Abstract
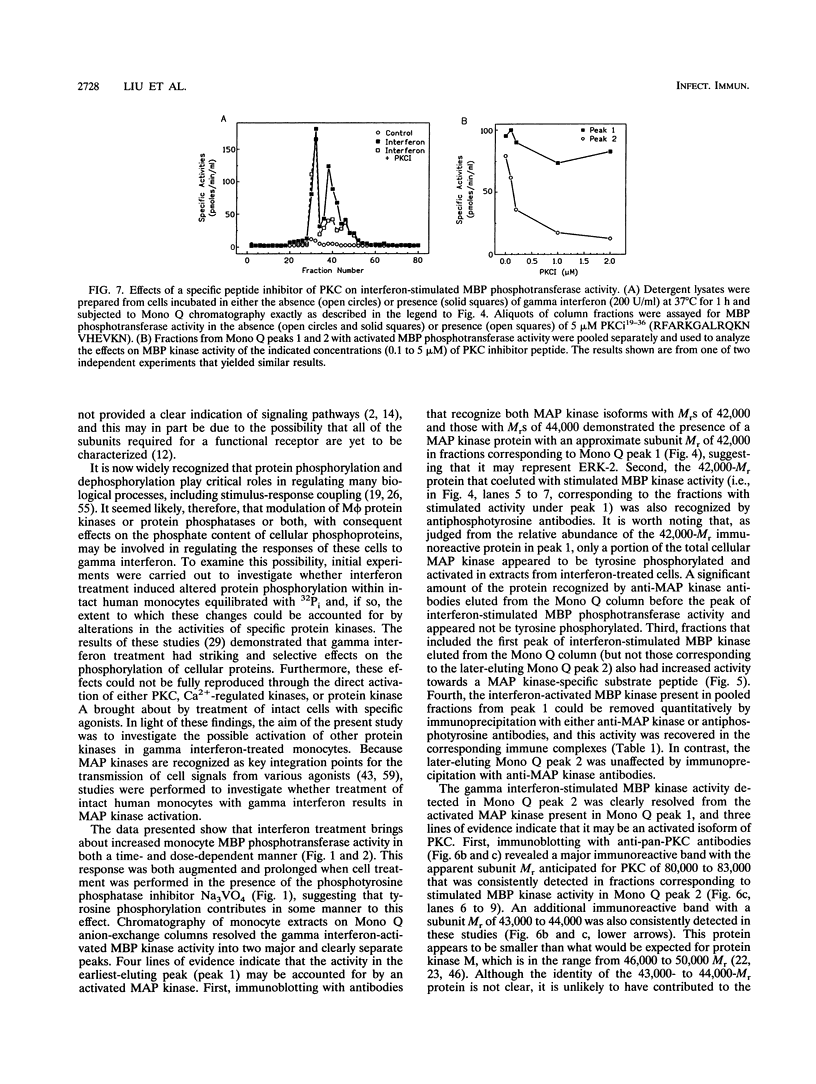
Gamma interferon plays an important role in regulating the functional properties of mononuclear phagocytes. In the present study, the role of activated protein kinases in the mechanism of action of gamma interferon cell signaling in human peripheral blood monocytes was investigated. Analysis in vitro of 100,000 x g cytosolic fractions from untreated and interferon-treated cells showed that agonist treatment resulted in time- and concentration-dependent increases in phosphotransferase activity when myelin basic protein (MBP) was used as the substrate. Anion-exchange chromatography of high-speed supernatants prepared from detergent extracts of interferon-treated cells revealed two discrete peaks of MBP phosphotransferase activity. Immunoblotting of fractions from these peaks with antiphosphotyrosine antibodies and with antibodies that specifically recognize the family of mitogen-activated protein (MAP) kinases detected a MAP kinase with a subunit M(r) of 42,000 in the earliest-eluting peak (peak 1). Phosphorylation of the 42,000-M(r) protein on tyrosine was observed only after treatment of cells with interferon. The contribution of MAP kinase to the interferon-stimulated activity in peak 1 was confirmed by quantitative immunoprecipitation with anti-MAP kinase and antiphosphotyrosine antibodies. The conclusion that the interferon-activated MBP kinase in peak 1 could be accounted for by an activated MAP kinase was also supported by the finding that fractions from Mono Q peak 1 demonstrated activity towards a MAP kinase-specific substrate. The later-eluting peak of interferon-activated MBP phosphotransferase activity appeared to be accounted for by an activated protein kinase C (PKC). This conclusion is based upon analyses of immunoblotting and immunoprecipitation experiments with antibodies to PKC and was also supported by the observed inhibition of this kinase with a PKC pseudosubstrate peptide. The interferon-stimulated PKC present in Mono Q peak 2 was active in the absence of calcium ions, suggesting that it is a calcium-independent isoform of PKC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Aguet M., Dembić Z., Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell. 1988 Oct 21;55(2):273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brickell P. M. The c-src family of protein-tyrosine kinases. Int J Exp Pathol. 1991 Feb;72(1):97–108. [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Receptor tyrosine kinase substrates: src homology domains and signal transduction. FASEB J. 1992 Nov;6(14):3283–3289. doi: 10.1096/fasebj.6.14.1385243. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Csermely P., Balint E., Grimley P. M., Aszalos A. Protein kinase C is involved in the early signals of interferon-alpha but not of interferon-gamma in U937 cells. J Interferon Res. 1990 Dec;10(6):605–611. doi: 10.1089/jir.1990.10.605. [DOI] [PubMed] [Google Scholar]
- Eilers A., Seegert D., Schindler C., Baccarini M., Decker T. The response of gamma interferon activation factor is under developmental control in cells of the macrophage lineage. Mol Cell Biol. 1993 Jun;13(6):3245–3254. doi: 10.1128/mcb.13.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. D., Goldberg M., Bloom B. R. Interferon-gamma-induced transcriptional activation is mediated by protein kinase C. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5122–5125. doi: 10.1073/pnas.85.14.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbloom D. S., Wahl L. M., Winestock K. D. The receptor for interferon-gamma on human peripheral blood monocytes consists of multiple distinct subunits. J Biol Chem. 1991 Nov 25;266(33):22545–22548. [PubMed] [Google Scholar]
- Grabarek J., Ware J. A. Protein kinase C activation without membrane contact in platelets stimulated by bryostatin. J Biol Chem. 1993 Mar 15;268(8):5543–5549. [PubMed] [Google Scholar]
- Gray P. W., Leong S., Fennie E. H., Farrar M. A., Pingel J. T., Fernandez-Luna J., Schreiber R. D. Cloning and expression of the cDNA for the murine interferon gamma receptor. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8497–8501. doi: 10.1073/pnas.86.21.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M., Hachiya T., Watanabe M., Usuda N., Iida F., Tamai K., Hidaka H. Assessment of protein kinase C isozymes by enzyme immunoassay and overexpression of type II in thyroid adenocarcinoma. Cancer Res. 1990 Sep 1;50(17):5515–5519. [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Hug H., Sarre T. F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993 Apr 15;291(Pt 2):329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Jayaraman S., Mensi N., Webb D. R., Dorf M. E. Involvement of protein kinase C in competence induction of macrophages to generate T suppressor cells. J Immunol. 1991 Jun 15;146(12):4085–4091. [PubMed] [Google Scholar]
- Kikkawa U., Ogita K., Ono Y., Asaoka Y., Shearman M. S., Fujii T., Ase K., Sekiguchi K., Igarashi K., Nishizuka Y. The common structure and activities of four subspecies of rat brain protein kinase C family. FEBS Lett. 1987 Nov 2;223(2):212–216. doi: 10.1016/0014-5793(87)80291-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi H., Imajoh-Ohmi S., Kanegasaki S. Novel antibodies specific for proteolyzed forms of protein kinase C: production of anti-peptide antibodies available for in situ analysis of intracellular limited proteolysis. Biochim Biophys Acta. 1993 Mar 5;1162(1-2):171–176. doi: 10.1016/0167-4838(93)90144-g. [DOI] [PubMed] [Google Scholar]
- Kishimoto A. Limited proteolysis of protein kinase C by calpain, its possible implication. Adv Second Messenger Phosphoprotein Res. 1990;24:472–477. [PubMed] [Google Scholar]
- Kiyotaki C., Bloom B. R. Activation of murine macrophage cell lines. Possible involvement of protein kinases in stimulation of superoxide production. J Immunol. 1984 Aug;133(2):923–931. [PubMed] [Google Scholar]
- Klein J. B., Schepers T. M., Dean W. L., Sonnenfeld G., McLeish K. R. Role of intracellular calcium concentration and protein kinase C activation in IFN-gamma stimulation of U937 cells. J Immunol. 1990 Jun 1;144(11):4305–4311. [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Powers E. A., Ruff V. A., Jaken S., Kaufmann S. Type 3 protein kinase C localization to the nuclear envelope of phorbol ester-treated NIH 3T3 cells. J Cell Biol. 1989 Aug;109(2):685–695. doi: 10.1083/jcb.109.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage M., Frith D., Livneh E., Stabel S. Protein kinase C group B members PKC-delta, -epsilon, -zeta and PKC-L(eta). Comparison of properties of recombinant proteins in vitro and in vivo. Biochem J. 1992 May 1;283(Pt 3):781–787. doi: 10.1042/bj2830781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay R. J., Russell S. W. Protein changes associated with stages of activation of mouse macrophages for tumor cell killing. J Immunol. 1986 Aug 15;137(4):1392–1398. [PubMed] [Google Scholar]
- Martelli A. M., Neri L. M., Gilmour R. S., Barker P. J., Huskisson N. S., Manzoli F. A., Cocco L. Temporal changes in intracellular distribution of protein kinase C in Swiss 3T3 cells during mitogenic stimulation with insulin-like growth factor I and bombesin: translocation to the nucleus follows rapid changes in nuclear polyphosphoinositides. Biochem Biophys Res Commun. 1991 May 31;177(1):480–487. doi: 10.1016/0006-291x(91)92009-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi H., Exton J. H. Purification and characterization of the zeta isoform of protein kinase C from bovine kidney. J Biol Chem. 1992 Aug 15;267(23):16347–16354. [PubMed] [Google Scholar]
- Nakano M., Saito S., Nakano Y., Yamasu H., Matsuura M., Shinomiya H. Intracellular protein phosphorylation in murine peritoneal macrophages in response to bacterial lipopolysaccharide (LPS): effects of kinase-inhibitors and LPS-induced tolerance. Immunobiology. 1993 Apr;187(3-5):272–282. doi: 10.1016/S0171-2985(11)80344-X. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Schultz G. Tyrosine phosphorylation is an early and requisite signal induced by interferon-gamma in HL-60 cells. FEBS Lett. 1992 Oct 5;310(3):260–264. doi: 10.1016/0014-5793(92)81344-l. [DOI] [PubMed] [Google Scholar]
- Olivier M., Brownsey R. W., Reiner N. E. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7481–7485. doi: 10.1073/pnas.89.16.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–6932. [PubMed] [Google Scholar]
- Pazin M. J., Williams L. T. Triggering signaling cascades by receptor tyrosine kinases. Trends Biochem Sci. 1992 Oct;17(10):374–378. doi: 10.1016/0968-0004(92)90003-r. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Charest D. L., Howard S. L., Paddon H. B., Salari H. Protein kinase C activation by platelet-activating factor is independent of enzyme translocation. Biochim Biophys Acta. 1990 Jan 23;1051(1):100–107. doi: 10.1016/0167-4889(90)90179-h. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Samiei M., Charest D. L., Howard S. L., Salari H. Characterization of calcium-independent forms of protein kinase C-beta in phorbol ester-treated rabbit platelets. J Biol Chem. 1991 May 15;266(14):8696–8705. [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S., Daya-Makin M. Protein kinase cascades in meiotic and mitotic cell cycle control. Biochem Cell Biol. 1990 Dec;68(12):1297–1330. doi: 10.1139/o90-194. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992 Jun;17(6):233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- Politis A. D., Vogel S. N. Pharmacologic evidence for the requirement of protein kinase C in IFN-induced macrophage Fc gamma receptor and Ia antigen expression. J Immunol. 1990 Dec 1;145(11):3788–3795. [PubMed] [Google Scholar]
- Pontremoli S., Michetti M., Melloni E., Sparatore B., Salamino F., Horecker B. L. Identification of the proteolytically activated form of protein kinase C in stimulated human neutrophils. Proc Natl Acad Sci U S A. 1990 May;87(10):3705–3707. doi: 10.1073/pnas.87.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Nairn A. C., Greengard P., Cohn Z. A., Aderem A. Bacterial lipopolysaccharide regulates the phosphorylation of the 68K protein kinase C substrate in macrophages. J Biol Chem. 1989 Jun 5;264(16):9118–9121. [PubMed] [Google Scholar]
- Ryu K., Koide Y., Yamashita Y., Yoshida T. O. Inhibition of tyrosine phosphorylation prevents IFN-gamma-induced HLA-DR molecule expression. J Immunol. 1993 Feb 15;150(4):1253–1262. [PubMed] [Google Scholar]
- Samelson L. E., Klausner R. D. Tyrosine kinases and tyrosine-based activation motifs. Current research on activation via the T cell antigen receptor. J Biol Chem. 1992 Dec 15;267(35):24913–24916. [PubMed] [Google Scholar]
- Sanghera J. S., Aebersold R., Morrison H. D., Bures E. J., Pelech S. L. Identification of the sites in myelin basic protein that are phosphorylated by meiosis-activated protein kinase p44mpk. FEBS Lett. 1990 Oct 29;273(1-2):223–226. doi: 10.1016/0014-5793(90)81090-b. [DOI] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Sengupta A., Liu W. K., Yeung Y. G., Yeung D. C., Frackelton A. R., Jr, Stanley E. R. Identification and subcellular localization of proteins that are rapidly phosphorylated in tyrosine in response to colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8062–8066. doi: 10.1073/pnas.85.21.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S. Control of cell function by reversible protein phosphorylation. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(7):531–541. [PubMed] [Google Scholar]
- Shuai K., Schindler C., Prezioso V. R., Darnell J. E., Jr Activation of transcription by IFN-gamma: tyrosine phosphorylation of a 91-kD DNA binding protein. Science. 1992 Dec 11;258(5089):1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- Smith M. K., Colbran R. J., Soderling T. R. Specificities of autoinhibitory domain peptides for four protein kinases. Implications for intact cell studies of protein kinase function. J Biol Chem. 1990 Feb 5;265(4):1837–1840. [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Trilivas I., McDonough P. M., Brown J. H. Dissociation of protein kinase C redistribution from the phosphorylation of its substrates. J Biol Chem. 1991 May 5;266(13):8431–8438. [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways D. K., Cook P. P., Webster C., Parker P. J. Effect of phorbol esters on protein kinase C-zeta. J Biol Chem. 1992 Mar 5;267(7):4799–4805. [PubMed] [Google Scholar]
- Weiel J. E., Hamilton T. A., Adams D. O. LPS induces altered phosphate labeling of proteins in murine peritoneal macrophages. J Immunol. 1986 Apr 15;136(8):3012–3018. [PubMed] [Google Scholar]
- Weinstein S. L., Gold M. R., DeFranco A. L. Bacterial lipopolysaccharide stimulates protein tyrosine phosphorylation in macrophages. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4148–4152. doi: 10.1073/pnas.88.10.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S. L., Sanghera J. S., Lemke K., DeFranco A. L., Pelech S. L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992 Jul 25;267(21):14955–14962. [PubMed] [Google Scholar]