Abstract
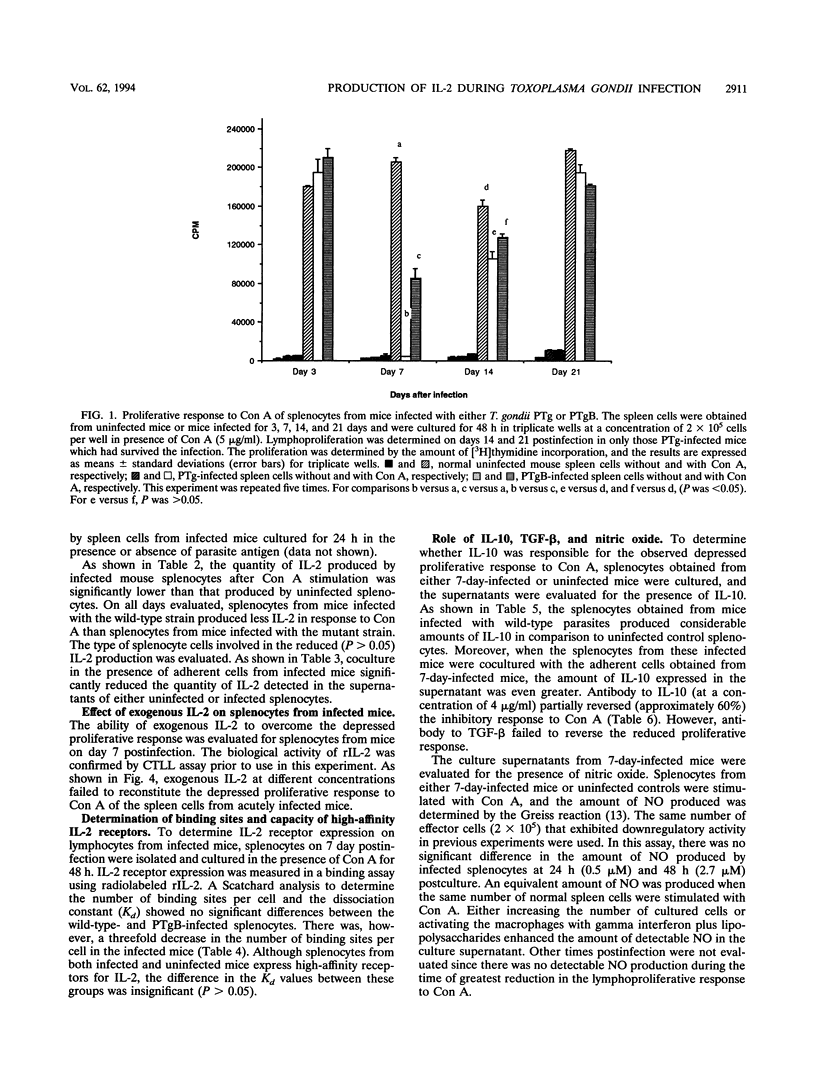
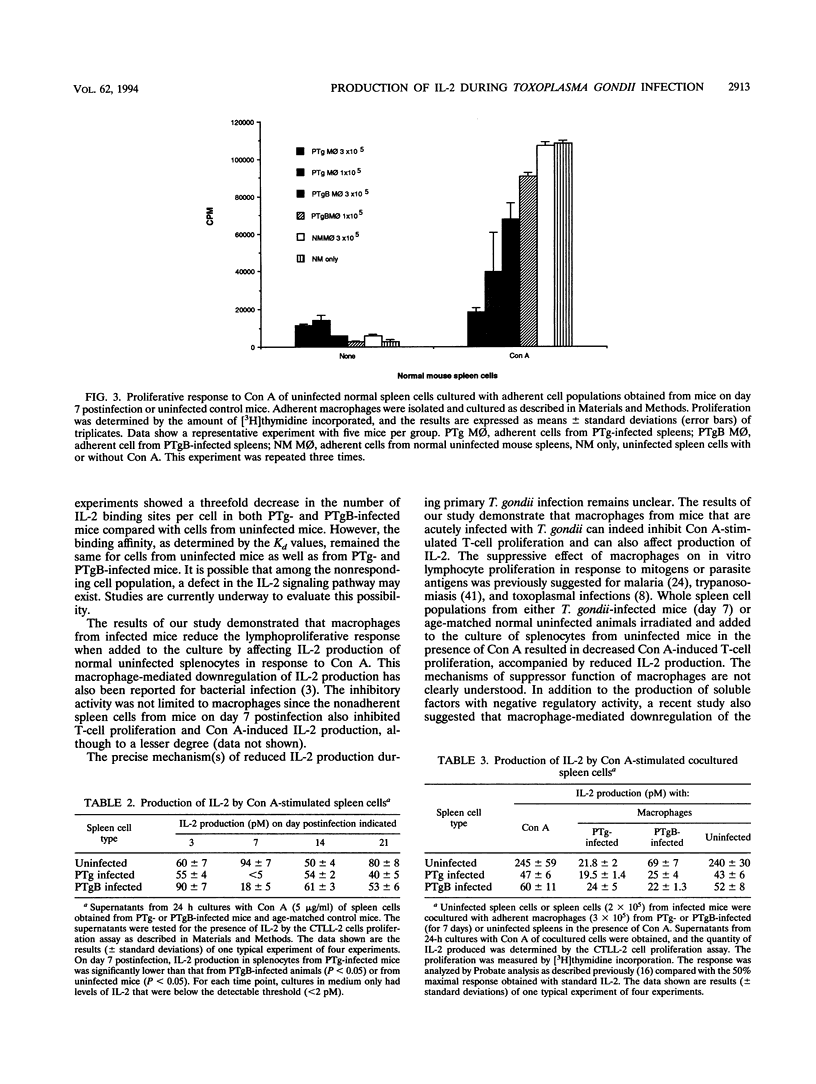
Depression of the cellular immune response to Toxoplasma gondii has been reported in both mice and humans. The present study was undertaken to determine the kinetics and mechanism of the observed downregulation of interleukin 2 (IL-2) production during experimental murine toxoplasmosis. For these investigations, the cell-mediated immune response to the wild type (PTg) was compared with that to the less-virulent mutant parasite (PTgB), which is deficient in the major surface antigen, p30 (SAG-1). Spleen cells from infected A/J mice failed to proliferate in response to Toxoplasma antigens during the first week of infection. Both PTg- and PTgB-infected A/J mice exhibited a significant reduction in the concanavalin A (Con A)-induced lymphoproliferative response. Further, the response of splenocytes from mice infected with the wild-type parasite was significantly diminished compared with that of mice infected with PTgB. The lymphoproliferative response to Con A reached its nadir at day 7 and remained below control levels for at least 14 days postinfection. By day 21 postinfection, the response to Con A and to Toxoplasma antigens was restored to the level observed prior to day 7. Con A-stimulated culture supernatants of spleen cells from mice on day 7 postinfection contained significantly less IL-2 than normal mice. There was no significant difference in the numbers of binding sites or capacity of high-affinity IL-2 receptors between infected and normal mouse splenocytes as determined by Scatchard analysis. Exogenous IL-2 at different concentrations failed to restore the proliferative response of lymphocytes from infected mice to Con A. Adherent macrophages from 7-day-infected mice were able to suppress IL-2 production by normal splenocytes following stimulation with Con A. The inhibitory activity mediated by infected cells was reversed by the antibody to IL-10 but not transforming growth factor beta. There were insignificant levels of nitric oxide production in both infected and normal splenocytes. These results indicate that during acute murine toxoplasmosis, there is a well-defined period (day 7) during which both the T-cell mitogen and parasite antigen-associated lymphoproliferative response are reduced. Further, there is a reduction in the production of IL-2 and an increase in IL-10, which appear to mediate, in part, the observed downregulation of immunity to T. gondii.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Albina J. E., Abate J. A., Henry W. L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991 Jul 1;147(1):144–148. [PubMed] [Google Scholar]
- Anderson S. E., Jr, Krahenbuhl J. L., Remington J. S. Longitudinal studies of lymphocyte response to Toxoplasma antigen in humans infected with T. gondii. J Clin Lab Immunol. 1979 Nov;2(4):293–297. [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Mencacci A., Spaccapelo R., Schiaffella E., Puccetti P., Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993 May;23(5):1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- Cerrone M. C., Ritter D. M., Kuhn R. E. Effect of antigen-specific T helper cells or interleukin-2 on suppressive ability of macrophage subsets detected in spleens of Trypanosoma cruzi-infected mice as determined by limiting dilution-partition analysis. Infect Immun. 1992 Apr;60(4):1489–1498. doi: 10.1128/iai.60.4.1489-1498.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Siegel J. P., Luft B. J. Demonstration of T-cell dysfunction during acute toxoplasma infection. Cell Immunol. 1986 Apr 1;98(2):422–433. doi: 10.1016/0008-8749(86)90301-1. [DOI] [PubMed] [Google Scholar]
- Chantry D., Turner M., Abney E., Feldmann M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989 Jun 15;142(12):4295–4300. [PubMed] [Google Scholar]
- Chizzolini C., Geinoz A., Schrijvers D. Interleukin-2 reverses T cell unresponsiveness to Plasmodium falciparum-antigen in malaria immune subjects. Cell Immunol. 1990 Jun;128(1):1–10. doi: 10.1016/0008-8749(90)90001-8. [DOI] [PubMed] [Google Scholar]
- Choromanski L., Kuhn R. E. Use of parasite antigens and interleukin-2 to enhance suppressed immune responses during Trypanosoma cruzi infection in mice. Infect Immun. 1987 Feb;55(2):403–408. doi: 10.1128/iai.55.2.403-408.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Shevach E. M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992 May 15;148(10):3133–3139. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Gazzinelli R. T., Hartley J. W., Fredrickson T. N., Chattopadhyay S. K., Sher A., Morse H. C., 3rd Opportunistic infections and retrovirus-induced immunodeficiency: studies of acute and chronic infections with Toxoplasma gondii in mice infected with LP-BM5 murine leukemia viruses. Infect Immun. 1992 Oct;60(10):4394–4401. doi: 10.1128/iai.60.10.4394-4401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L. H., Crabb J. H., Pfefferkorn E. R. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol. 1983 May;130(5):2407–2412. [PubMed] [Google Scholar]
- Kasper L. H., Currie K. M., Bradley M. S. An unexpected response to vaccination with a purified major membrane tachyzoite antigen (P30) of Toxoplasma gondii. J Immunol. 1985 May;134(5):3426–3431. [PubMed] [Google Scholar]
- Kasper L. H. Isolation and characterization of a monoclonal anti-P30 antibody resistant mutant of Toxoplasma gondii. Parasite Immunol. 1987 Jul;9(4):433–445. doi: 10.1111/j.1365-3024.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Kasper L. H., Khan I. A., Ely K. H., Buelow R., Boothroyd J. C. Antigen-specific (p30) mouse CD8+ T cells are cytotoxic against Toxoplasma gondii-infected peritoneal macrophages. J Immunol. 1992 Mar 1;148(5):1493–1498. [PubMed] [Google Scholar]
- Kasper L. H., Khan I. A. Role of P30 in host immunity and pathogenesis of T. gondii infection. Res Immunol. 1993 Jan;144(1):45–48. doi: 10.1016/s0923-2494(05)80097-5. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Rose G., Playfair J. H. Changes in the capacity of macrophages and T cells to produce interleukins during murine malaria infection. Cell Immunol. 1984 Apr 1;84(2):253–263. doi: 10.1016/0008-8749(84)90097-2. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Kansas G., Engleman E. G., Remington J. S. Functional and quantitative alterations in T lymphocyte subpopulations in acute toxoplasmosis. J Infect Dis. 1984 Nov;150(5):761–767. doi: 10.1093/infdis/150.5.761. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992 Aug;15(2):211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- McLeod R., Beem M. O., Estes R. G. Lymphocyte anergy specific to Toxoplasma gondii antigens in a baby with congenital toxoplasmosis. J Clin Lab Immunol. 1985 Jul;17(3):149–153. [PubMed] [Google Scholar]
- McLeod R., Eisenhauer P., Mack D., Brown C., Filice G., Spitalny G. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J Immunol. 1989 May 1;142(9):3247–3255. [PubMed] [Google Scholar]
- Mills C. D. Molecular basis of "suppressor" macrophages. Arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991 Apr 15;146(8):2719–2723. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Santoli D., Phillips P. D., Colt T. L., Zurier R. B. Suppression of interleukin 2-dependent human T cell growth in vitro by prostaglandin E (PGE) and their precursor fatty acids. Evidence for a PGE-independent mechanism of inhibition by the fatty acids. J Clin Invest. 1990 Feb;85(2):424–432. doi: 10.1172/JCI114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saron M. F., Colle J. H., Dautry-Varsat A., Truffa-Bachi P. Activated T lymphocytes from mice infected by lymphocytic choriomeningitis virus display high affinity IL-2 receptors but do not proliferate in response to IL-2. J Immunol. 1991 Dec 15;147(12):4333–4337. [PubMed] [Google Scholar]
- Saron M. F., Shidani B., Nahori M. A., Guillon J. C., Truffa-Bachi P. Lymphocytic choriomeningitis virus-induced immunodepression: inherent defect of B and T lymphocytes. J Virol. 1990 Sep;64(9):4076–4083. doi: 10.1128/jvi.64.9.4076-4083.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. W., Mansfield J. M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993 Nov 15;151(10):5492–5503. [PubMed] [Google Scholar]
- Silva J. S., Twardzik D. R., Reed S. G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J Exp Med. 1991 Sep 1;174(3):539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong L., Tarleton R. L. Selective suppressive effects of Trypanosoma cruzi infection on IL-2, c-myc, and c-fos gene expression. J Immunol. 1992 Sep 15;149(6):2095–2102. [PubMed] [Google Scholar]
- Strickland G. T., Ahmed A., Sell K. W. Blastogenic response of Toxoplasma-infected mouse spleen cells to T- and B-cell mitogens. Clin Exp Immunol. 1975 Oct;22(1):167–176. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Kobayashi A. Macrophage-mediated suppression of immune responses in Toxoplasma-infected mice. I. Inhibition of proliferation of lymphocytes in primary antibody responses. Cell Immunol. 1984 May;85(2):417–427. doi: 10.1016/0008-8749(84)90255-7. [DOI] [PubMed] [Google Scholar]
- Taga K., Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol. 1992 Feb 15;148(4):1143–1148. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wong H. L., Dougherty S., McCartney-Francis N., Wahl L. M., Ellingsworth L., Schmidt J. A., Hall G., Roberts A. B. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988 May 1;140(9):3026–3032. [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren H. S., Pembrey R. G. Human allogeneic responses: lymphokine requirement for the in vitro generation of specific cytotoxic responses to a malignant melanoma cell line. Aust J Exp Biol Med Sci. 1982 Apr;60(Pt 2):215–217. doi: 10.1038/icb.1982.25. [DOI] [PubMed] [Google Scholar]
- al-Ramadi B. K., Chen Y. W., Meissler J. J., Jr, Eisenstein T. K. Immunosuppression induced by attenuated Salmonella. Reversal by IL-4. J Immunol. 1991 Sep 15;147(6):1954–1961. [PubMed] [Google Scholar]


