, and Talaromyces species. In contrast, StuA is evolutionary widely conserved and orthologs have been shown to regulate development in A. fumigatus, Penicillium marneffei, Neurospora crassa, Fusarium oxysporum, Glomerella cingulata, Saccharomyces cerevisiae, and Candida albicans (6, 8, 22, 50, 58, 62, 67, 72). In Aspergillus fumigatus, 6 of 22 putative secondary metabolite-encoding gene clusters were found to be StuA dependent (15, 70), including clusters for biosynthesis of gliotoxin, pseurotin A, and the alkaloids fumigaclavin and fumitremorgin, whereas BrlA affects expression of only 2 of these gene clusters: fumigaclavin positively and fumitremorgin negatively. In A. nidulans, the synthesis of the mycotoxin sterigmatocystin is influenced positively by FluG and FlbA but negatively by FadA. In contrast, penicillin production is positively affected by FadA (26, 57, 65).
The genetic program controlling development in P. chrysogenum appears to be highly similar to that of aspergilli, as the P. chrysogenum genome encodes orthologs of all regulators identified in A. nidulans (Table 1). In this study, we demonstrate that in P. chrysogenum inactivation of either brlA or stuA blocks conidiation but affects expression of genes involved in development and secondary metabolism partially differently. In particular, penicillin production was found to be absolutely dependent on stuA but not brlA.
TABLE 1.
P. chrysogenum orthologs of genes previously implicated in conidiogenesis of A. nidulans or A. fumigatus
| Gene | ID | Species | P. chrysogenum gene | ID | e valuea | Reference |
|---|---|---|---|---|---|---|
| abaA | AAA33286 | A. nidulans | abaA | Pc16g09610 | 0.0 | 5 |
| abr1 | XP_756089.2 | A. fumigatus | abrA | Pc21g16380 | 0.0 | 68 |
| abr2 | ACJ13064.1 | A. fumigatus | yA | Pc22g08420 | 0.0 | 68 |
| alb1 | EAL94057 | A. fumigatus | wA | Pc21g16000 | 0.0 | 68 |
| arp1 | XP_756093.1 | A. fumigatus | arpA | Pc21g16420 | 4e-65 | 68 |
| arp2 | XP_756091.1 | A. fumigatus | arpB | Pc21g16430 | 7e-100 | 68 |
| asp f4 | XP_749515 | A. fumigatus | asp f4 | Pc16g11310 | 6e-77 | 16 |
| ayg1 | EDP55259.1 | A. fumigatus | aygA | Pc21g16440 | 6e-162 | 68 |
| brlA | AAB48671 | A. nidulans | brlA | Pc06g00470 | 2e-147 | 1 |
| catA | AAC49254 | A. nidulans | catA | Pc20g06360 | 0.0 | 45 |
| dewA | CBF73692 | A. nidulans | dewA | Pc16g06690 | 3e-17 | 64 |
| fadA | AAC49476 | A. nidulans | pga1 | Pc13g12240 | 0.0 | 19 |
| flbA | AAA73955 | A. nidulans | flbA | Pc15g00600 | 0.0 | 36 |
| flbB | CAM35586.1 | A. nidulans | flbB | Pc12g01640 | 7e-138 | 18 |
| flbC | ACP28867 | A. nidulans | flbC | Pc12g12190 | 5e-124 | 75 |
| flbD | AAA61913 | A. nidulans | flbD | Pc13g03170 | 5e-93 | 21 |
| flbE | ACP28868 | A. nidulans | flbE | Pc16g12460 | 2e-47 | 20 |
| fluG | AAC37414 | A. nidulans | fluG | Pc20g02420 | 1e-120 | 37 |
| laeA | AAQ95166 | A. nidulans | laeA | Pc16g14010 | 9e-134 | 7 |
| medA | AAC31205 | A. nidulans | medA | Pc22g13450 | 0.0 | 10 |
| nimX | AAA20597 | A. nidulans | nimX | Pc20g10270 | 7e-145 | 54 |
| pclA | CAC06384 | A. nidulans | pclA1 | Pc16g08700 | 1e-118 | 55 |
| pclA2 | Pc03g00090 | 8e-63 | ||||
| pclA3 | Pc22g26190 | 2e-69 | ||||
| phiA | CAA09585 | A. nidulans | phiA | Pc22g00190 | 3e-52 | 40 |
| ppoC | AAT36614 | A. nidulans | ppoC | Pc18g00240 | 0.0 | 69 |
| rodA | AAA33321 | A. nidulans | rodA | Pc22g14290 | 5e-52 | 63 |
| rodB | XP_753093.1 | A. fumigatus | rodB | Pc21g18350 | 2e-19 | 51 |
| sfgA | AAY99779 | A. nidulans | sfgA | Pc21g18560 | 3e-177 | 57 |
| stuA | CBF70741 | A. nidulans | stuA | Pc13g04920 | 9e-180 | 41 |
| tmpA | AAP13095 | A. nidulans | tmpA | Pc21g07830 | 2e-170 | 38 |
| veA | AAD42946 | A. nidulans | velA | Pc13g13200 | 2e-112 | 61 |
| velB | ABQ17967 | A. nidulans | velB | Pc22g22320 | 2e-77 | 11 |
| vosA | ABI51618 | A. nidulans | vosA | Pc22g06890 | 3e-104 | 46 |
| wetA | AAA33330 | A. nidulans | wetA | Pc22g03220 | 3e-138 | 39 |
Homology (at the amino acid level) of the P. chrysogenum gene product to the corresponding Aspergillus nidulans or Aspergillus fumigatus gene product.
MATERIALS AND METHODS
Strains and culture conditions.
Escherichia coli strain K-12 Fusion-Blue (recA endA) was used for plasmid construction (In-Fusion Dry-Down PCR cloning kit; Clontech). Reproduction and maintenance of plasmids were done according to standard protocols (53). P. chrysogenum carrying ΔPcku70 (herein referred to as the Δku70 mutant) (28), derived from P2niaD18 (herein referred to as the niaD18 mutant) carrying a mutated niaD gene (29), served as the parental strain in all transformation experiments (see Table S1 in the supplemental material). Additionally, most assays included the niaD18 mutant as an additional control. Fungal strains were cultivated in liquid complete CCM (43) or minimal MM1 medium for 48 to 120 h at 25°C and 230 rpm or on solid minimal medium MM2 as already described (23). For sporulation assays, strains were grown on solid complete medium CM2 [3.5 g/liter (NH4)2SO4, 2 g/liter K2SO4, 0.2 g/liter KH2PO4, 1 g/liter of soy flour, 5 g/liter limestone powder, 50 g/liter lactose, 20 g/liter agar] for transcript analysis on CM2 on sterile membranes (Supor-200; Pall Life Sciences, USA) or in liquid production medium (28). Cultures were inoculated with 5 × 104 spores for point inoculations on plates, with 2 × 106 spores for inoculation of whole plates (sporulation assay and microarrays) and 1 × 107 spores for liquid cultures. In studies including nonsporulating mutants, cultures were inoculated with 5 μl, 200 μl, and 500 μl of liquid 72-h CCM cultures for point inoculation, whole plate assays, and liquid cultures, respectively.
Transformation of P. chrysogenum.
Transformation was performed as described by Windhofer et al. (77) with some modifications. Liquid CCM medium cultures were incubated for 48 h at 25°C and 230 rpm. Protoplasts were transformed by linear PCR, restriction fragments, or circular plasmids 4.103, 4.104, 4.122, 4.127, 4.128, 4.196, 4.181, 4.182, and 4.177 (see Table S2 in the supplemental material). Transformants were selected on either terbinafine (0.9 μg/ml) or phleomycin (45 μg/ml) containing MM2 supplemented with 0.5 g/liter arginine and either 2.5% xylose or 2.5% glucose depending on the promoter used for the selection marker gene. Screening of positive clones was performed by PCR (data not shown). To obtain homokaryotic transformants, colonies from single homokaryotic spores were picked and homologous integration of the constructs was verified by Southern blot analysis.
Identification of P. chrysogenum genes stuA and brlA.
The genomic sequence of P. chrysogenum ATCC 28089 (71) served as a source for the sequences used here. The sequences for the P. chrysogenum genes brlA (CAP79040.1) and stuA (CAP91561.1) were obtained from the public database NCBI Entrez (http://www.ncbi.nlm.nih.gov/sites/gquery). Protein sequence alignments were performed with the ClustalW program (66).
Preparation of nucleic acids, hybridization protocol, and PCR.
Fungal genomic DNA for Southern blot analysis was isolated from hyphal cells, grown for 3 days at 25°C and 230 rpm in liquid CCM medium, based on protoplasting as previously described (80). RNA extraction for microarray and quantitative real-time PCR (qRT-PCR) analysis from mycelia grown on solid complete CM2 or in liquid production medium was done using Trizol reagent (no. 15596-018; Invitrogen), the RNeasy midikit (no. 75144; Qiagen) and the DNase I kit (no. 79254; Qiagen) for DNase I treatment according to manufacturer's protocols. For PCR analysis, transformants were grown on solid complete CM2 medium for 6 days at 25°C and mycelia or spores were washed off with H2O. A 5-μl portion of this suspension was used for DNA extraction with the REDExtract-N-Amp seed PCR kit (no. 086K6825; Sigma).
For clonings, PCR amplifications were performed with the Accu Prime Pfx DNA polymerase (no. 12344-024; Invitrogen) with “proofreading” activity, for screening approaches with the REDTaq DNA polymerase (no. R4775; Sigma), according to the manufacturer's instructions.
Southern hybridization was carried out with Duralon-UV membranes (no. 420101; Stratagene) and digoxigenin-labeled probes (PCR DIG probe synthesis kit, no. 11636090910; Roche). The attached probes were detected with the DIG luminescence detection kit (no. 11363514910; Roche). All techniques were used according to the method of Sambrook and Russell (53).
Quantification of penicillin (penicillin V) and biomass production.
For quantification of penicillin productivity, 5 g culture broth from shake flask cultures grown for 144 h at 25°C and 230 rpm in a production medium were analyzed by high-performance liquid chromatography (HPLC) (56). For quantification of biomass production, 5-ml portions of culture broth (grown as mentioned above) from each sample were filtered and dried for 18 h at 100°C.
Quantification of conidiation.
Spores (2 × 106) were spread on four independent CM2 plates per strain and incubated for 168 h at 25°C. Areas of 1 cm in diameter were cut out from each plate, pooled, and vortexed together with five small glass balls in 5 ml M3 (8.5 g/liter NaCl, 1 ml/liter Tween 80) for 1.5 min. Spores of each sample were counted three times in the Thoma chamber.
Microarray analysis.
The custom-designed Affymetrix GeneChip DSM_PENa520255F, representing the genome of P. chrysogenum Wisconsin54-1255, was used for gene expression quantification (71). RNA was isolated from the strains carrying the Δku70, ΔbrlA, and ΔstuA mutations after 36, 48, 60, 72, and 96 h of growth on solid complete CM2 plates separated from the medium by dialysis membranes. RNA of three biological replicates was pooled for sample preparation and microarray hybridization (28). Differentially expressed genes were determined using the R/Bioconductor packages limma and affylmGUI (60, 74); raw data were preprocessed using the implemented RMA algorithm (31, 32). Correlation analysis was performed using GeneSpring 7.2 (Silicon Genetics) and a correlation coefficient of ≥0.9. Heat maps were generated with the program TreeView by Michael Eisen (version 1.60; Howard Hughes Medical Institute, University of California at Berkeley [http://rana.lbl.gov/EisenSoftware.htm]).
Quantitative real-time PCR (qRT-PCR).
RNA was extracted from three biological replicates of the niaD18, Δku70, ΔbrlA and ΔstuA strains in a time course experiment on solid CM2 plates (see microarray analysis). Furthermore, from three biological replicates of the Δku70 and stuA-OE strains, RNA was isolated in a time course experiment in liquid production medium after 48, 72, 96, 120 and 144 h. From all RNA samples, cDNA was synthesized with the High-Capacity cDNA archive kit (no. 4322171; ABI). qRT-PCR was carried out with 4 to 8 ng/μl cDNA. Detection was based on the SYBR green I dye detection system (no. 4309155; ABI) and measured with a 7900HT Fast-Realtime PCR system (Applied Biosystems). qRT-PCR assays were performed in triplicate for each sample. Data analysis was performed according to the method of Nowrousian et al. (48). All values were normalized to the housekeeping gene actA (Pc20g11630) encoding γ-actin.
Scanning electron microscopy.
Strains were grown on solid complete CM2 plates at 25°C for 48 h and fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.3). A brief washing in the same buffer was followed by 1 h of postfixation with 1% aqueous OsO4, gradual dehydration with ethanol, and critical point drying with liquid CO2 (Bal-Tec CPD 030; Balzers, Liechtenstein). Specimens were mounted on aluminum stubs with Leit C (Göcke, Plano GmbH), sputtered with 10 nm Au/Pd (MED 020; Balzers, Liechtenstein), and examined with a Zeiss field emission scanning electron microscope (FESEM, Gemini 982).
RESULTS AND DISCUSSION
Identification of the P. chrysogenum stuA and brlA orthologs.
P. chrysogenum brlA and stuA were identified by homology searches as described in Materials and Methods. P. chrysogenum brlA encodes a protein of 433 amino acids showing 63% and 62% identity to the orthologs of A. nidulans and A. fumigatus, respectively (see Fig. S1 in the supplemental material). P. chrysogenum stuA encodes a protein of 818 amino acids. Alignment of the predicted P. chrysogenum StuA protein shows 62% to 67% identity with the corresponding published amino acid sequences of A. flavus, Neosartorya fischeri, A. nidulans, and A. fumigatus, with A. flavus StuA being the most similar ortholog. Interestingly, compared to the A. nidulans and A. fumigatus orthologs, which are annotated as proteins 622 and 635 amino acids in length, respectively, the annotated StuA orthologs of P. chrysogenum, A. flavus, and N. fischeri contain an “N-terminal extension” of about 200 amino acids. TBLASTN searches revealed that the amino acid sequence of the N-terminal extension also is highly conserved in the A. nidulans and A. fumigatus orthologs, indicating misannotation of these two StuA-encoding orthologs (see Fig. S2 in the supplemental material).
Generation of brlA and stuA deletion strains.
Deletion of either the brlA or the stuA locus was performed by replacement of the respective coding region with the terbinafine resistance gene ergA under the control of the xylose-inducible xylP promoter (59, 80) using the bipartite marker technique (47) in a Δku70 mutant (28), as described in Fig. S3A and B and “Generation of transformation fragments” in the supplemental materials. A total of 42 brlA knockout strains out of 221 transformants and 27 stuA knockout strains out of 50 transformants were verified by Southern blot analysis after reisolation. Southern blot analysis confirmed the homologous integration of the selection marker as well as the loss of the gene to be deleted (see Fig. S3A and B in the supplemental material). In conclusion, the homologous brlA and stuA recombination frequencies correspond to about 19 and 54%, respectively, clearly reinforcing the qualification of the P. chrysogenum Δku70 mutant as a valuable recipient for gene deletion experiments. For simplicity, only three deletants each of brlA and stuA were used for further analysis.
For reconstitution of the ΔbrlA mutant, a functional copy of the brlA gene was inserted ectopically. This was achieved by transforming the ΔbrlA mutant with plasmid p4.122 containing the brlA gene and the ergA selection marker gene driven by the P. chrysogenum gene γ-actin promoter (Pc20g11630). This promoter exchange enables a second selection on terbinafine with glucose instead of xylose as a carbon source in the selection medium, which represses the xylP-driven expression of ergA (80). Four transformants, designated brlA-kom, were proved to carry the brlA gene after single-spore reisolation and Southern blot analysis (see Fig. S3C in the supplemental material). For reconstitution, the ΔstuA strain was transformed with plasmid p4.196 carrying a functional copy of the stuA gene and the Tn5 phleomycin selection marker gene of E. coli driven by the ipnA promoter of P. chrysogenum. Three transformants were identified as positive complementation strains (designated stuA-kom), after single-spore reisolation and Southern blot analysis (see Fig. S3D). Interestingly, further PCR and Southern blot analyses (data not shown) demonstrated that in all three stuA-kom strains the entire plasmid p4.196 integrated homologously at the stuA locus by single crossover.
Inactivation of either brlA or stuA blocks conidiation and causes aberrant hyphal morphology.
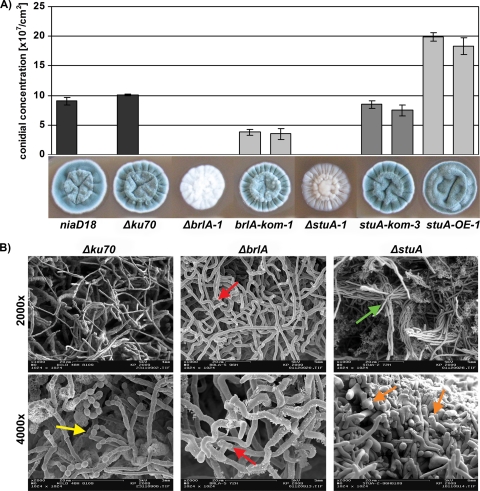
Primary transformants lacking brlA or stuA did not produce conidia and therefore had to be propagated in hyphal form. Growth tests starting with point inoculation of defined amounts of vegetative hyphae on CM2 plates confirmed that both ΔbrlA and ΔstuA strains are completely blocked in conidiation, as evident from visual (Fig. 1 A) and light microscopic inspection (data not shown). In contrast, stuA deficiency in both A. nidulans and A. fumigatus results in the production of extremely shortened conidiophores that lack metulae and phialides but produce conidia directly from buds formed on the conidiophore vesicle (2, 58).
FIG. 1.
Characterization of ΔbrlA and ΔstuA strains compared to the recipient Δku70 and niaD18 mutant strains. (A) Colony phenotypes on solid complete CM2 after growth for 168 h at 25°C. Quantification of conidial formation in mutant and complemented strains was performed in triplicate and repeated twice with two independent primary transformants of each strain; bars show standard deviations. (B) Scanning electron microscopy of ΔbrlA and ΔstuA mutants. Both mutants lack conidia and conidiophores (yellow arrow indicates conidia on conidiophores in the Δku70 mutant); the ΔbrlA strain displayed zigzag growth and increased and abnormal branching (red arrows); ΔstuA strain hyphae were bundled at the surface of the cultures (green arrow) and showed increased fragmentation (orange arrow).
The complemented brlA-kom and stuA-kom strains showed restored conidiation, demonstrating that the conidiation defect was indeed caused by the deletion of the respective gene. Interestingly, the ΔbrlA strain formed wild-type-like white mycelia whereas the ΔstuA mycelia were brown pigmented, indicating deregulation of pigment biosynthesis (see below).
Scanning electron microscopy of 48-h surface cultures visualized formation of conidiophores and conidia in the Δku70 mutant, while the ΔbrlA and ΔstuA mutants lacked these structures (Fig. 1B). Moreover, hyphae of the ΔbrlA mutant showed zigzag growth and hyperbranching; hyphae of the ΔstuA mutant congregated to thick bundles and displayed numerous stumps, possibly indicating increased susceptibility to fragmentation (Fig. 1B). Measurement of the biomass production after 144 h in submerged culture indicated that inactivation of either brlA or stuA has no significant effect on the vegetative growth rate (see Fig. S4 in the supplemental material).
Inactivation of either brlA or stuA decreases expression of developmental regulators.
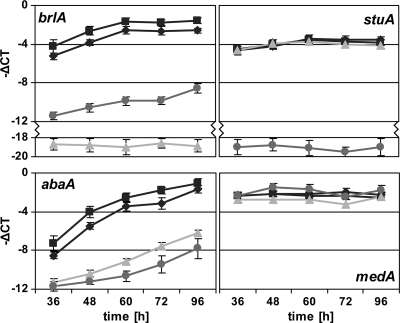
In a time course experiment, samples were taken after 36 h to 96 h of growth on solid medium. qRT-PCR measurements revealed an increase of brlA and abaA transcript levels, whereas stuA and medA levels remained largely constant in both Δku70 and niaD18 strains (Fig. 2). A difference in threshold cycle values (−ΔCT) of <−18 (i.e., a decrease of <18 in CT) confirmed the deletion of the respective genes in ΔbrlA and ΔstuA mutants (Fig. 2). Remarkably, brlA was severely downregulated in the ΔstuA mutant (−ΔCT of −8, equaling about 250-fold), while stuA transcript levels remained unaltered in the ΔbrlA mutant, which indicates that StuA regulates the brlA transcript level but not vice versa. Moreover, inactivation of either brlA or stuA decreased expression of abaA (−ΔCT of up to −6, equaling about 60-fold) but did not affect the medA transcript level. Interestingly, the brlA transcript level was still upregulated during the time course in the ΔstuA mutant and the abaA transcript level was still upregulated during the time course in the ΔstuA and ΔbrlA mutants, demonstrating that expression of both brlA and abaA is responsive to more than one regulatory mechanism. Taken together, these data reveal a regulatory cascade with BrlA activating expression of abaA and StuA activating expression of brlA and abaA during conidiogenesis, whereby StuA might influence abaA indirectly via BrlA.
FIG. 2.
Expression of brlA, stuA, abaA, and medA in niaD18, Δku70, ΔbrlA, and ΔstuA strains in a time course experiment measured by qRT-PCR. The results are given as means ± standard deviations of results for independent biological triplicates with technical triplicates. Black diamonds, niaD18; black squares, Δku70; light gray triangles, ΔbrlA; medium gray circles, ΔstuA.
Microarray analysis of ΔbrlA and ΔstuA mutants reveals a complex regulatory network.
In order to gain more insight into molecular changes associated with the gene deletions of brlA and stuA, the Δku70, ΔbrlA and ΔstuA mutant strains were compared by genome-wide transcriptome analysis. For a time course study, RNA was isolated from all strains grown on solid CM2 for 36, 48, 60, 72 and 96 h. Formation of conidia was observed after 48 h of growth for the Δku70 mutant but not the ΔbrlA and ΔstuA mutants. All strains showed similar radial growth rates, and therefore differences in the expression profiles of the two mutant strains are not expected to be caused by differences in growth rate.
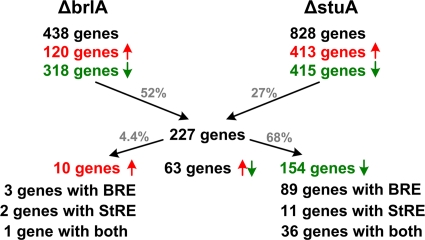
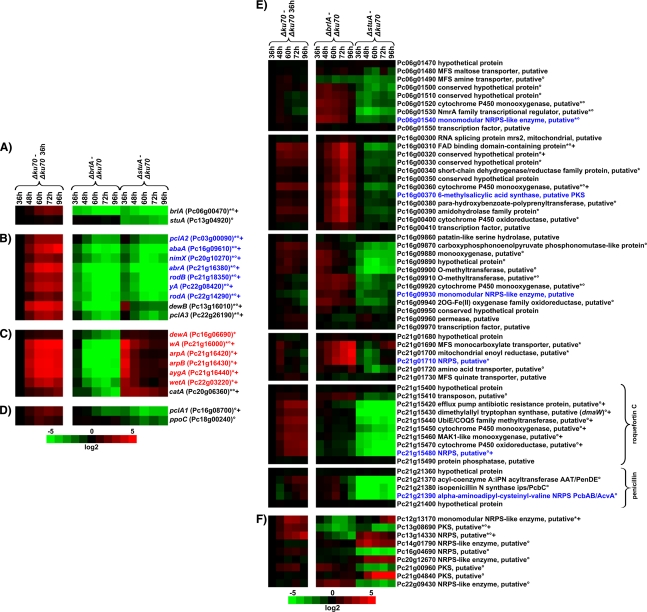
In the Δku70 strain, orthologs of 17 genes previously found to encode regulatory and structural genes upregulated during conidiogenesis in A. nidulans or A. fumigatus (2, 51, 68) were upregulated ≥3-fold at three or more time points compared to their status at the 36-h time point, e.g., the hydrophobin-encoding genes and the pigment biosynthetic genes (Table 1; see Fig. 4A to D). In contrast, transcript levels of stuA and medA did not significantly change during the time course. The microarray-based expression profiles of brlA, abaA, stuA, and medA nicely fit to the qRT-PCR analyses shown here (Fig. 2). A schematic summary of the transcription profiling of the Δku70, ΔbrlA, and ΔstuA mutants is shown in Fig. 3. Compared to the results for the Δku70 mutant, 438 genes were ≥3-fold deregulated at three or more consecutive time points in the ΔbrlA mutant, with 27% of these genes being upregulated and 73% being downregulated. In the ΔstuA mutant, 828 genes were deregulated, with 50% being upregulated and 50% being downregulated. A total of 227 deregulated genes are common to both ΔbrlA and ΔstuA mutants, a majority, 68%, of which were downregulated (see Table S4 in the supplemental material). Within 1 kb of the 5′ upstream region, 58% of these genes contain at least one BrlA response element (BRE) (MRAGGGR) (13), 7% contain at least one StuA response element (StRE) (WCGCGWNM) (17), and 23% contain both elements. Taking into account the downregulation of brlA by inactivation of stuA (see above), these data indicate that StuA might regulate the majority of these genes rather indirectly via BrlA. The genes downregulated in both ΔbrlA and ΔstuA mutants enclosed orthologs of nine genes, including abaA, previously found to be involved in conidiogenesis in A. nidulans (Table 1; Fig. 4 B). Gene expression correlation analysis indicated that 93 genes are coregulated with abaA (see Table S5 in the supplemental material), 85 of which are downregulated in both ΔbrlA and ΔstuA mutants, including seven of the nine orthologs previously identified to be involved in conidiogenesis in A. nidulans (Fig. 4B). Of the 154 genes downregulated in both ΔbrlA and ΔstuA mutants and of the 93 genes coregulated with abaA, 93.5% and 94.6%, respectively, contain at least one AbaA-responsive element (CATTCY, identified in A. nidulans [5]) within 1 kb of the 5′ upstream region. These data indicate that the majority of genes downregulated in the ΔbrlA and ΔstuA mutants and coregulated with abaA are regulated via AbaA. Interestingly, this gene set also includes an ortholog of Asp F4 (Table 1), an allergen of A. fumigatus (16), which indicates developmental regulation of allergen formation. Orthologs of seven other genes previously found to be involved in conidiogenesis in A. nidulans, including wetA, were downregulated in the ΔbrlA mutant but upregulated at early time points in the ΔstuA mutant (Table 1, Fig. 4C). Analysis of wetA coregulated genes identified six of these seven genes and additional 38 genes (Fig. 4C; see Table S6 in the supplemental material). This suggests that these genes are activated by BrlA (possibly via AbaA) but are temporally repressed by StuA, in both cases most likely via WetA. A proposed regulatory scheme is summarized in Fig. S5 in the supplemental material.
FIG. 3.
Schematic summary of the genome-wide comparative expression profiling of ΔbrlA and ΔstuA strains compared to the Δku70 strain. Genes were scored as deregulated when their expression was ≥3-fold changed at ≥3 consecutive time points compared to the Δku70 strain. BRE and StRE elements in forward, reverse, or both orientations within 1,000 bp upstream of the start codons were considered.
FIG. 4.
Heat map representation of the microarray analysis of ΔbrlA and ΔstuA mutants compared to the Δku70 mutant, including orthologs of genes previously implicated in conidiogenesis in A. nidulans or A. fumgatus and genes potentially involved in secondary metabolism. (A) brlA and stuA mutants. (B) Conidiogenesis-related genes downregulated in both ΔbrlA and ΔstuA mutants. (C) Conidiogenesis-related genes downregulated in the ΔbrlA mutant. (D) Conidiogenesis-related genes downregulated in ΔstuA mutant. (E) Gene clusters containing NRPS- or PKS-encoding genes deregulated in either the ΔbrlA or the ΔstuA mutant or both. (F) Individual NRPS- or PKS-encoding genes deregulated in either the ΔbrlA or ΔstuA mutant. The left column displays the time course expression in the Δku70 mutant, with all values normalized to the respective 36-h value; +, genes upregulated ≥3-fold at three or more consecutive time points compared to results at the 36-h time. The right column displays the change in transcript levels in ΔbrlA and ΔstuA mutants, respectively, normalized to the same time point as the Δku70 mutant. Values are shown in log 2 scale in green for downregulation and red for upregulation. * and °, genes deregulated ≥3-fold at three or more consecutive time points compared to Δku70 in ΔbrlA and ΔstuA strains, respectively. abaA coregulated and wetA coregulated genes are shown in blue and red, respectively. Gene clusters were regarded as deregulated when three or more genomically neighboring genes were ≥3-fold deregulated at three or more consecutive time points; gaps of single less-regulated genes were allowed for three or more neighboring genes; the heat map representation of regulated gene clusters includes the flanking unregulated genes. NRPS and PKS in panel E are shown in blue.
The derepression of genes encoding early steps of conidial melanin synthesis (wA, arpA, arpB, and aygA) combined with the repression of genes encoding late steps (abrA and yA) might explain the brownish pigmentation of the ΔstuA hyphae (Fig. 1A and 4B and C), in particular as deletion of abrA and yA changes the conidial color from green to brown in A. fumigatus (34, 68). In agreement, in the ΔbrlA mutant, all these pigment genes were repressed, resulting in white hyphae (Fig. 1A and 4B and C).
The 212 genes downregulated in the ΔstuA mutant but not the ΔbrlA mutant included pclA1 and ppoC (Fig. 4D). Interestingly, P. chrysogenum possesses three genes (pclA1, pclA2, and pclA3) homologous to the single pcl-like cyclin-encoding pclA of A. nidulans (55), whereas the three paralogs display different expression patterns (Fig. 4B and D). ppoC encodes a fatty acid oxygenase, contributing to the production of a secreted lipogenic signal molecule, that together with other factors (ppoA, ppoB) governs timing and balance of sexual and asexual development in A. nidulans (69).
Other genes implicated in conidiogenesis in A. nidulans were largely unaffected or affected ≤3-fold by inactivation of brlA and stuA, e.g., medA, phiA, sfgA, pga1, velA, laeA, and the ”fluffy” genes fluG, tmpA, flbA, flbB, flbC, flbD, and flbE (Table 1 and data not shown).
Similarly to P. chrysogenum, a complex regulatory network involving BrlA, StuA, AbaA, and WetA with several feedback loops controlling conidiogenesis has been characterized previously in A. nidulans and, in a more fragmentary form, in other fungal species (1, 8, 49, 58). The numerous genes identified in this study to be affected by deletion of brlA, stuA, or both, as well as the genes found to be coregulated with abaA and wetA, will now aid further elucidation of conidiogenesis of P. chrysogenum and other fungal species.
Inactivation of stuA or brlA affects expression of secondary metabolite-encoding genes.
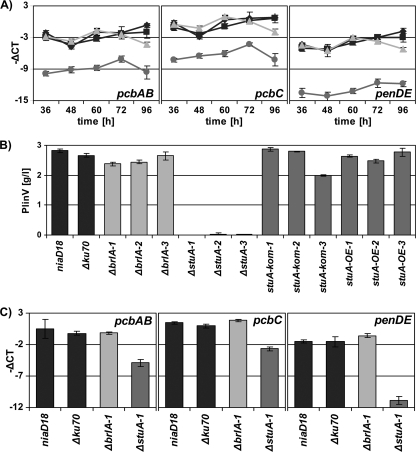
van den Berg et al. (71) identified 51 genes encoding putative nonribosomal peptide synthases (NRPS), polyketide synthases (PKS), or hybrid enzymes postulated to be part of 39 gene clusters. This indicates that P. chrysogenum has the potential to produce a variety of secondary metabolites. The genome-wide expression profiling revealed that inactivation of either BrlA or StuA deregulates expression ≥3-fold of six of these putative secondary metabolite-encoding clusters (for the cluster definition, see the legend to Fig. 4) and nine single genes encoding NRPS or PKS (Fig. 4E and F). Of these gene clusters, only one has been functionally characterized and shown to be responsible for biosynthesis of the β-lactam antibiotic penicillin. The cluster containing the putative NRPS Pc21g15480 and the tryptophan dimethylallyl transferase Pc21g15430 (dmaW) most likely encodes the biosynthesis of the mycotoxin roquefortine C (33, 71). Roquefortine C, a prenylated indole derivative containing tryptophan and histidine moieties, was isolated first from Penicillium roqueforti and identified later in many Penicillium strains, including P. chrysogenum. Roquefortine C is also the precursor for further prenylated indole alkaloids such as meleagrine, oxaline, and glandicolines (79). Among all StuA- or BrlA-regulated gene clusters, the pencillin and roquefortine C biosynthetic gene clusters were affected most significantly. Expression of both clusters was largely unaffected in the ΔbrlA strain but drastically downregulated in the ΔstuA strain (Fig. 4E). qRT-PCR analysis confirmed downregulation of the penicillin biosynthetic genes pcbAB (−ΔCT of −7, equaling about 130-fold), pcbC (−ΔCT of −7, equaling about 130-fold), and penDE (−ΔCT of −9, equaling about 500-fold) in the ΔstuA mutant but not the ΔbrlA mutant at all time points (Fig. 5 A).
FIG. 5.
Penicillin biosynthesis by ΔbrlA or ΔstuA. (A) qRT-PCR analysis of the expression of penicillin biosynthetic genes in a time course experiment. (B) Quantification of PENV production by HPLC analysis. Mean values ± standard deviations are derived from biological sextuplicates. (C) qRT-PCR analysis of the expression of the penicillin biosynthetic genes in panel B. The results are given as means ± standard deviations of three independent biological with three technical triplicates. Black diamonds, niaD1; black squares, Δku70; light gray triangles, ΔbrlA; medium gray circles, ΔstuA.
stuA but not brlA is essential for PENV production.
To further analyze the involvement of stuA and brlA in secondary metabolism, penicillin V (PENV) production was measured in liquid cultures. In agreement with expression patterns of PENV biosynthesis genes scored on solid medium, PENV production was wild-type-like in the ΔbrlA mutant and 99% reduced in the ΔstuA mutant (Fig. 5B). Complementation of ΔstuA with a functional stuA copy restored productivity to wild-type levels, reinforcing the crucial role of stuA in penicillin biosynthesis. qRT-PCR analysis confirmed that inactivation of stuA but not brlA blocks PENV production by transcriptional downregulation of the penicillin biosynthetic genes pcbAB, pcbC, and penDE in liquid medium cultures (Fig. 5C) as well as in solid medium cultures as shown above (Fig. 5A). These data also show that the developmental regulator StuA, which is essential for conidiation, also plays a role during submerged growth. StuA has the most severe influence on PENV production among all identified regulators yet. Recently, deficiency in LaeA and VelA was shown to decrease PENV production in the same P. chrysogenum strain grown under identical conditions by approximately 80% (27). In Aspergillus fumigatus, 6 of 22 putative secondary metabolite-encoding gene clusters were found to be StuA dependent (15, 70), including those for synthesis of gliotoxin, pseurotin A, and the alkaloids fumigaclavin and fumitremogen. Moreover, the StuA ortholog was found to play a crucial role in production of the mycotoxin alternariol in Stagnospora nodorum (30), underlining the impact of StuA orthologs on secondary metabolism.
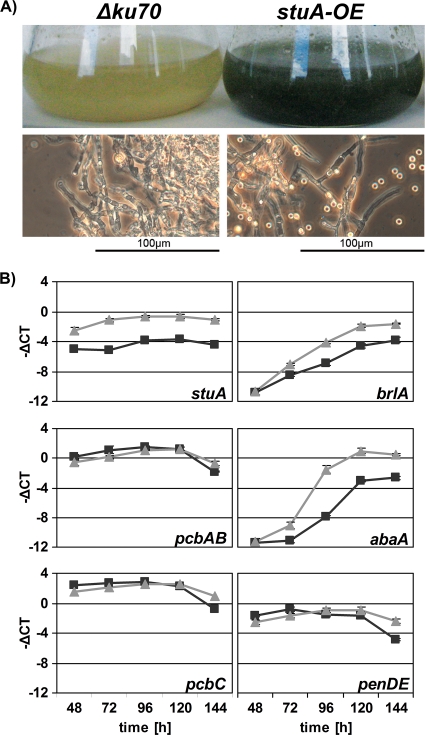
Overexpression of stuA derepresses conidiation during submerged growth but does not affect PENV production.
To further analyze the role of stuA in conidiogenesis and PENV production, stuA was overexpressed by replacement of its native promoter by the Pc22g-P promoter, which drives the constitutively highly expressed gene Pc22g10010 (28) in the Δku70 strain as described in “Generation of transformation fragments” in the supplemental material. Six of 13 transformants, termed stuA-OE, were proved, by single-spore reisolation and Southern blot analysis, to carry the promoter replacement (see Fig. S3E in the supplemental material). stuA-OE colonies appeared phenotypically identical on CM2 plates compared to the recipient strain but displayed an approximately doubled production of conidia (Fig. 1A). Remarkably, overexpression of stuA resulted in heavy conidiation during submerged growth (Fig. 6 A), causing dark green staining of the culture broth. qRT-PCR demonstrated that the promoter replacement indeed caused overexpression of stuA, up to 4 −ΔCT units, equaling about 16-fold (Fig. 6B). Moreover, qRT-PCR analyses proved that stuA overexpression causes upregulation of brlA (−ΔCT of up to 3, equaling about 8-fold) and abaA (−ΔCT of up to 6, equaling about 60-fold), which underlines the positive role of stuA in regulation of brlA and abaA identified in the stuA deletion analysis (see above). Consequently, the induction of conidiation during submerged growth might be due to upregulation of stuA, brlA, abaA, or a combination. In A. nidulans, overexpression of either fluG, flbA, tmpA, flbC, or flbD but not flbB was shown to induce brlA expression and conidiation in submerged cultures (2, 18, 38). Moreover, overexpression of brlA induces the expression of abaA and wetA and conidiation during submerged growth in A. nidulans (1, 24), while overexpression of abaA, stuA, and wetA does not result in conidiation (17, 24, 39). In further contrast to results with P. chrysogenum, forced expression of stuA in submerged cultures leads to repression of abaA in A. nidulans, indicating differences despite the general conservation of the regulatory system in these two species.
FIG. 6.
(A) stuA overexpression derepresses conidiation in submerged growth in minimal medium. MM1-containing flasks were inoculated with Δku70 and stuA-OE-1 strains and incubated for 120 h at 25°C. (B) Expression of penicillin biosynthetic genes, stuA, brlA, and abaA in stuA-OE-1 and Δku70 strains, measured by qRT-PCR. The results are given as means ± standard deviations of independent biological triplicates with technical triplicates. Black squares, Δku70; light gray triangles, stuA-OE-1.
In contrast to conidiation, stuA overexpression did not influence PENV production (Fig. 5B). In agreement, the transcript levels of the penicillin biosynthesis genes pcbAB, pcbC, and penDE were similar in stuA-OE and Δku70 strains (Fig. 6B).
Analysis of a putative StuA-binding site in the penDE promoter.
A search in the promoter regions of pcbAB, pcbC, and penDE strains for a putative StuA response element (StRE) (WCGCGWNM) identified in A. nidulans (17) revealed a single putative StRE 50 bp upstream of the penDE start codon. In order to analyze its functionality, StuA-binding activity was inactivated by mutation of two nucleotides within the core sequence, as described previously in A. nidulans (17). Native and mutated penDE promoters were fused to the E. coli uidA reporter gene and integrated at the Pcku70 locus using the terbinafine resistance gene ergA as a selection marker, driven by the acnP promoter, as described in “Generation of transformation fragments” in the supplemental material. After single-spore reisolation, Southern blot analysis demonstrated that 3 out of 9 transformants carried the reporter constructs with the native promoter (strain GUS) and 9 out of 27 transformants with the mutated promoter (strain GUS-m) (see Fig. S3F in the supplemental material). Notably, growth rate, conidiation, and penicillin production were wild-type-like in all reporter strains, indicating that the transformation procedure did not result in side effects (data not shown). qRT-PCR analyses did not reveal differences in the expression of uidA in the reporter strains carrying the native and mutated penDE promoter (see Fig. S6 in the supplemental material), indicating either that the putative StuA-binding site is not functional or that the performed mutation did not inactivate StuA binding.
Conclusion.
Taken together, these data indicate an intimate but not exclusive link between regulation of conidiation and secondary metabolism in P. chrysogenum. Inactivation of stuA was demonstrated to block both conidiation and PENV production. Consistently, deficiency in LaeA and VelA was recently shown to decrease conidiation and PENV production in the same P. chrysogenum strain grown under identical conditions by 80% (27), underlining the link between development and secondary metabolism. On the other hand, inactivation of the Gα subunit of a heterotrimeric G protein, Pga1, increases conidiation but decreases production of penicillin and roquefortine C (19), and inactivation of brlA blocks conidiation but does not affect PENV biosynthesis in P. chrysogenum (see above).
Supplementary Material
Acknowledgments
We thank Monika Handler, Georg Sprenger, Inga Schulte, and Karoline Rupp for their excellent technical assistance. Furthermore, we thank Markus Schrettl, Rudolf Mitterbauer, Birgit Hoff, Ulrich Kück, and Heiko Eichhorn for their interest and support.
This work was funded by Sandoz GmbH (Kundl, Austria).
Footnotes
Published ahead of print on 10 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adams, T. H., M. T. Boylan, and W. E. Timberlake. 1988. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54:353-362. [DOI] [PubMed] [Google Scholar]
- 2.Adams, T. H., J. K. Wieser, and J. H. Yu. 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62:35-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams, T. H., and J. H. Yu. 1998. Coordinate control of secondary metabolite production and asexual sporulation in Aspergillus nidulans. Curr. Opin. Microbiol. 1:674-677. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Andrianopoulos, A., and W. E. Timberlake. 1994. The Aspergillus nidulans abaA gene encodes a transcriptional activator that acts as a genetic switch to control development. Mol. Cell. Biol. 14:2503-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aramayo, R., Y. Peleg, R. Addison, and R. Metzenberg. 1996. Asm-1+, a Neurospora crassa gene related to transcriptional regulators of fungal development. Genetics 144:991-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayram, O., et al. 2008. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320:1504-1506. [DOI] [PubMed] [Google Scholar]
- 8.Borneman, A. R., M. J. Hynes, and A. Andrianopoulos. 2002. A basic helix-loop-helix protein with similarity to the fungal morphological regulators, Phd1p, Efg1p and StuA, controls conidiation but not dimorphic growth in Penicillium marneffei. Mol. Microbiol. 44:621-631. [DOI] [PubMed] [Google Scholar]
- 9.Boylan, M. T., P. M. Mirabito, C. E. Willett, C. R. Zimmerman, and W. E. Timberlake. 1987. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans. Mol. Cell. Biol. 7:3113-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busby, T. M., K. Y. Miller, and B. L. Miller. 1996. Suppression and enhancement of the Aspergillus nidulans medusa mutation by altered dosage of the bristle and stunted genes. Genetics 143:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo, A. M. 2008. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 45:1053-1061. [DOI] [PubMed] [Google Scholar]
- 12.Calvo, A. M., R. A. Wilson, J. W. Bok, and N. P. Keller. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang, Y. C., and W. E. Timberlake. 1993. Identification of Aspergillus BrlA response elements (BREs) by genetic selection in yeast. Genetics 133:29-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clutterbuck, A. J. 1969. A mutational analysis of conidial development in Aspergillus nidulans. Genetics 63:317-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle, C. M., S. C. Kenaley, W. R. Rittenour, and D. G. Panaccione. 2007. Association of ergot alkaloids with conidiation in Aspergillus fumigatus. Mycologia 99:804-811. [DOI] [PubMed] [Google Scholar]
- 16.Crameri, R., and K. Blaser. 1996. Cloning Aspergillus fumigatus allergens by the pJuFo filamentous phage display system. Int. Arch. Allergy Immunol. 110:41-45. [DOI] [PubMed] [Google Scholar]
- 17.Dutton, J. R., S. Johns, and B. L. Miller. 1997. StuAp is a sequence-specific transcription factor that regulates developmental complexity in Aspergillus nidulans. EMBO J. 16:5710-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etxebeste, O., et al. 2008. Basic-zipper-type transcription factor FlbB controls asexual development in Aspergillus nidulans. Eukaryot. Cell 7:38-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Rico, R. O., et al. 2008. The heterotrimeric G alpha protein Pga1 regulates biosynthesis of penicillin, chrysogenin and roquefortine in Penicillium chrysogenum. Microbiology 154:3567-3578. [DOI] [PubMed] [Google Scholar]
- 20.Garzia, A., et al. 2009. Aspergillus nidulans FlbE is an upstream developmental activator of conidiation functionally associated with the putative transcription factor FlbB. Mol. Microbiol. 71:172-184. [DOI] [PubMed] [Google Scholar]
- 21.Garzia, A., O. Etxebeste, E. Herrero-Garcia, U. Ugalde, and E. A. Espeso. 2010. The concerted action of bZip and cMyb transcription factors FlbB and FlbD induces brlA expression and asexual development in Aspergillus nidulans. Mol. Microbiol. 75:1314-1324. [DOI] [PubMed] [Google Scholar]
- 22.Gimeno, C. J., and G. R. Fink. 1994. Induction of pseudohyphal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol. Cell. Biol. 14:2100-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas, H., K. Albermann, I. Zadra, and G. Stöffler. 1997. Overexpression of nreB, a new GATA factor-encoding gene of Penicillium chrysogenum, leads to repression of the nitrate assimilatory gene cluster. J. Biol. Chem. 272:22576-22582. [DOI] [PubMed] [Google Scholar]
- 24.Han, S., and T. H. Adams. 2001. Complex control of the developmental regulatory locus brlA in Aspergillus nidulans. Mol. Genet. Genomics 266:260-270. [DOI] [PubMed] [Google Scholar]
- 25.Han, S., J. Navarro, R. A. Greve, and T. H. Adams. 1993. Translational repression of brlA expression prevents premature development in Aspergillus. EMBO J. 12:2449-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicks, J. K., J. H. Yu, N. P. Keller, and T. H. Adams. 1997. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 16:4916-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoff, B., et al. 2010. Two components of a velvet-like complex control hyphal morphogenesis, conidiophore development and penicillin biosynthesis in Penicillium chrysogenum. Eukaryot. Cell 9:1236-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoff, B., J. Kamerewerd, C. Sigl, I. Zadra, and U. Kück. 2010. Homologous recombination in the antibiotic producer Penicillium chrysogenum: strain Delta Pcku70 shows up-regulation of genes from the HOG pathway. Appl. Microbiol. Biotechnol. 85:1081-1094. [DOI] [PubMed] [Google Scholar]
- 29.Hoff, B., S. Pöggeler, and U. Kück. 2008. Eighty years after its discovery, Fleming's Penicillium strain discloses the secret of its sex. Eukaryot. Cell 7:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IpCho, S. V. S., et al. 2010. The transcription factor SnStuA regulates central carbon metabolism, mycotoxin production and effector gene expression in the wheat pathogen Stagonospora nodorum. Eukaryot. Cell 9:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irizarry, R. A., et al. 2003. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irizarry, R. A., et al. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249-264. [DOI] [PubMed] [Google Scholar]
- 33.Kosalkova, K., et al. 2009. The global regulator LaeA controls penicillin biosynthesis, pigmentation and sporulation, but not roquefortine C synthesis in Penicillium chrysogenum. Biochimie 91:214-225. [DOI] [PubMed] [Google Scholar]
- 34.Langfelder, K., M. Streibel, B. Jahn, G. Haase, and A. A. Brakhage. 2003. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38:143-158. [DOI] [PubMed] [Google Scholar]
- 35.Lara-Ortiz, T., H. Riveros-Rosas, and J. Aguirre. 2003. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 50:1241-1255. [DOI] [PubMed] [Google Scholar]
- 36.Lee, B. N., and T. H. Adams. 1994. Overexpression of flbA, an early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Mol. Microbiol. 14:323-334. [DOI] [PubMed] [Google Scholar]
- 37.Lee, B. N., and T. H. Adams. 1996. fluG and flbA function interdependently to initiate conidiophore development in Aspergillus nidulans through brlAβ activation. EMBO J. 15:299-309. [PMC free article] [PubMed] [Google Scholar]
- 38.Mah, J. H., and J. H. Yu. 2006. Upstream and downstream regulation of asexual development in Aspergillus fumigatus. Eukaryot. Cell 5:1585-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall, M. A., and W. E. Timberlake. 1991. Aspergillus nidulans WetA activates spore-specific gene expression. Mol. Cell. Biol. 11:55-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melin, P., J. Schnurer, and E. G. Wagner. 2003. Characterization of phiA, a gene essential for phialide development in Aspergillus nidulans. Fungal Genet. Biol. 40:234-241. [DOI] [PubMed] [Google Scholar]
- 41.Miller, K. Y., T. M. Toennis, T. H. Adams, and B. L. Miller. 1991. Isolation and transcriptional characterization of a morphological modifier: the Aspergillus nidulans stunted (stuA) gene. Mol. Gen. Genet. 227:285-292. [DOI] [PubMed] [Google Scholar]
- 42.Miller, K. Y., J. Wu, and B. L. Miller. 1992. StuA is required for cell pattern formation in Aspergillus. Genes Dev. 6:1770-1782. [DOI] [PubMed] [Google Scholar]
- 43.Minuth, W., P. Tudzynski, and K. Esser. 1982. Extrachromosomal genetics of Cephalosporium acremonium. Curr. Genet. 25:34-40. [DOI] [PubMed] [Google Scholar]
- 44.Mirabito, P. M., T. H. Adams, and W. E. Timberlake. 1989. Interactions of three sequentially expressed genes control temporal and spatial specificity in Aspergillus development. Cell 57:859-868. [DOI] [PubMed] [Google Scholar]
- 45.Navarro, R. E., M. A. Stringer, W. Hansberg, W. E. Timberlake, and J. Aguirre. 1996. catA, a new Aspergillus nidulans gene encoding a developmentally regulated catalase. Curr. Genet. 29:352-359. [PubMed] [Google Scholar]
- 46.Ni, M., and J. H. Yu. 2007. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One 2:e970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen, M. L., L. Albertsen, G. Lettier, J. B. Nielsen, and U. H. Mortensen. 2006. Efficient PCR-based gene targeting with a recyclable marker for Aspergillus nidulans. Fungal Genet. Biol. 43:54-64. [DOI] [PubMed] [Google Scholar]
- 48.Nowrousian, M., C. Ringelberg, J. C. Dunlap, J. L. Loros, and U. Kück. 2005. Cross-species microarray hybridization to identify developmentally regulated genes in the filamentous fungus Sordaria macrospora. Mol. Genet. Genomics 273:137-149. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa, M., M. Tokuoka, F. J. Jin, T. Takahashi, and Y. Koyama. 2010. Genetic analysis of conidiation regulatory pathways in koji-mold Aspergillus oryzae. Fungal Genet. Biol. 47:10-18. [DOI] [PubMed] [Google Scholar]
- 50.Ohara, T., and T. Tsuge. 2004. FoSTUA, encoding a basic helix-loop-helix protein, differentially regulates development of three kinds of asexual spores, macroconidia, microconidia, and chlamydospores, in the fungal plant pathogen Fusarium oxysporum. Eukaryot. Cell 3:1412-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paris, S., et al. 2003. Conidial hydrophobins of Aspergillus fumigatus. Appl. Environ. Microbiol. 69:1581-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prade, R. A., and W. E. Timberlake. 1993. The Aspergillus nidulans brlA regulatory locus consists of overlapping transcription units that are individually required for conidiophore development. EMBO J. 12:2439-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 54.Schier, N., and R. Fischer. 2002. The Aspergillus nidulans cyclin PclA accumulates in the nucleus and interacts with the central cell cycle regulator NimXCdc2. FEBS Lett. 523:143-146. [DOI] [PubMed] [Google Scholar]
- 55.Schier, N., R. Liese, and R. Fischer. 2001. A Pcl-like cyclin of Aspergillus nidulans is transcriptionally activated by developmental regulators and is involved in sporulation. Mol. Cell. Biol. 21:4075-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt, E. K., et al. 2004. Winged helix transcription factor CPCR1 is involved in regulation of beta-lactam biosynthesis in the fungus Acremonium chrysogenum. Eukaryot. Cell 3:121-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seo, J. A., Y. J. Guan, and J. H. Yu. 2006. FluG-dependent asexual development in Aspergillus nidulans occurs via derepression. Genetics 172:1535-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheppard, D. C., et al. 2005. The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol. Biol. Cell 16:5866-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sigl, C., M. Handler, G. Sprenger, H. Kürnsteiner, and I. Zadra. 2010. A novel homologous dominant selection marker for genetic transformation of Penicillium chrysogenum: overexpression of squalene epoxidase-encoding ergA. J. Biotechnol. 150:307-311. [DOI] [PubMed] [Google Scholar]
- 60.Smyth, G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:article 3. [DOI] [PubMed] [Google Scholar]
- 61.Spröte, P., and A. A. Brakhage. 2007. The light-dependent regulator velvet A of Aspergillus nidulans acts as a repressor of the penicillin biosynthesis. Arch. Microbiol. 188:69-79. [DOI] [PubMed] [Google Scholar]
- 62.Stoldt, V. R., A. Sonneborn, C. E. Leuker, and J. F. Ernst. 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stringer, M. A., R. A. Dean, T. C. Sewall, and W. E. Timberlake. 1991. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 5:1161-1171. [DOI] [PubMed] [Google Scholar]
- 64.Stringer, M. A., and W. E. Timberlake. 1995. DewA encodes a fungal hydrophobin component of the Aspergillus spore wall. Mol. Microbiol. 16:33-44. [DOI] [PubMed] [Google Scholar]
- 65.Tag, A., et al. 2000. G-protein signalling mediates differential production of toxic secondary metabolites. Mol. Microbiol. 38:658-665. [DOI] [PubMed] [Google Scholar]
- 66.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tong, X. Z., et al. 2007. GcSTUA, an APSES transcription factor, is required for generation of appressorial turgor pressure and full pathogenicity of Glomerella cingulata. Mol. Plant Microbe Interact. 20:1102-1111. [DOI] [PubMed] [Google Scholar]
- 68.Tsai, H. F., M. H. Wheeler, Y. C. Chang, and K. J. Kwon-Chung. 1999. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 181:6469-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsitsigiannis, D., T. M. Kowieski, R. Zarnowski, and N. P. Keller. 2004. Endogenous lipogenic regulators of spore balance in Aspergillus nidulans. Eukaryot. Cell 3:1398-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Twumasi-Boateng, K., et al. 2009. Transcriptional profiling identifies a role for BrlA in the response to nitrogen depletion and for StuA in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot. Cell 8:104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van den Berg, M. A., et al. 2008. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat. Biotechnol. 26:1161-1168. [DOI] [PubMed] [Google Scholar]
- 72.Ward, M. P., C. J. Gimeno, G. R. Fink, and S. Garrett. 1995. SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol. Cell. Biol. 15:6854-6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reference deleted.
- 74.Wettenhall, J. M., K. M. Simpson, K. Satterley, and G. K. Smyth. 2006. affylmGUI: a graphical user interface for linear modeling of single channel microarray data. Bioinformatics 22:897-899. [DOI] [PubMed] [Google Scholar]
- 75.Wieser, J., and T. H. Adams. 1995. flbD encodes a Myb-like DNA-binding protein that coordinates initiation of Aspergillus nidulans conidiophore development. Genes Dev. 9:491-502. [DOI] [PubMed] [Google Scholar]
- 76.Wieser, J., B. N. Lee, J. Fondon III, and T. H. Adams. 1994. Genetic requirements for initiating asexual development in Aspergillus nidulans. Curr. Genet. 27:62-69. [DOI] [PubMed] [Google Scholar]
- 77.Windhofer, F., K. Hauck, D. E. Catcheside, U. Kück, and F. Kempken. 2002. Ds-like restless deletion derivates occur in Tolypocladium inflatum and two foreign hosts, Neurospora crassa and Penicillium chrysogenum. Fungal Genet. Biol. 35:171-182. [DOI] [PubMed] [Google Scholar]
- 78.Wu, J. G., and B. L. Miller. 1997. Aspergillus asexual reproduction and sexual reproduction are differentially affected by transcriptional and translational mechanisms regulating stunted gene expression. Mol. Cell. Biol. 17:6191-6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yin, W.-B., A. Grundmann, J. Cheng, and S.-M. Li. 2009. Acetylaszonalenin biosynthesis in Neosartorya fischeri: identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J. Biol. Chem. 284:100-109. [DOI] [PubMed] [Google Scholar]
- 80.Zadra, I., B. Abt, W. Parson, and H. Haas. 2000. xylP promoter-based expression system and its use for antisense downregulation of the Penicillium chrysogenum nitrogen regulator NRE. Appl. Environ. Microbiol. 66:4810-4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.