Abstract
The second messenger phosphatidylinositol (3,4,5)-trisphosphate (PIP3), formed by the p110 family of PI3-kinases, promotes cellular growth, proliferation, and survival, in large part by activating the protein kinase Akt/PKB. We show that inositol polyphosphate multikinase (IPMK) physiologically generates PIP3 as well as water soluble inositol phosphates. IPMK deletion reduces growth factor-elicited Akt signaling and cell proliferation caused uniquely by loss of its PI3-kinase activity. Inhibition of p110 PI3-kinases by wortmannin prevents IPMK phosphorylation and activation. Thus, growth factor stimulation of Akt signaling involves PIP3 generation through the sequential activations of the p110 PI3-kinases and IPMK. As inositol phosphates inhibit Akt signaling, IPMK appears to act as a molecular switch, inhibiting or stimulating Akt via its inositol phosphate kinase or PI3-kinase activities, respectively. Drugs regulating IPMK may have therapeutic relevance in influencing cell proliferation.
Keywords: signal transduction, cancer
A large family of inositol phosphates serves multiple functions, with inositol 1,4,5-trisphosphate (IP3) being well known as a second messenger releasing intracellular calcium (1). Inositol diphosphates, incorporating an energetic pyrophosphate bond, display numerous physiological roles, including pyrophosphorylation of a variety of protein targets (2–4). These inositol pyrophosphates are synthesized by a family of IP6 kinase enzymes (5). Recently, novel isomers of inositol pyrophosphates have been described that are synthesized by a distinct inositol phosphate kinase enzyme designated Vip1 (6, 7).
Inositol polyphosphate multikinase (IPMK) is a member of the IP6 kinase family of enzymes but is not primarily associated with the formation of inositol pyrophosphates. Instead it generates several inositol phosphates, converting IP3 to IP4 and IP4 to IP5, with its primary physiologic role in this pathway being to form the bulk of IP5 in cells (5, 8–11). IPMK also possesses phosphatidylinositol 3-kinase (PI3K) activity in vitro (12), specifically phosphorylating phosphatidylinositol(4,5)-bisphosphate (PIP2) to generate phosphatidylinositol (3,4,5)-trisphosphate (PIP3), a second messenger known to promote cellular growth, proliferation, survival, and migration (13). The physiologic role of this activity has not heretofore been established. The principal PI3K activity in cells has been attributed to a family of enzymes identified by Cantley and associates (reviewed in ref. 14), whose catalytic subunits are designated p110. PIP3 generated by p110 in response to extracellular stimuli, such as growth factors, is a principal stimulus of the Akt/mammalian target of rapamycin (mTOR) signaling pathway, which in turn regulates protein synthesis and plays a role in some cancers (15–17).
We wondered whether the PI3K activity of IPMK contributes to the generation of PIP3 under physiologic conditions to influence Akt signaling and cell growth. There is good reason to assume that IPMK is crucial for cellular physiology, as its deletion is lethal in early embryonic stages of mice (10). In the present study we demonstrate that IPMK is a physiologic PI3K responsible for generating a substantial portion of cellular PIP3. Thus, genetic deletion of IPMK impairs Akt signaling and diminishes cell growth, consequences that are determined by the PI3K activity of IPMK.
Results and Discussion
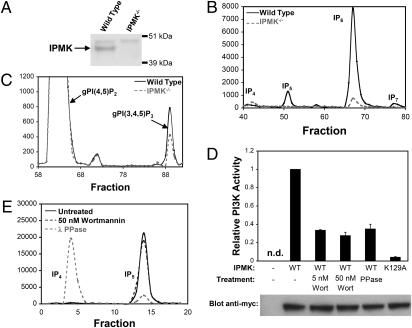
Because the very early embryonic lethality (E9.5) of conventional IPMK knockouts precludes harvesting tissues, such as fibroblasts (10), we obtained murine embryonic fibroblasts (MEFs) from mice harboring a conditionally targeted IPMK allele (Fig. S1). Expression of Cre recombinase in these MEFs abolished IPMK expression (Fig. 1A). IPMK is a rate-limiting enzyme in the formation of multiple inositol phosphates. In MEFs lacking IPMK, synthesis of IP5 and IP7 is abolished, but IP6 formation is reduced about 90% (Fig. 1B), resembling findings of York and colleagues (10) in embryonic stem cells of IPMK knockout mice.
Fig. 1.
IPMK physiologically produces inositol phosphates and PIP3 in MEFs. (A) Western immunoblot demonstrating the absence of the 44 kDa IPMK protein in IPMK−/− MEFs. (B) Comparison of higher-order inositol phosphate profiles of wild-type and IPMK−/− MEFs. Equal numbers of each cell type were labeled with [3H]myo-inositol. After extraction, inositol phosphates were resolved by HPLC. (C) Comparison of cellular PIP3 levels in wild-type and IPMK−/− MEFs. Equal numbers of each cell type were labeled with [3H]myo-inositol. Cells were starved of serum overnight and stimulated with 10% FBS for 5 min to induce PIP3 formation. After extraction, cellular phosphoinositides were deacylated and the polar head groups were resolved by HPLC. The identities of the peaks were confirmed by comigration with standards. (D) Treatment of cells with wortmannin reduces the PI3K activity of IPMK. HEK293T cells overexpressing myc-IPMK were treated with wortmannin before lysis and immunoprecipitation. Alternatively, IPMK was immunoprecipitated from untreated cells and then dephosphorylated with Lambda protein phosphatase. After immunoprecipitation, in vitro PI3K activity was assayed. A control reaction with a catalytically inactive mutant (K129A) form of IPMK confirms that the immunoprecipitates are not contaminated with other PI3Ks. Western blots with an anti-myc antibody were used to normalize data to IPMK levels. The no-IPMK lane is from an immunoprecipitate from empty vector transfected cells. The data are means of three independent experiments; error bars represent SEs. (E) Treatment of cells with wortmannin does not reduce the inositol phosphate kinase activity of IPMK. IPMK was immunoprecipitated as described in D, and the in vitro formation of IP5 from [3H]myo-inositol 1,3,4,5-tetrakisphosphate (IP4) was assayed.
To ascertain whether IPMK possesses physiologic PI3K activity in intact cells, we labeled MEFs with [3H]myo-inositol and examined the formation of PIP3 (Fig. 1C). In cells treated with FBS, we observe a 50% decrease in PIP3 generation in IPMK-deleted cells. Thus, IPMK appears to be a physiologic PI3K.
Wortmannin, a potent inhibitor of nearly all known PI3Ks, abolishes PIP3 formation in a wide range of cells (18). It does not, however, inhibit IPMK directly, even at high concentrations (12). Accordingly, we postulated that IPMK is positively regulated by a wortmannin-sensitive PI3K, and that treatment of cells with wortmannin could thereby decrease the formation of PIP3 by IPMK. To test this hypothesis, we isolated IPMK from HEK293T cells treated with 5 or 50 nM wortmannin and monitored PIP3 formation by the purified enzyme (Fig. 1D). The PI3K activity of IPMK is reduced by about 70% at both concentrations. Thus, wortmannin inhibits IPMK in vivo with potency similar to that for p110 inhibition. Given that many protein kinases are activated in response to PIP3 production, we hypothesize that phosphorylation of IPMK by such a kinase is required to switch on the PI3K activity of IPMK in cells. This notion is supported by our observation that dephosphorylation of IPMK with λ protein phosphatase after purification from untreated HEK293T cells reduces the PI3K activity of the enzyme to a similar extent as wortmannin treatment.
These experiments establish that IPMK is a physiologic PI3K interposed within a PIP3/protein kinase signaling pathway, wherein phosphorylation of IPMK in a wortmannin-sensitive fashion stimulates its PI3K activity. We wondered whether the inositol phosphate kinase activity of IPMK is similarly regulated. Treatment of HEK293T cells with 50 nM wortmannin fails to reduce the inositol phosphate kinase activity of IPMK, indicating that this activity is regulated in a different fashion than IPMK's PI3K activity (Fig. 1E). However, a distinct mechanism involving phosphorylation of IPMK appears to positively regulate its inositol phosphate kinase activity, as λ protein phosphatase treatment dramatically reduces IPMK's inositol phosphate kinase activity.
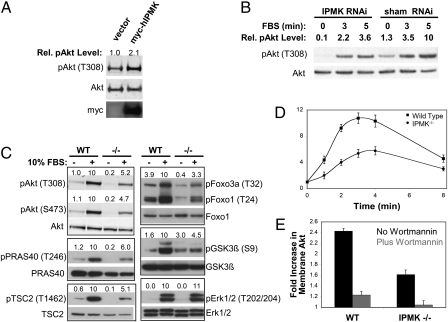
If IPMK is a physiologically important PI3K, then it ought to influence Akt signaling. Akt activation requires PIP3-dependent phosphorylaton at two sites, T308 and S473 (19). As IPMK appears to be activated by PIP3 generated by another PI3K, we examined U87MG glioma cells in which the tumor-suppressor PTEN, a well-characterized phosphoinositide 3-phosphatase (20), is inactivated, leading to high intracellular levels of PIP3 (21). Overexpression of IPMK in these cells increases phosphorylation of Akt at T308 (Fig. 2A) by twofold. This finding contrasts with our previous inability to detect an effect of IPMK overexpression on Akt activation in HEK293T cells (12), in which PTEN is expressed at physiologic levels. As a more stringent test for a physiologic role for IPMK in Akt signaling, we depleted IPMK by RNA interference in U87MG cells and observed a 65% decrease in serum-induced Akt phosphorylation (Fig. 2B). Measurement of cellular IP5 levels in these cells indicates a 50% loss of IPMK function compared with control cells (Fig. S2). These results are in agreement with a previous report that IPMK depletion by RNA interference enhances the cytotoxicity of an Akt inhibitor (22).
Fig. 2.
IPMK promotes PIP3-dependent activation of Akt. (A) Overexpression of IPMK increases basal Akt phosphorylation in U87MG glioma cells. Cells were transfected with myc-hIPMK or control vector. After 24 h, cells were serum-starved overnight, lysed, and analyzed for phospho- and total Akt and myc-IPMK expression by immunoblotting. Relative phospho-Akt (T308) levels were determined by densitometry and corrected for total Akt levels. (B) Depletion of IPMK in U87MG cells by RNA interference reduces basal and serum-induced Akt phosphorylation. Cells were stably transfected with either a siRNA targeting IPMK or a control siRNA. Equal numbers of each stable cell line were plated, starved of serum overnight, stimulated with 10% FBS for the indicated times, and lysed. Lysates were analyzed for phospho- and total Akt by immunoblotting. Relative phospho-Akt (T308) levels were determine by densitometry and corrected for total Akt levels. As the anti-mouse IPMK antibody does not recognize the human isoform well, knock-down was confirmed by cellular IP5 measurement (Fig. S2). (C) Deletion of IPMK in MEFs reduces serum-induced Akt phosphorylation and activation. Equal numbers of wild-type and IPMK−/− MEFs were plated, serum-starved overnight, stimulated with 10% FBS for 5 min, and lysed. Lysates were analyzed by immunoblotting with the indicated antibodies. Relative phospho-protein levels (above each blot) were determined by densitometry and corrected for total levels of the respective protein. (D) Time course of EGF-induced Akt phosphorylation. Equal numbers of wild-type and IPMK−/− MEFs were plated, serum-starved overnight, stimulated with EGF for the indicated times, and lysed. Lysates were analyzed for total Akt and phospho-Akt-T308 by immunoblotting. Data are means of three independent experiments and error bars represent SEs. For representative raw data, see Fig. S3B. (E) IPMK promotes the EGF-dependent translocation of Akt to the plasma membrane. Equal numbers of wild-type and IPMK−/− MEFs were plated, serum-starved overnight, stimulated with EGF for 3 min, and fractionated into S100 and P100 fractions. Fractions were analyzed for total Akt by immunoblotting. Wortmannin treatment of cells before and during EGF treatment abolishes the EGF-dependent translocation of Akt into the P100 fractions, indicating that this experiment reflects the levels of PIP3 in the plasma membrane. Data are means of three independent experiments and error bars represent SEs. For representative raw data, see Fig. S4A.
To explore in greater depth the role of IPMK in Akt signaling, we used IPMK-null MEFs (Fig. 2C). Phosphorylation of Akt at both T308 and S473 in response to FBS is reduced by 50% in IPMK-deleted MEFs. IPMK mediates signaling to multiple Akt targets, as levels of phospho-PRAS40, phospho-TSC2, phospho-FoxO1/3A, and phospho-GSK3β are diminished in IPMK-deleted MEFs. The decreased signaling is selective for the Akt pathway, because levels of phospho-Erk1/2, which are not Akt targets, are not altered in the IPMK knockouts.
We also examined Akt activation in response to the growth factors EGF, IGF, and insulin (Fig. S3A). Phospho-Akt-T308 levels in response to these growth factors are substantially reduced in IPMK-deleted MEFs with the greatest reduction being for EGF signaling. We evaluated the time course of EGF-dependent Akt phosphorylation (Fig. 2D and Fig. S3B). In both wild-type and IPMK-deleted MEFs, EGF markedly increases Akt signaling with substantial augmentation evident within one minute and maximal at 3 to 4 min. Akt activation is reduced about 50% in the IPMK-null cells.
Growth factor-dependent Akt phosphorylation and activation requires translocation of Akt from the cytosol to the plasma membrane by virtue of the specific affinity of the Akt plekstrin homology domain for PIP3 (23). We investigated this process in IPMK-deleted MEFs (Fig. 2E and Fig. S4A). EGF elicits a 2.4-fold increase in membrane levels of Akt in wild-type MEFs with a 60% reduction in this response evident in IPMK-deleted cells. In both wild-type and IPMK-null cells, the translocation is virtually abolished following wortmannin treatment. We detect substantial amounts of endogenous IPMK in both membrane and cytoplasmic fractions similar to the disposition of Akt, although the relative distribution between these two fractions appears insensitive to growth factors and wortmannin (Fig. S4A). Earlier studies conducted primarily with overexpressed IPMK reported a predominant nuclear localization (9, 12). We detect endogenous IPMK in both nuclear and cytoplasmic fractions in MEFs (Fig. S4B).
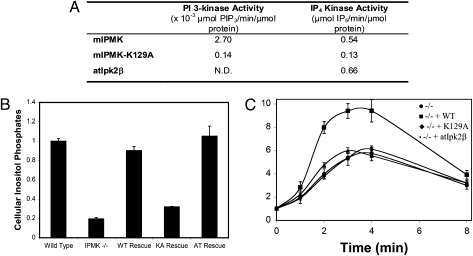
We wondered whether regulation of Akt by IPMK is because of its PI3K or inositol phosphate kinase activities. To explore this question, we sought a form of IPMK that might possess only one of these two activities. We noted that the Arabidopsis thaliana ortholog of IPMK, atIpk2β, possesses inositol phosphate kinase activity comparable to mouse IPMK but is devoid of PI3K activity (Fig. 3A). In contrast, mutating lysine 129 of mouse IPMK (mIPMK-K129A) virtually abolishes both PI3K and inositol phosphate kinase activities. In IPMK-deleted MEFs, ectopic expression of mIPMK or atIpk2β, but not mIPMK-K129A, restores inositol phosphate production to normal levels (Fig. 3B). We next explored the ability of these various forms of IPMK to rescue the loss of Akt signaling in IPMK-deleted MEFs (Fig. 3C and Fig. S5). As observed previously, in wild-type cells phospho-Akt-T308 levels increase 10-fold 3 to 4 min following treatment with EGF. This activation is reduced 40 to 50% in IPMK-deleted MEFs. Overexpressing wild-type IPMK restores phospho-Akt-T308 to normal levels, but no rescue is evident with mIPMK-K129A or atIpk2β. Thus, although some catalytic activity is required, the inositol phosphate kinase activity of atIpk2β does not suffice to stimulate Akt signaling. Accordingly, we conclude that it is the PI3K activity of IPMK that is predominantly responsible for mediating IPMK's augmentation of Akt signaling.
Fig. 3.
The PI3K activity of IPMK is required for full activation of Akt in response to EGF. (A) Comparison of in vitro specific activities of mouse IPMK, mouse IPMK-K129A, and atIpk2β. His-tagged recombinant proteins were purified from Escherichia coli, as previously described for IP6K1 (35). All reactions were monitored over time and specific activities were calculated based on the linear range of the reaction curves. (B) Wild-type IPMK, and atIpk2β fully restore higher inositol phosphate production in IPMK−/− MEFs, but IPMK-K129A does not. Wild-type IPMK, IPMK-K129A, and atIpk2β were stably expressed in IPMK−/− MEFs. Equal numbers of each cell type were labeled to with [3H]myo-inositol. After extraction, inositol phosphates were resolved by HPLC and quantified by scintillation counting. Data represent the sums of cellular IP5, IP6, and IP7, and are means of three independent experiments. Error bars represent SEs. (C) Wild-type IPMK restores EGF-induced Akt phosphorylation in IPMK−/− MEFs, but IPMK-K129A and atIpk2β do not. Equal numbers of the same stable cell lines used in the experiment shown in B were plated, serum-starved overnight, stimulated with EGF, and lysed. Lysates were analyzed for phospho-Akt-T308, total Akt, and myc-IPMK expression by immunoblotting. Data are means of three independent experiments and error bars are SEs. For representative raw data, see Fig. S5.
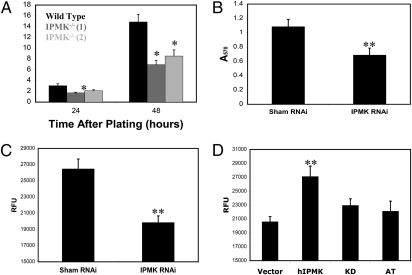
These findings establish that the PI3K activity of IPMK physiologically enhances Akt signaling. Akt is a major determinant of cell proliferation. Accordingly, we examined the influence of IPMK on this process. In IPMK-deleted MEFs, the rate of cell proliferation is reduced about 50% (Fig. 4A). In U87MG glioma cells, depletion of IPMK by RNA interference results in a 32% reduction in the rate of proliferation (Fig. 4B). IPMK-depleted U87MG cells also exhibit a reduced rate of anchorage-independent growth relative to control cells (Fig. 4C). Consistent with this finding, overexpression of IPMK causes a 35% increase in the rate of anchorage-independent growth of U87MG cells (Fig. 4D). This process requires the PI3K activity of the enzyme, as a kinase-dead variant and atIpk2β fail to elicit an effect. Thus, IPMK appears to promote cell proliferation in both cancerous and noncancerous cells.
Fig. 4.
IPMK promotes the proliferation of both MEFs and U87MG glioma cells. (A) Comparison of proliferation of wild-type and IPMK−/− MEFs. Equal numbers of each cell type were plated, and then harvested and counted at the indicated time intervals. Trypan blue staining indicated that viability was >98% throughout the experiment for all cell lines. Data are means of three independent experiments and error bars represent SEs. Two IPMK−/− cell lines were tested to control for clonal variation. In all panels, P values were calculated using a two-tailed unpaired student's t test. Statistical comparisons were made between wild-type and IPMK−/− cells at each time point. (B) Depletion of IPMK by RNA interference impairs the proliferation of U87MG cells stably expressing either control or IPMK-targeting siRNAs. Equal numbers of each cell line were plated, allowed to proliferate for 72 h, and analyzed for relative cell number by the MTT assay (Millipore). Data are means of eight replicates and the error bars represent the SDs. (C) Depletion of IPMK by RNA interference reduces anchorage-independent growth of U87MG cells. Anchorage-independent growth was assayed using the Cytoselect soft agar colony-formation assay (Cell Bioloabs), as recommended by the manufacturer. Data are means of six replicates and error bars represent SEs. (D) The PI3K activity of IPMK promotes anchorage-independent growth of U87MG cells. U87MG cells were transfected with myc vector, myc-hIPMK, a kinase dead variant of myc-hIPMK (KD) or myc-atIpk2β (AT), and analyzed for anchorage-independent growth. Data are means of six replicates and error bars represent SEs. Only wild-type IPMK causes a statistically significant increase in anchorage-independent growth relative to control cells. *P < 0.05, **P < 0.01; no designation indicates P > 0.05.
In summary, we established that IPMK is a physiologic PI3K interposed in a PIP3/protein kinase signaling pathway. Thus, depletion of PIP3 by wortmannin markedly reduces IPMK's PI3K activity by decreasing IPMK phosphorylation, which appears to be important for its catalytic activity. IPMK is a major determinant of Akt signaling, as its deletion leads to a 50% decrease in growth-factor dependent Akt activation. The regulation of Akt by IPMK is selective, as IPMK knockout does not affect the ERK signaling system. IPMK's regulation of Akt is attributable to its PI3K activity and not its inositol phosphate kinase activity. Finally, the regulation of Akt signaling by IPMK impacts cell growth, which is markedly diminished with IPMK deletion.
The inositol phosphate kinase and the PI3K activities of IPMK appear to be differentially regulated. For example, in intact cells wortmannin inhibits the PI3K activity but not the inositol phosphate kinase activity. The products of these two activities generally oppose one another in their effects on cellular physiology and the Akt pathway. PIP3 promotes cellular proliferation and survival, largely via activation of Akt. In contrast, a number of reports indicate that higher-order inositol phosphates antagonize proliferation and tumorigenesis and promote apoptosis (24–29). Moreover, several studies indicate that higher inositol phosphates specifically decrease Akt activity (26, 27, 30–32). The differential regulation of PIP3 and inositol phosphate production by IPMK may provide a process enabling cells to switch between Akt activation and inhibition, with corresponding influences upon cellular proliferation and survival (Fig. 5). Recently we showed that IP7 generated by IP6 kinase-1 physiologically inhibits Akt by preventing its phosphorylation and activation by PDK1 (30). This finding suggests that IP6K1 and IPMK, both members of the same family, have opposing physiologic activities. Interestingly, IPMK−/− cells have depleted levels of PIP3 and IP7 (Fig. 1), yet still have reduced Akt activity, which suggests that the fraction of Akt activation attributable to IPMK could be greater than what we have observed here. At the very least, it appears that, under normal growth conditions in embryonic fibroblasts, the ability of IPMK to activate Akt is dominant to the inhibition of Akt by IP7. Key to understanding the interplay between these opposing activities will be elucidation of the mechanism whereby IPMK switches between PI3K and inositol phosphate production. It is thus essential to identify the kinases responsible for phosphorylation of IPMK. Many kinases, both proximal and distal, are activated by PIP3. Given that IPMK localizes to plasma membranes, we hypothesize that a proximal kinase, such as Akt or PDK1, could be responsible for activating its PI3K activity. However, we cannot rule out a downstream kinase, such as mTOR or S6 kinase. Regarding activation of IPMK's inositol phosphate kinase activity, it is tempting to speculate that a conventional isoform of PKC is responsible. Given that elevated IP3 levels lead to activation of such kinases, phosphorylation and activation of IPMK would coincide with elevated levels of a substrate necessary for higher order inositol phosphate production. We are currently pursuing both pharmacological and genetic approaches to address these possibilities.
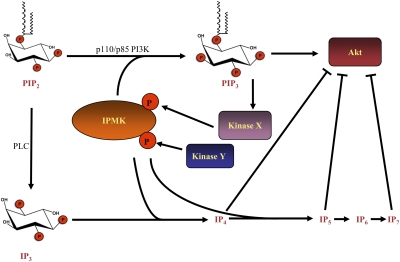
Fig. 5.
Model describing a role for IPMK in both activation and inhibition of Akt. In response to extracellular stimuli such as growth factors, the p110/p85 PI3Ks produce PIP3 in the plasma membrane, which results in the activation of a host of kinases, including Akt. One such kinase phosphorylates IPMK, activating its PI3K activity and resulting in a feed-forward loop necessary for maximal PIP3 levels and Akt activity. A distinct kinase, perhaps in response to some other specific signal, phosphorylates IPMK on a different site, thus activating its inositol phosphate kinase activity. Inositol phosphates including IP4, IP5, and IP7 have been reported to antagonize Akt signaling.
Finally, these findings may have therapeutic relevance. Depletion of IPMK either by genetic deletion or RNA interference leads to decreased cell growth. Accordingly, drugs that inhibit IPMK might offer therapeutic utility in inhibiting the growth of tumors.
Materials and Methods
Generation and Maintenance of Mice and Cell Lines.
For generation and maintenance of mice and cell lines, see SI Materials and Methods.
Measurement of Cellular Inositol Phosphates and Phosphoinositides.
For inositol phosphate measurements, MEFs were plated at a density of 2 × 105 per well in six-well plates and incubated for 3 d in complete culture medium in the presence of 60 μCi per well [3H]myo-inositol (PerkinElmer). After washing three times with ice-cold PBS, inositol phosphates were extracted into 200 μL ice-cold lysis buffer (0.6 M perchloric acid, 0.2 mg/mL IP6) and neutralized with 1 M K2CO3. Extracts were clarified by centrifugation and inositol phosphate species were resolved by anion exchange HPLC, as previously described (12).
For phosphoinositide measurements, isolated glycerophosphoinositides were prepared as in ref. 12 after 72 h of labeling using 20 uCi/mL [3H]inositol (Perkin-Elmer).
Preparation of Immunoprecipitates for in Vitro Enzyme Activity Assays.
For assays using immunoprecipitated enzyme, HEK-293T cells were transiently transfected with either empty myc vector, myc-WT-mIPMK, or myc-mIPMK-K129A using PolyFect (Qiagen) according to the manufacturer's recommendations. Thirty-six hours after transfection, cells were treated with either wortmannin or DMSO vehicle. Cells were washed twice in cold PBS and lysed in 40 mM Hepes, pH 7.5, 120 mM NaCl, 1 mM EDTA, 0.3% CHAPS, protease inhibitor mixtures 1 and 2 (Sigma), and phosphatase inhibitor mixtures 1 and 2 (Sigma). Immunoprecipitations were performed using 1 mg of cell lysates, 0.4 μg of anti-myc antibody (Roche), and 40 μL of protein G Plus/protein A-agarose suspension (Calbiochem). Immnoprecipitates were washed three times in lysis buffer and then once in 1× NEBuffer for protein metallophosphatases (PMP). Immunoprecipitates were then incubated in 1× NEBuffer for PMP supplemented with 1 mM MnCl2 in the presence or absence of lambda protein phosphatase (New England Biolabs) for 45 min at 30 °C. Immunoprecipitates were again washed three times in lysis buffer, and then washed once in 1× IPMK reaction buffer. Reactions were performed as described below. After removing the reaction mixture, immunoprecipitates were washed twice with PBS and then resuspended in 2× NuPAGE LDS Sample Buffer (Invitrogen). HRP-conjugated anti-myc antibody (Roche) was used in immunoblots to confirm the presence of equal amounts of immunoprecipitated enzyme in each assay.
In Vitro PI3-Kinase Activity Assays.
PI(4,5)P2 was resuspended via sonication in of 20 mM Hepes (pH 7.4), 1 mM EDTA, and 0.5% deoxycholate. Reactions were performed with immunoprecipitates or with 200 nM His-tagged purified recombinant protein in a total volume of 50 μL containing 10 μL of lipid resuspension, providing a final concentration of 0.03 mg/mL purified synthetic PI(4,5)P2 (Avanti Polar Lipids). Reaction buffer consisted of 20 mM Hepes (pH 7.4), 6 mM MgCl2, and 10 μCi of [γ-32P]ATP (PerkinElmer–NEN; 6,000 mCi/mmol; 1 Ci = 37 GBq) in a carrier of 100 μM unlabeled ATP. Reactions were incubated at 37 °C for 15 min, and stopped by adding the reaction mixture to 90 μL of 1 M HCl/methanol (1:1, by volume). Lipids were extracted twice with 100 μL of choloroform, dried down, and resuspended in chloroform/methanol (2:1, by volume). Lipids were resolved on silica gel 60 TLC plates in a solvent system consisting of water/n-propanol/glacial acetic acid (34:65:1, by volume). Alternatively, reactions were stopped by spotting aliquots on nitrocellulose for analysis by the membrane capture method, as described (33). For all in vitro PI3K assays, reactions were monitored as a function of time and activities were calculated based on the linear range.
In Vitro Inositol Phosphate Kinase Activity Assays.
Reactions were carried out with immunoprecipitates or with 500 nM purified His-tagged enzyme, as previously described (12).
Growth Factor Treatments and Immunoblotting.
Before growth factor treatment, MEFs were plated at a density of 5 × 105 cells per well in six-well plates. To minimize the impact of differences in growth rates of various cell lines, cells were growth-arrested by overnight serum-starvation 3 h after plating (the minimum time necessary for attachment to the plates). Cells were then stimulated (for 5 min if not otherwise indicated) with 10% FBS, 33 nM EGF, 10 nM IGF, or 20 nM insulin. For EGF time courses, 0.4 nM EGF was used. Treatment was stopped by washing with ice-cold PBS and then flash-freezing the plates in liquid nitrogen. Cells were thawed into PBS, 0.1% Triton X-100, protease inhibitor mixtures 1 and 2, and protein phosphatase inhibitor mixtures 1 and 2 (all inhibitors from Sigma). Lysates were normalized for total protein content using Coomassie Plus Protein Assay Reagent (Thermo Scientific). No systematic differences were apparent in total protein yields from different cell lines, suggesting that the number of cells present during growth factor treatment were very similar. Visual inspection of plates under a microscope also confirmed that all cell lines were at nearly identical confluence before growth factor treatment.
After addition of LDS loading buffer to a final concentration of 1×, 20 μg of each lysate were analyzed by Western immunoblotting. Blots were blocked for 1 h at room temperature with 1% fish-skin gelatin in TBS and then incubated overnight with primary antibody at 4 °C. Secondary antibody incubations were for 1 h at room temperature. For phospho- and total Akt blots, infrared dye-conjugated secondary antibodies were used for two-color detection using a Licor imaging system. For all other blots, HRP-conjugated secondary antibodies were used for detection with SuperSignal West Pico chemiluminescence reagent (Thermo Scientific).
Primary antibodies were total Akt (R&D Systems; MAB2055), myc (Roche; 11667203001), caveolin (BD Transduction Laboratories; 610059), pPRAS40 (Upstate; 07–888), and GAPDH (Biogenesis; MCA4739). The following were from Cell Signaling Technologies: HDACII (2540), pAkt-T308 (2965), pAkt-S473 (4058), pGSK3β (9331), GSK3β (9315), pFoxO1/3a (9464), FoxO1 (9462), pTSC2 (3617), TSC2 (3612), pErk1/2 (4376), Erk1/2 (4695), and PRAS40 (2610). Anti-IPMK was a custom rabbit polyclonal antibody from Covance raised against a synthetic peptide starting with Cys followed by mouse IPMK amino acids 295–311 (SKAYSRHRKLYAKKHQS).
Subcellular Fractionation.
For determination of Akt membrane translocation, MEFs were plated at a density of 6 × 106 per 10-cm plate. After 3 h to allow the cells to attach, cells were serum-starved overnight. After stimulation with 0.4 nM EGF for 3 min, cells were washed with ice-cold PBS and fractionated into S100 and P100 fractions, as previously described (34). Fractions were analyzed by immunoblotting. Caveolin was used as a marker for plasma membrane and GAPDH was used as a marker for cytoplasm. For determination of cytoplasmic/nuclear distribution of IPMK, 6 × 106 MEFs were fractionated into cytoplasmic and nuclear fractions using a Nuclear/Cytosol Fractionation Kit (BioVision), as described by the manufacturer.
Supplementary Material
Acknowledgments
We thank Charles Brearley, Rashna Bhandari, Michael Koldobskiy, Sangwon Kim, and the members of the S.H.S. laboratory for helpful discussions. This work was funded by US Public Health Service Grant DA-00266 and Research Scientist Award DA-00074 (to S.H.S.) and a Damon Runyon Cancer Research fellowship (to D.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017831108/-/DCSupplemental.
References
- 1.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Phosphorylation of proteins by inositol pyrophosphates. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 3.Azevedo C, Burton A, Ruiz-Mateos E, Marsh M, Saiardi A. Inositol pyrophosphate mediated pyrophosphorylation of AP3B1 regulates HIV-1 Gag release. Proc Natl Acad Sci USA. 2009;106:21161–21166. doi: 10.1073/pnas.0909176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhandari R, et al. Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc Natl Acad Sci USA. 2007;104:15305–15310. doi: 10.1073/pnas.0707338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- 6.Mulugu S, et al. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 7.Lin H, et al. Structural analysis and detection of biological inositol pyrophosphates reveal that the family of VIP/diphosphoinositol pentakisphosphate kinases are 1/3-kinases. J Biol Chem. 2009;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiardi A, et al. Mammalian inositol polyphosphate multikinase synthesizes inositol 1,4,5-trisphosphate and an inositol pyrophosphate. Proc Natl Acad Sci USA. 2001;98:2306–2311. doi: 10.1073/pnas.041614598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalaskowski MM, Deschermeier C, Fanick W, Mayr GW. The human homologue of yeast ArgRIII protein is an inositol phosphate multikinase with predominantly nuclear localization. Biochem J. 2002;366:549–556. doi: 10.1042/BJ20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frederick JP, et al. An essential role for an inositol polyphosphate multikinase, Ipk2, in mouse embryogenesis and second messenger production. Proc Natl Acad Sci USA. 2005;102:8454–8459. doi: 10.1073/pnas.0503706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang SC, Miller AL, Feng Y, Wente SR, Majerus PW. The human homolog of the rat inositol phosphate multikinase is an inositol 1,3,4,6-tetrakisphosphate 5-kinase. J Biol Chem. 2002;277:43836–43843. doi: 10.1074/jbc.M206134200. [DOI] [PubMed] [Google Scholar]
- 12.Resnick AC, et al. Inositol polyphosphate multikinase is a nuclear PI3-kinase with transcriptional regulatory activity. Proc Natl Acad Sci USA. 2005;102:12783–12788. doi: 10.1073/pnas.0506184102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 15.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powis G, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 19.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 20.Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27:5464–5476. doi: 10.1038/onc.2008.243. [DOI] [PubMed] [Google Scholar]
- 21.Haas-Kogan D, et al. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 22.Morgan-Lappe S, et al. RNAi-based screening of the human kinome identifies Akt-cooperating kinases: A new approach to designing efficacious multitargeted kinase inhibitors. Oncogene. 2006;25:1340–1348. doi: 10.1038/sj.onc.1209169. [DOI] [PubMed] [Google Scholar]
- 23.Currie RA, et al. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337:575–583. [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison BH, et al. Gene deletion of inositol hexakisphosphate kinase 2 predisposes to aerodigestive tract carcinoma. Oncogene. 2009;28:2383–2392. doi: 10.1038/onc.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata E, et al. Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J Biol Chem. 2005;280:1634–1640. doi: 10.1074/jbc.M409416200. [DOI] [PubMed] [Google Scholar]
- 26.Maffucci T, et al. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway by inositol pentakisphosphate results in antiangiogenic and antitumor effects. Cancer Res. 2005;65:8339–8349. doi: 10.1158/0008-5472.CAN-05-0121. [DOI] [PubMed] [Google Scholar]
- 27.Piccolo E, et al. Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway. Oncogene. 2004;23:1754–1765. doi: 10.1038/sj.onc.1207296. [DOI] [PubMed] [Google Scholar]
- 28.Razzini G, et al. Novel functional PI 3-kinase antagonists inhibit cell growth and tumorigenicity in human cancer cell lines. FASEB J. 2000;14:1179–1187. doi: 10.1096/fasebj.14.9.1179. [DOI] [PubMed] [Google Scholar]
- 29.Koldobskiy MA, et al. p53-mediated apoptosis requires inositol hexakisphosphate kinase-2. Proc Natl Acad Sci USA. 2010;107:20947–20951. doi: 10.1073/pnas.1015671107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborty A, et al. Inositol pyrophosphates mediate obesity and insulin resistance by physiologically inhibiting Akt signaling. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falasca M, et al. A novel inhibitor of the PI3K/Akt pathway based on the structure of inositol 1,3,4,5,6-pentakisphosphate. Br J Cancer. 2010;102:104–114. doi: 10.1038/sj.bjc.6605408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia Y, Schurmans S, Luo HR. Regulation of innate immunity by inositol 1,3,4,5-tetrakisphosphate. Cell Cycle. 2008;7:2803–2808. doi: 10.4161/cc.7.18.6688. [DOI] [PubMed] [Google Scholar]
- 33.Knight ZA, Feldman ME, Balla A, Balla T, Shokat KM. A membrane capture assay for lipid kinase activity. Nat Protoc. 2007;2:2459–2466. doi: 10.1038/nprot.2007.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill MM, Hemmings BA. Analysis of protein kinase B/Akt. Methods Enzymol. 2002;345:448–463. doi: 10.1016/s0076-6879(02)45037-9. [DOI] [PubMed] [Google Scholar]
- 35.Azevedo C, Burton A, Bennett M, Onnebo SM, Saiardi A. Synthesis of InsP7 by the inositol hexakisphosphate kinase 1 (IP6K1) Methods Mol Biol. 2010;645:73–85. doi: 10.1007/978-1-60327-175-2_5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.