Abstract
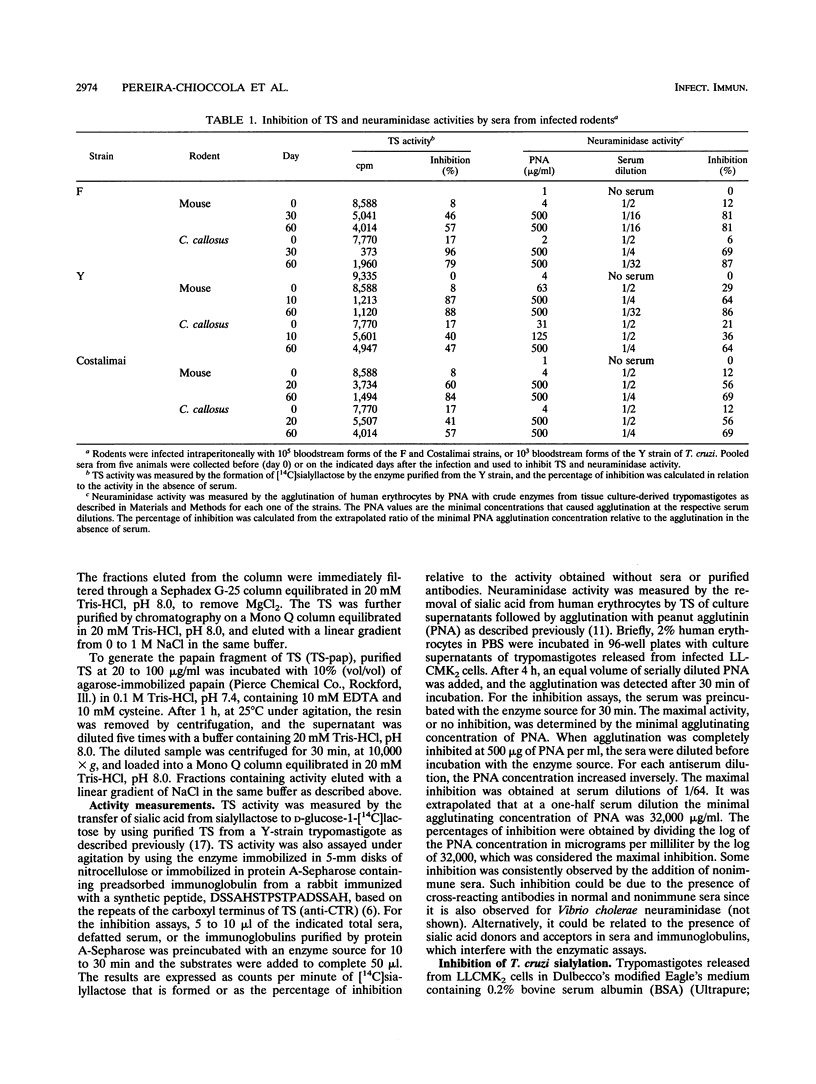
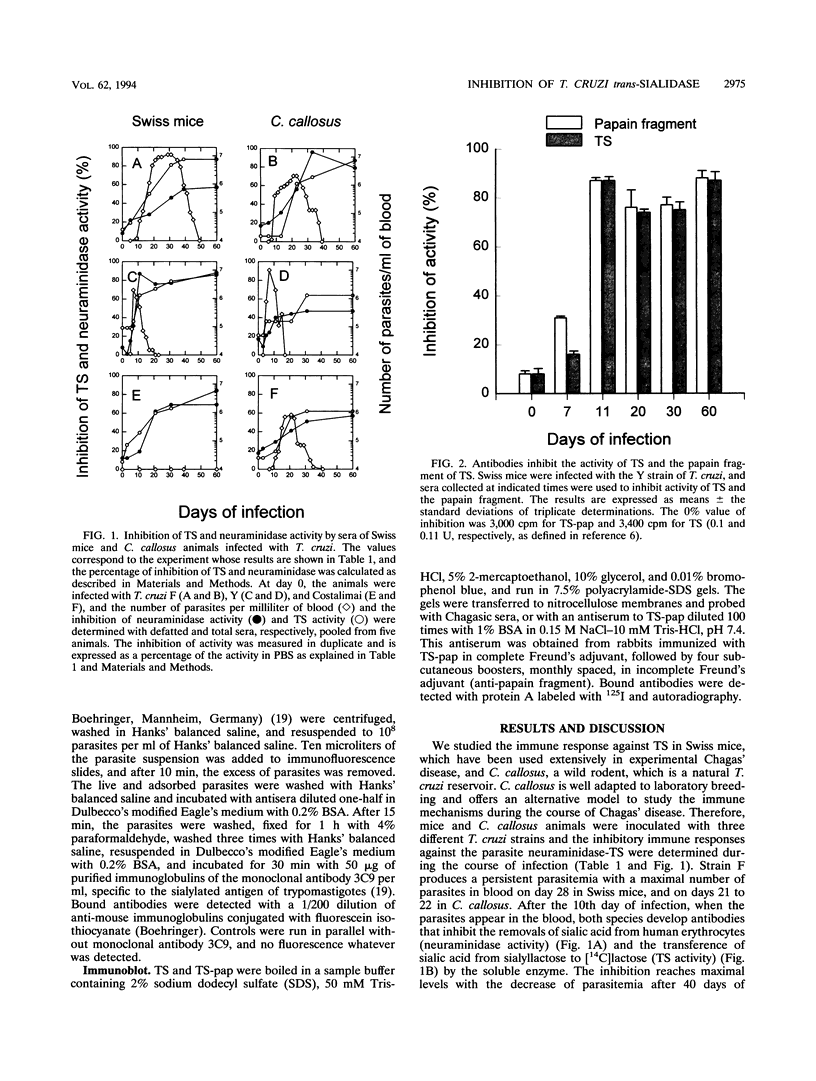
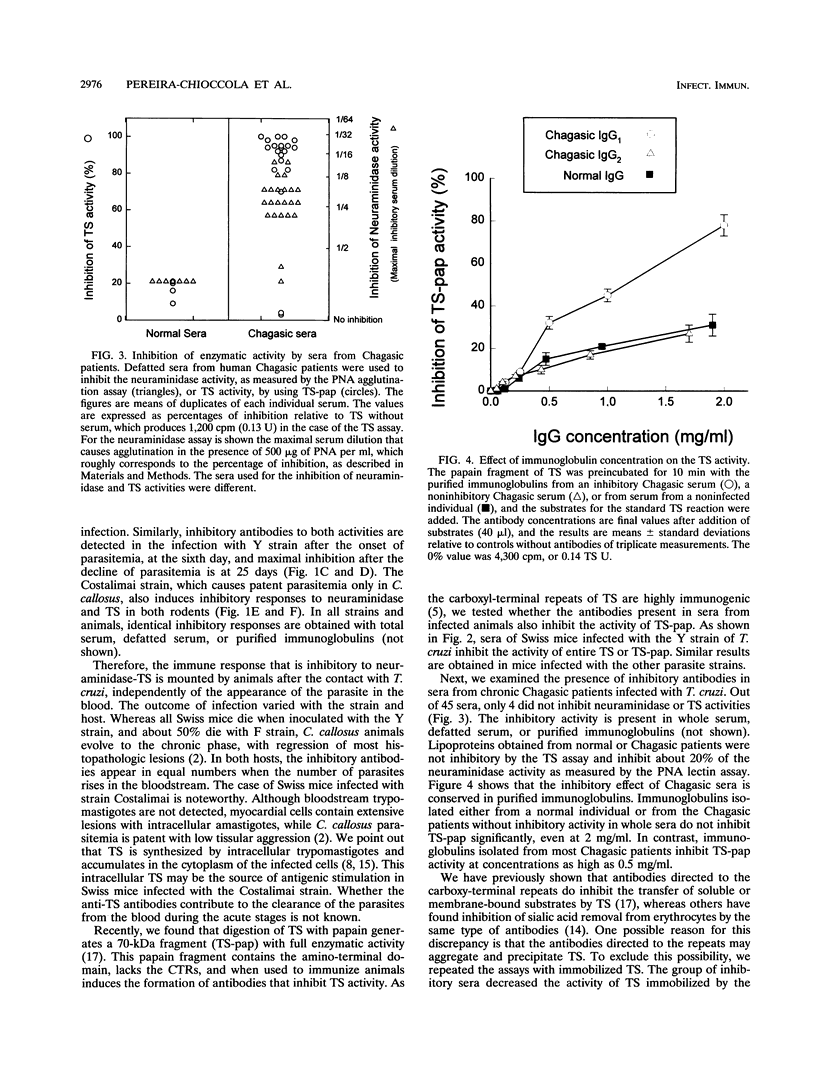
We investigated whether sera from chronic Chagasic patients and animals infected with Trypanosoma cruzi inhibit the removal of sialic acid from human erythrocytes and the transfer of sialic acid from sialyllactose to [14C]lactose in the reactions catalyzed by the parasite trans-sialidase. Sera from Swiss mice and Calomys callosus animals infected with three different T. cruzi strains inhibit both reactions. Inhibition increases during the infection, reaching maximal levels when the parasitemia decreases. Among 44 sera of untreated chronic Chagasic patients, 40 inhibit both reactions. Inhibition is observed with total, defatted sera or with purified immunoglobulins. Whereas most of the inhibitory antibodies from Chagasic patients react with the papain fragment of trans-sialidase in immunoblots, a few patients have noninhibitory antibodies that react only with the entire trans-sialidase. These findings may be relevant for the pathology of Chagas' disease.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affranchino J. L., Ibañez C. F., Luquetti A. O., Rassi A., Reyes M. B., Macina R. A., Aslund L., Pettersson U., Frasch A. C. Identification of a Trypanosoma cruzi antigen that is shed during the acute phase of Chagas' disease. Mol Biochem Parasitol. 1989 May 15;34(3):221–228. doi: 10.1016/0166-6851(89)90050-9. [DOI] [PubMed] [Google Scholar]
- BRENER Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1962 Nov-Dec;4:389–396. [PubMed] [Google Scholar]
- Borges M. M., De Andrade S. G., Pilatti C. G., do Prado Júnior J. C., Kloetzel J. K. Macrophage activation and histopathological findings in Calomys callosus and Swiss mice infected with several strains of Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1992 Oct-Dec;87(4):493–502. doi: 10.1590/s0074-02761992000400006. [DOI] [PubMed] [Google Scholar]
- Campetella O., Sánchez D., Cazzulo J. J., Frasch A. C. A superfamily of Trypanosoma cruzi surface antigens. Parasitol Today. 1992 Nov;8(11):378–381. doi: 10.1016/0169-4758(92)90175-2. [DOI] [PubMed] [Google Scholar]
- Cazzulo J. J., Frasch A. C. SAPA/trans-sialidase and cruzipain: two antigens from Trypanosoma cruzi contain immunodominant but enzymatically inactive domains. FASEB J. 1992 Nov;6(14):3259–3264. [PubMed] [Google Scholar]
- Chaves L. B., Briones M. R., Schenkman S. Trans-sialidase from Trypanosoma cruzi epimastigotes is expressed at the stationary phase and is different from the enzyme expressed in trypomastigotes. Mol Biochem Parasitol. 1993 Sep;61(1):97–106. doi: 10.1016/0166-6851(93)90162-q. [DOI] [PubMed] [Google Scholar]
- Deane M. P., Kloetzel J. Lack of protection against Trypanosoma cruzi by multiple doses of T. lewisi culture forms. A discussion on some strains of "lewisi". Exp Parasitol. 1974 Jun;35(3):406–410. doi: 10.1016/0014-4894(74)90046-0. [DOI] [PubMed] [Google Scholar]
- Frevert U., Schenkman S., Nussenzweig V. Stage-specific expression and intracellular shedding of the cell surface trans-sialidase of Trypanosoma cruzi. Infect Immun. 1992 Jun;60(6):2349–2360. doi: 10.1128/iai.60.6.2349-2360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello D. A., Borges M. M. Primeiro encontro do Triatoma costalimai naturalmente infectado pelo Trypanosoma cruzi: estudo de aspectos biológicos da amostra isolada. Mem Inst Oswaldo Cruz. 1981 Jan-Mar;76(1):61–69. doi: 10.1590/s0074-02761981000100007. [DOI] [PubMed] [Google Scholar]
- Parodi A. J., Pollevick G. D., Mautner M., Buschiazzo A., Sanchez D. O., Frasch A. C. Identification of the gene(s) coding for the trans-sialidase of Trypanosoma cruzi. EMBO J. 1992 May;11(5):1705–1710. doi: 10.1002/j.1460-2075.1992.tb05221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E. A rapid and sensitive assay for neuraminidase using peanut lectin hemagglutination: application to Vibrio cholera and Trypanosoma cruzi. J Immunol Methods. 1983 Sep 30;63(1):25–34. doi: 10.1016/0022-1759(83)90206-5. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Mejia J. S., Ortega-Barria E., Matzilevich D., Prioli R. P. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low density lipoprotein receptor, and type III modules of fibronectin. J Exp Med. 1991 Jul 1;174(1):179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previato J. O., Andrade A. F., Pessolani M. C., Mendonça-Previato L. Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol Biochem Parasitol. 1985 Jun;16(1):85–96. doi: 10.1016/0166-6851(85)90051-9. [DOI] [PubMed] [Google Scholar]
- Prioli R. P., Ortega-Barria E., Mejia J. S., Pereira M. E. Mapping of a B-cell epitope present in the neuraminidase of Trypanosoma cruzi. Mol Biochem Parasitol. 1992 May;52(1):85–96. doi: 10.1016/0166-6851(92)90038-l. [DOI] [PubMed] [Google Scholar]
- Rosenberg I. A., Prioli R. P., Mejia J. S., Pereira M. E. Differential expression of Trypanosoma cruzi neuraminidase in intra- and extracellular trypomastigotes. Infect Immun. 1991 Jan;59(1):464–466. doi: 10.1128/iai.59.1.464-466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer R., Reuter G., Mühlpfordt H., Andrade A. F., Pereira M. E. The occurrence of N-acetyl- and N-glycoloylneuraminic acid in Trypanosoma cruzi. Hoppe Seylers Z Physiol Chem. 1983 Aug;364(8):1053–1057. doi: 10.1515/bchm2.1983.364.2.1053. [DOI] [PubMed] [Google Scholar]
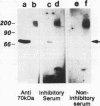
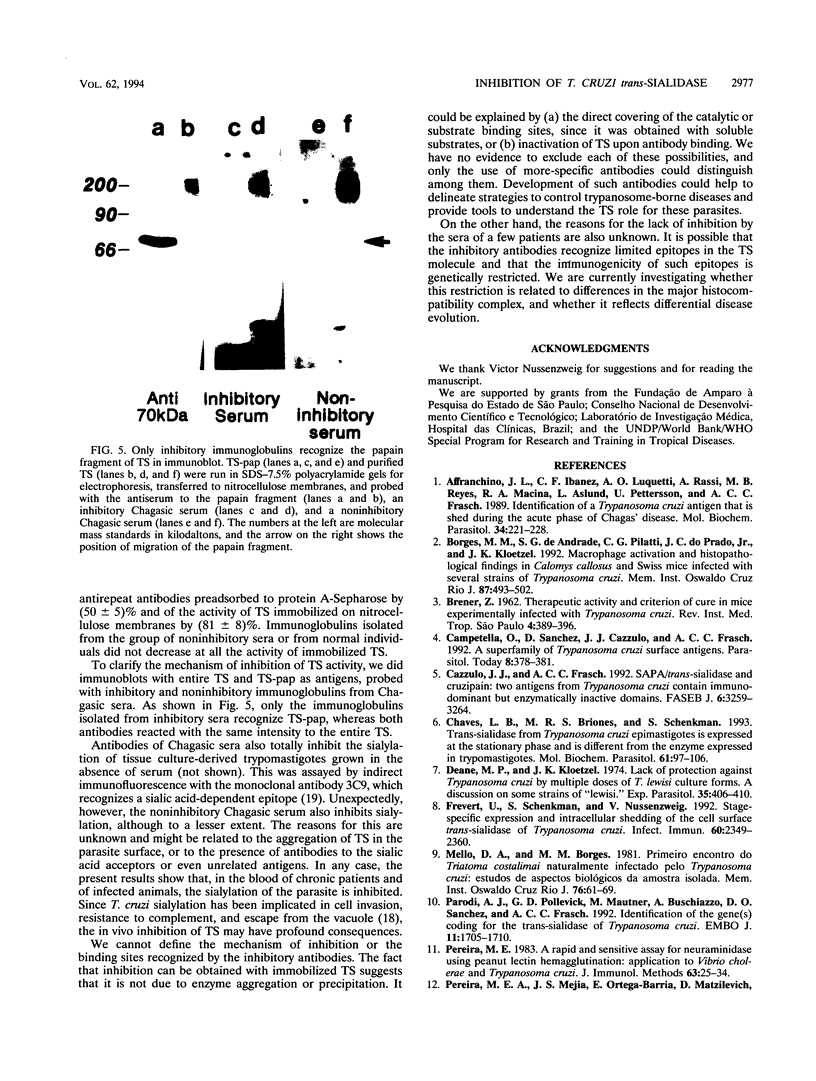
- Schenkman S., Chaves L. B., Pontes de Carvalho L. C., Eichinger D. A proteolytic fragment of Trypanosoma cruzi trans-sialidase lacking the carboxyl-terminal domain is active, monomeric, and generates antibodies that inhibit enzymatic activity. J Biol Chem. 1994 Mar 18;269(11):7970–7975. [PubMed] [Google Scholar]
- Schenkman S., Eichinger D. Trypanosoma cruzi trans-sialidase and cell invasion. Parasitol Today. 1993 Jun;9(6):218–222. doi: 10.1016/0169-4758(93)90017-a. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Jiang M. S., Hart G. W., Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991 Jun 28;65(7):1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Pontes de Carvalho L., Nussenzweig V. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J Exp Med. 1992 Feb 1;175(2):567–575. doi: 10.1084/jem.175.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]