Abstract
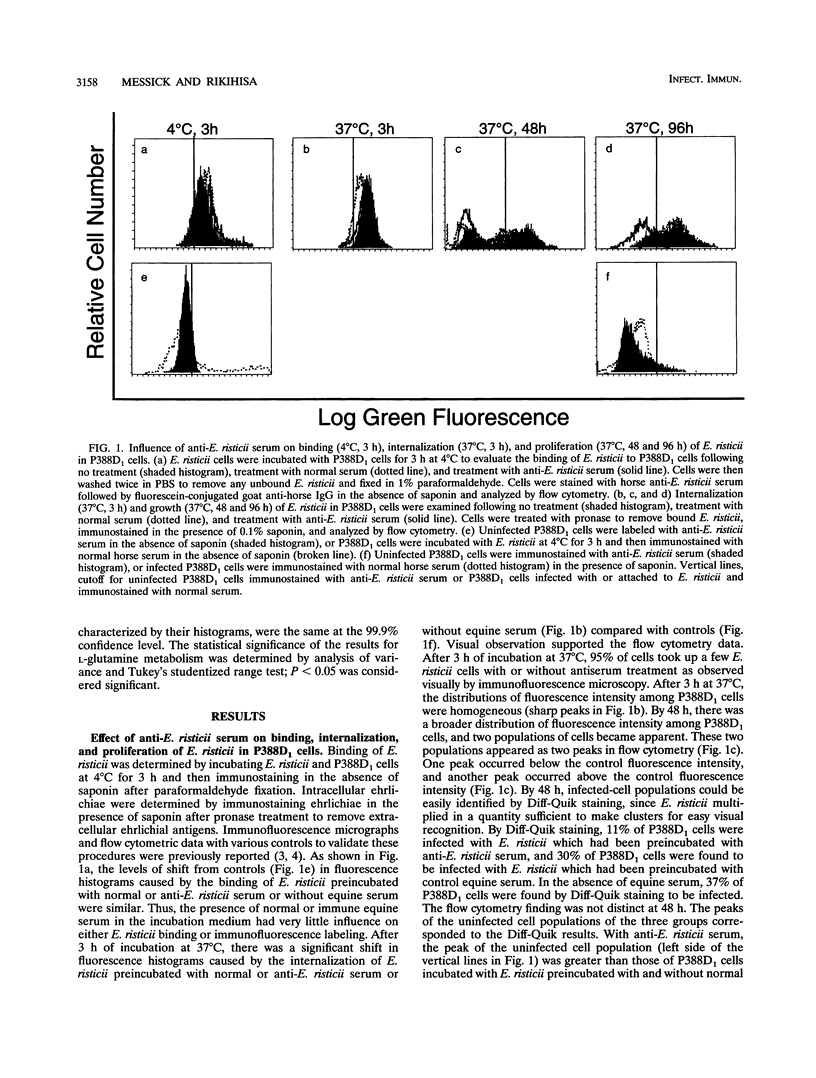
The effects of equine antiserum, immunoglobulin G (IgG) specific for Ehrlichia risticii, and its Fab fragment on E. risticii binding to, internalization into, and proliferation in P388D1 cells were studied by immunofluorescence flow cytometry. Anti-E. risticii equine serum or IgG inhibited E. risticii at a stage beyond binding and internalization. In contrast, monovalent anti-E. risticii equine Fab fragments inhibited E. risticii binding and internalization into P388D1 cells. In the presence of control equine serum, IgG, or its Fab fragment, E. risticii cells were bound, were internalized and subsequently grew within P388D1 cells, and eventually destroyed the host cells as effectively as was the case without equine serum, IgG, or Fab fragments. Anti-E. risticii IgG but not normal horse IgG inhibited L-[14C]glutamine metabolism in Percoll gradient-purified E. risticii. These findings suggest that the Fab fragment of intact anti-E. risticii IgG blocks the ligands on E. risticii responsible for non-IgG-mediated internalization and diverts them to bind via the Fc receptor. Following Fc-mediated entry of E. risticii, the antibody interfered with the metabolic activity of E. risticii cells, rendering them incapable of proliferation in P388D1 cells and resulting in the eventual destruction of the organisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kaylor P. S., Crawford T. B., McElwain T. F., Palmer G. H. Passive transfer of antibody to Ehrlichia risticii protects mice from ehrlichiosis. Infect Immun. 1991 Jun;59(6):2058–2062. doi: 10.1128/iai.59.6.2058-2062.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. N., Khan R. J., Posner B. I. A method for quantitation of protein in the presence of Percoll. Anal Biochem. 1981 Oct;117(1):108–112. doi: 10.1016/0003-2697(81)90699-0. [DOI] [PubMed] [Google Scholar]
- Messick J. B., Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and proliferation in host cells by flow cytometry. Infect Immun. 1993 Sep;61(9):3803–3810. doi: 10.1128/iai.61.9.3803-3810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messick J. B., Rikihisa Y. Presence of parasite antigen on the surface of P388D1 cells infected with Ehrlichia risticii. Infect Immun. 1992 Aug;60(8):3079–3086. doi: 10.1128/iai.60.8.3079-3086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretzman C. I., Rikihisa Y., Ralph D., Gordon J. C., Bech-Nielsen S. Enzyme-linked immunosorbent assay for Potomac horse fever disease. J Clin Microbiol. 1987 Jan;25(1):31–36. doi: 10.1128/jcm.25.1.31-36.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Johnson G. C., Burger C. J. Reduced immune responsiveness and lymphoid depletion in mice infected with Ehrlichia risticii. Infect Immun. 1987 Sep;55(9):2215–2222. doi: 10.1128/iai.55.9.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D., Cordes D. O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985 Sep;49(3):505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Pretzman C. I., Johnson G. C., Reed S. M., Yamamoto S., Andrews F. Clinical, histopathological, and immunological responses of ponies to Ehrlichia sennetsu and subsequent Ehrlichia risticii challenge. Infect Immun. 1988 Nov;56(11):2960–2966. doi: 10.1128/iai.56.11.2960-2966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y. Protection against murine potomac horse fever by an inactivated Ehrlichia risticii vaccine. Vet Microbiol. 1991 May;27(3-4):339–350. doi: 10.1016/0378-1135(91)90159-d. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y., Reed S. M., Sams R. A., Gordon J. C., Pretzman C. I. Serosurvey of horses with evidence of equine monocytic ehrlichiosis. J Am Vet Med Assoc. 1990 Nov 15;197(10):1327–1332. [PubMed] [Google Scholar]
- Rikihisa Y., Wada R., Reed S. M., Yamamoto S. Development of neutralizing antibody in horses infected with Ehrlichia risticii. Vet Microbiol. 1993 Jul;36(1-2):139–147. doi: 10.1016/0378-1135(93)90135-t. [DOI] [PubMed] [Google Scholar]
- Weiss E., Dasch G. A., Kang Y. H., Westfall H. N. Substrate utilization by Ehrlichia sennetsu and Ehrlichia risticii separated from host constituents by renografin gradient centrifugation. J Bacteriol. 1988 Nov;170(11):5012–5017. doi: 10.1128/jb.170.11.5012-5017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Williams J. C., Dasch G. A., Kang Y. H. Energy metabolism of monocytic Ehrlichia. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1674–1678. doi: 10.1073/pnas.86.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. Y., Rikihisa Y. Lack of lysosomal fusion with phagosomes containing Ehrlichia risticii in P388D1 cells: abrogation of inhibition with oxytetracycline. Infect Immun. 1988 Dec;56(12):3209–3215. doi: 10.1128/iai.56.12.3209-3215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. M., Timoney P. J. In vitro killing of Ehrlichia risticii by activated and immune mouse peritoneal macrophages. Infect Immun. 1993 Mar;61(3):861–867. doi: 10.1128/iai.61.3.861-867.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. T. Proof without prejudice: use of the Kolmogorov-Smirnov test for the analysis of histograms from flow systems and other sources. J Histochem Cytochem. 1977 Jul;25(7):935–941. doi: 10.1177/25.7.894009. [DOI] [PubMed] [Google Scholar]


