Abstract
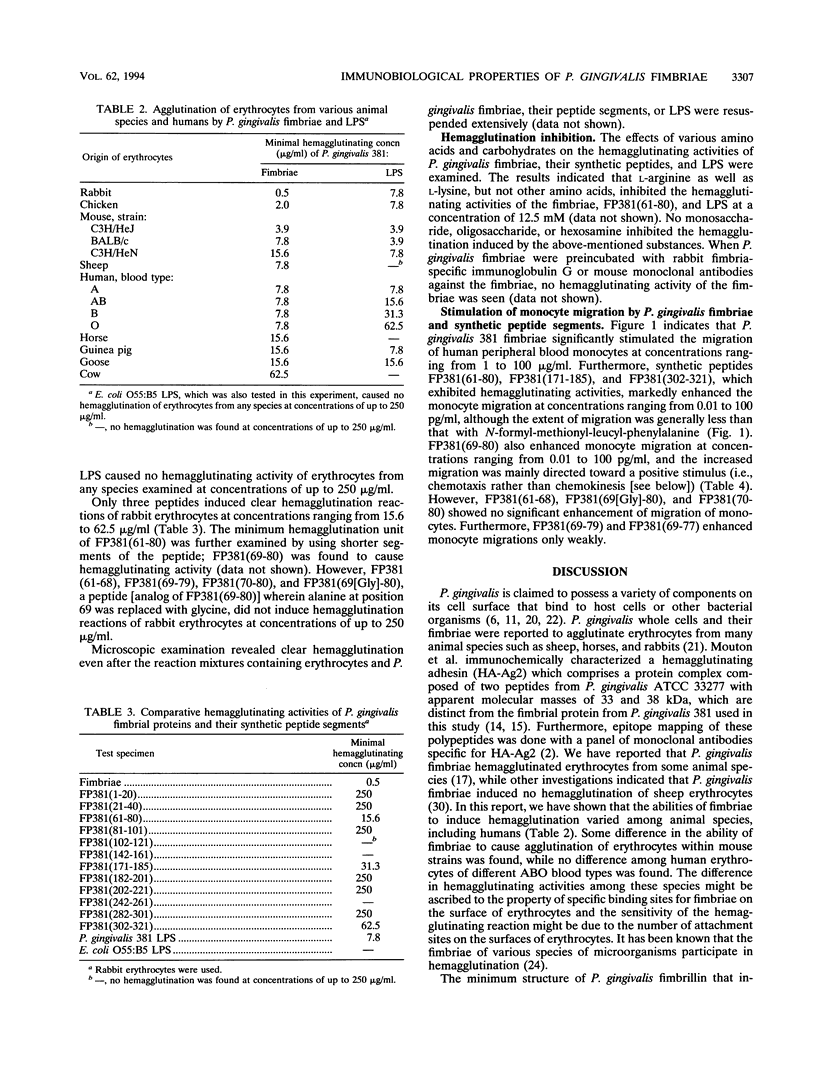
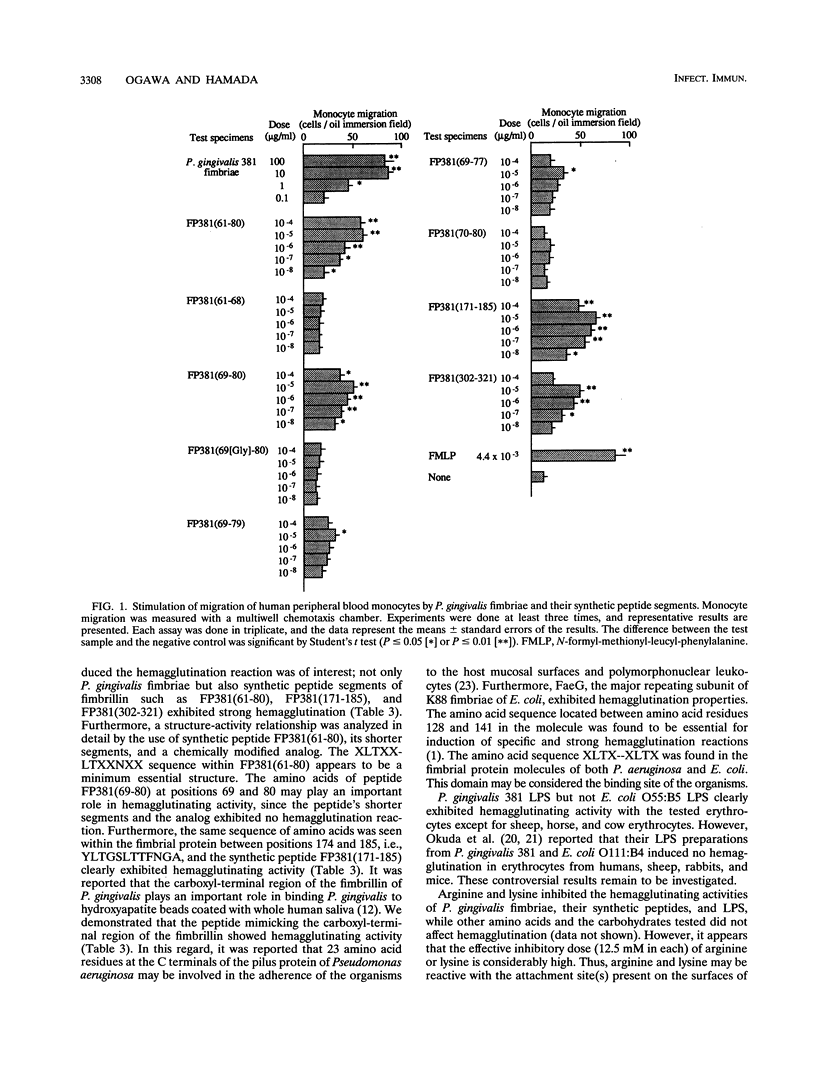
Porphyromonas gingivalis 381 fimbriae, their synthetic peptide segments, and lipopolysaccharide (LPS) were examined for hemagglutinating and migration-stimulating activities. P. gingivalis 381 fimbriae clearly caused hemagglutination, and several oligopeptide segments such as FP381(61-80), FP381(171-185), and FP381(302-321), corresponding to the amino acid residue numbers based on the amino acid sequence of fimbrillin proposed by Dickinson et al. (D. P. Dickinson, M. A. Kubiniec, F. Yoshimura, and R. J. Genco, J. Bacteriol. 170:1658-1665, 1988), were also demonstrated to agglutinate erythrocytes although less effectively than the native fimbriae. Furthermore, P. gingivalis 381 LPS but not Escherichia coli O55:B5 LPS definitely exhibited hemagglutination. P. gingivalis fimbriae as well as their synthetic peptides possessing hemagglutinating activity enhanced the chemotaxically induced migration of human peripheral blood monocytes. The results of the analyses using synthetic peptide FP381(61-80), its related compounds, and an analog suggested that the amino acid sequence XLTXXLTXXNXX within fimbrial protein molecules may play an important role structurally in the attachment of the protein to host cells such as erythrocytes and monocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker D., Willemsen P. T., Simons L. H., van Zijderveld F. G., de Graaf F. K. Characterization of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol Microbiol. 1992 Jan;6(2):247–255. doi: 10.1111/j.1365-2958.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Deslauriers M., Mouton C. Epitope mapping of hemagglutinating adhesion HA-Ag2 of Bacteroides (Porphyromonas) gingivalis. Infect Immun. 1992 Jul;60(7):2791–2799. doi: 10.1128/iai.60.7.2791-2799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Ogawa T., Sobue S., Hamada S. Chemical, immunobiological and antigenic characterizations of lipopolysaccharides from Bacteroides gingivalis strains. J Gen Microbiol. 1990 Feb;136(2):319–326. doi: 10.1099/00221287-136-2-319. [DOI] [PubMed] [Google Scholar]
- Genco R. J., Slots J. Host responses in periodontal diseases. J Dent Res. 1984 Mar;63(3):441–451. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Ogawa T., Shimauchi H., Kusumoto Y. Induction of mucosal and serum immune responses to a specific antigen of periodontal bacteria. Adv Exp Med Biol. 1992;327:71–81. doi: 10.1007/978-1-4615-3410-5_9. [DOI] [PubMed] [Google Scholar]
- Hanazawa S., Hirose K., Ohmori Y., Amano S., Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988 Jan;56(1):272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai H., Isogai E., Yoshimura F., Suzuki T., Kagota W., Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33(7):479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Vail T. A., Switalski L. M., Hook M. Specific cell components of Bacteroides gingivalis mediate binding and degradation of human fibrinogen. J Bacteriol. 1991 Jan;173(2):495–504. doi: 10.1128/jb.173.2.495-504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Sojar H. T., Bedi G. S., Genco R. J. Synthetic peptides analogous to the fimbrillin sequence inhibit adherence of Porphyromonas gingivalis. Infect Immun. 1992 Apr;60(4):1662–1670. doi: 10.1128/iai.60.4.1662-1670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Bouchard D., Deslauriers M., Lamonde L. Immunochemical identification and preliminary characterization of a nonfimbrial hemagglutinating adhesin of Bacteroides gingivalis. Infect Immun. 1989 Feb;57(2):566–573. doi: 10.1128/iai.57.2.566-573.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Ni Eidhin D., Deslauriers M., Lamy L. The hemagglutinating adhesin HA-Ag2 of Bacteroides gingivalis is distinct from fimbrilin. Oral Microbiol Immunol. 1991 Feb;6(1):6–11. doi: 10.1111/j.1399-302x.1991.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Fukuda K., Tsukamoto Y., Mori M., Kusumoto S., Shiba T. Stimulation of migration of human monocytes by bacterial cell walls and muramyl peptides. Infect Immun. 1982 Dec;38(3):817–824. doi: 10.1128/iai.38.3.817-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kusumoto Y., Uchida H., Nagashima S., Ogo H., Hamada S. Immunobiological activities of synthetic peptide segments of fimbrial protein from Porphyromonas gingivalis. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1335–1341. doi: 10.1016/s0006-291x(05)81342-7. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Mukai T., Yasuda K., Shimauchi H., Toda Y., Hamada S. Distribution and immunochemical specificities of fimbriae of Porphyromonas gingivalis and related bacterial species. Oral Microbiol Immunol. 1991 Dec;6(6):332–340. doi: 10.1111/j.1399-302x.1991.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Shimauchi H., Hamada S. Mucosal and systemic immune responses in BALB/c mice to Bacteroides gingivalis fimbriae administered orally. Infect Immun. 1989 Nov;57(11):3466–3471. doi: 10.1128/iai.57.11.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Kato T. Hemagglutinating activity of lipopolysaccharides from subgingival plaque bacteria. Infect Immun. 1987 Dec;55(12):3192–3196. doi: 10.1128/iai.55.12.3192-3196.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Yamamoto A., Naito Y., Takazoe I., Slots J., Genco R. J. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986 Dec;54(3):659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Volpel K., Loh B. A., Speert D. P. Fimbriae (pili): molecular basis of Pseudomonas aeruginosa adherence. Clin Invest Med. 1986;9(2):113–118. [PubMed] [Google Scholar]
- Saglie F. R., Pertuiset J., Rezende M. T., Nestor M., Marfany A., Cheng J. In situ correlative immuno-identification of mononuclear infiltrates and invasive bacteria in diseased gingiva. J Periodontol. 1988 Oct;59(10):688–696. doi: 10.1902/jop.1988.59.10.688. [DOI] [PubMed] [Google Scholar]
- Sinden P. R., Walker D. M. Inflammatory cells extracted from chronically inflamed gingiva. J Periodontal Res. 1979 Nov;14(6):467–474. doi: 10.1111/j.1600-0765.1979.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Stoufi E. D., Taubman M. A., Ebersole J. L., Smith D. J. Preparation and characterization of human gingival cells. J Periodontal Res. 1987 Mar;22(2):144–149. doi: 10.1111/j.1600-0765.1987.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Yamaji Y., Umemoto T. Correlation between cell-adherent activity and surface structure in Porphyromonas gingivalis. Oral Microbiol Immunol. 1992 Dec;7(6):357–363. doi: 10.1111/j.1399-302x.1992.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


