Abstract
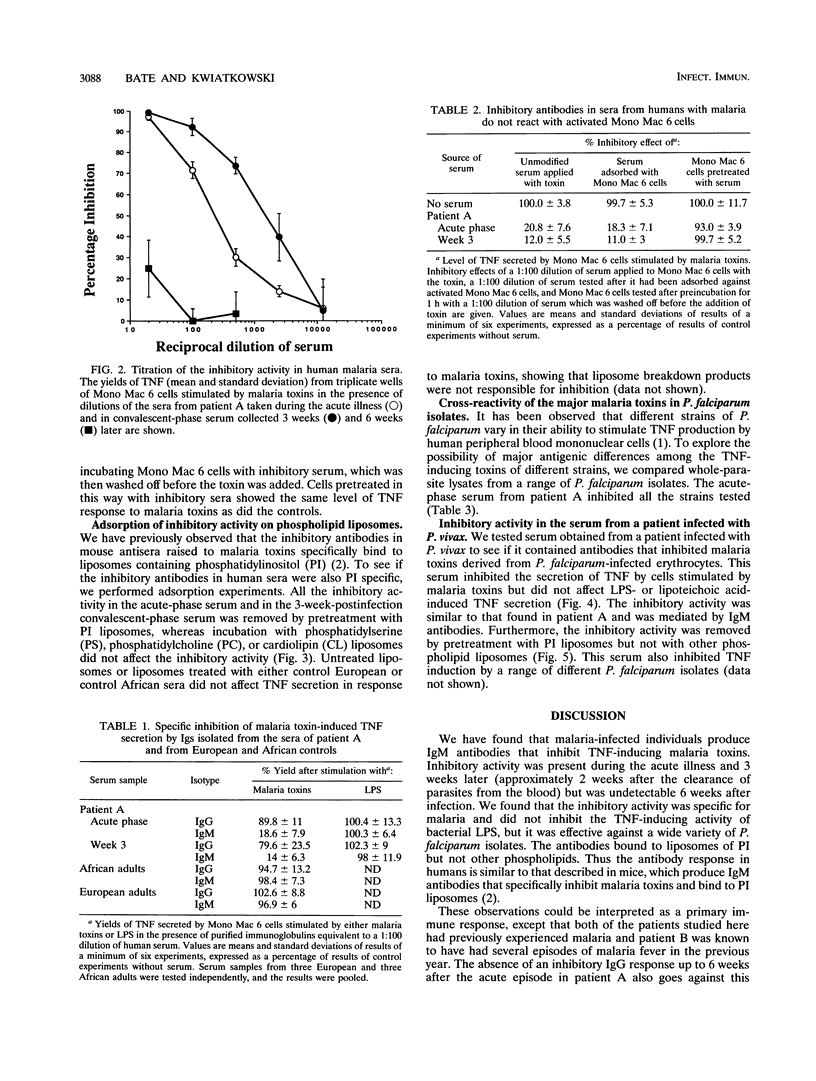
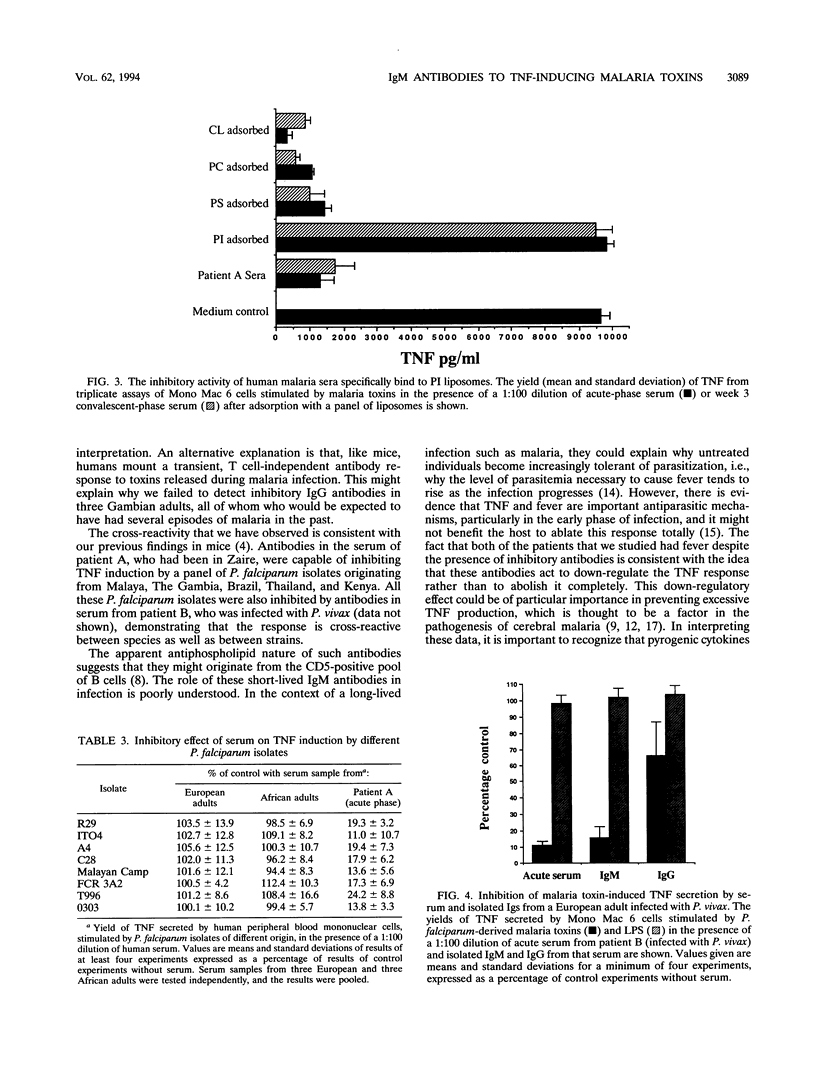
Cytokines such as tumor necrosis factor alpha (TNF) appear to play an important role in the pathogenesis of malaria. We have previously shown that TNF is produced in response to substances released at schizont rupture, which we have called malaria toxins. In mice these toxins stimulate a T cell-independent antibody response, generating short-lived immunoglobulin M (IgM) antibodies that inhibit the TNF-inducing activity of the toxins. We report here that a similar antibody response is seen in humans. Serum from a European adult infected with Plasmodium falciparum inhibited the induction of TNF by malaria toxins derived from P. falciparum-infected erythrocytes. We found that IgM antibodies were responsible for the inhibitory activity. These inhibitory antibodies could not be detected in convalescent-phase serum collected from the same patient 6 weeks later or in sera from healthy European and African controls. The antibodies appeared to be malaria specific in that they inhibited TNF induction by a variety of P. falciparum isolates but failed to inhibit TNF induction by bacterial lipopolysaccharide or lipoteichoic acid. The inhibitory antibodies bound to liposomes containing phosphatidylinositol but not other phospholipids. Serum from a European adult infected with P. vivax also inhibited the activity of toxins derived from P. falciparum-infected erythrocytes, and this too was mediated by IgM antibodies which were malaria specific and bound to phosphatidylinositol liposomes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. J., Rowe A., Kwiatkowski D. Plasmodium falciparum varies in its ability to induce tumor necrosis factor. Infect Immun. 1993 Nov;61(11):4772–4776. doi: 10.1128/iai.61.11.4772-4776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Bootsma H. J., Mason R. C., Skalko N., Gregoriadis G., Playfair J. H. Antibodies against phosphatidylinositol and inositol monophosphate specifically inhibit tumour necrosis factor induction by malaria exoantigens. Immunology. 1992 May;76(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Davé A., Playfair J. H. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology. 1990 Jul;70(3):315–320. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Karunaweera N. D., Mendis K. N., Kwiatkowski D., Playfair J. H. Serological relationship of tumor necrosis factor-inducing exoantigens of Plasmodium falciparum and Plasmodium vivax. Infect Immun. 1992 Mar;60(3):1241–1243. doi: 10.1128/iai.60.3.1241-1243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Detoxified exoantigens and phosphatidylinositol derivatives inhibit tumor necrosis factor induction by malarial exoantigens. Infect Immun. 1992 May;60(5):1894–1901. doi: 10.1128/iai.60.5.1894-1901.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Román E., Moreno C., Playfair J. H. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992 Jan;75(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Cell-mediated immunity in protection and pathology of malaria. Parasitol Today. 1987 Oct;3(10):300–305. doi: 10.1016/0169-4758(87)90187-6. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Karunaweera N. D., Grau G. E., Gamage P., Carter R., Mendis K. N. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3200–3203. doi: 10.1073/pnas.89.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D., Cannon J. G., Manogue K. R., Cerami A., Dinarello C. A., Greenwood B. M. Tumour necrosis factor production in Falciparum malaria and its association with schizont rupture. Clin Exp Immunol. 1989 Sep;77(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D. Cytokines and anti-disease immunity to malaria. Res Immunol. 1991 Oct;142(8):707–712. doi: 10.1016/0923-2494(91)90154-b. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Molyneux M. E., Stephens S., Curtis N., Klein N., Pointaire P., Smit M., Allan R., Brewster D. R., Grau G. E. Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med. 1993 Feb;86(2):91–98. [PubMed] [Google Scholar]
- Playfair J. H., Taverne J., Bate C. A., de Souza J. B. The malaria vaccine: anti-parasite or anti-disease? Immunol Today. 1990 Jan;11(1):25–27. doi: 10.1016/0167-5699(90)90007-v. [DOI] [PubMed] [Google Scholar]
- Pradines-Figueres A., Raetz C. R. Processing and secretion of tumor necrosis factor alpha in endotoxin-treated Mono Mac 6 cells are dependent on phorbol myristate acetate. J Biol Chem. 1992 Nov 15;267(32):23261–23268. [PubMed] [Google Scholar]
- Roberts D. J., Craig A. G., Berendt A. R., Pinches R., Nash G., Marsh K., Newbold C. I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992 Jun 25;357(6380):689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993 Jan 1;177(1):145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]


