Abstract
Phytochromes are red and far-red light photoreceptors that regulate various aspects of plant development. One of the less-understood roles of phytochromes is the inhibition of hypocotyl negative gravitropism, which refers to the loss of hypocotyl gravitropism and resulting random growth direction in red or far-red light. This light response allows seedlings to curve toward blue light after emergence from the soil and enhances seedling establishment in the presence of mulch. Phytochromes inhibit hypocotyl negative gravitropism by inhibiting four phytochrome-interacting factors (PIF1, PIF3, PIF4, PIF5), as shown by hypocotyl agravitropism of dark-grown pif1 pif3 pif4 pif5 quadruple mutants. We show that phytochromes inhibit negative gravitropism by converting starch-filled gravity-sensing endodermal amyloplasts to other plastids with chloroplastic or etioplastic features in red or far-red light, whereas PIFs promote negative gravitropism by inhibiting the conversion of endodermal amyloplasts to etioplasts in the dark. By analyzing transgenic plants expressing PIF1 with an endodermis-specific SCARECROW promoter, we further show that endodermal PIF1 is sufficient to inhibit the conversion of endodermal amyloplasts to etioplasts and hypocotyl negative gravitropism of the pif quadruple mutant in the dark. Although the functions of phytochromes in gravitropism and chloroplast development are normally considered distinct, our results indicate that these two functions are closely related.
Phytochromes are red and far-red light plant photoreceptors that regulate various light responses, including seed germination, seedling photomorphogenesis, and shade avoidance. Phytochromes regulate light responses partly by inhibiting phytochrome-interacting factors (PIFs), a set of bHLH transcription factors that negatively regulate various light responses (1, 2). A series of experiments has demonstrated that phytochromes promote light responses partly by activating degradation of these PIFs (3). Consistent with these findings, pif1 pif3 pif4 pif5 quadruple mutants (pifQ) display constitutive photomorphogenic phenotypes and ectopic expression of chloroplast-related genes in the dark, and pif4 pif5 double mutants show suppressed shade avoidance responses and associated repression of shade-avoidance marker genes in a low-red/far-red light (4–8). At the molecular level, PIFs bind to G-box elements (CACGTG) and regulate the expression of various genes associated with G-box–containing promoters (9–11). In the case of PIF1, a genome-wide analysis of its binding sites and an associated gene-expression analysis indicated that PIF1 binds to 748 sites and directly regulates the expression of at least 166 genes either positively (105 genes) or negatively (61 genes) during the seed imbibition period (12). The 166 genes include many hormone-signaling genes, such as RGA, ABI3, ABI5, JAZ1, and ARF18, as well as various cell wall-modifying enzyme genes. In addition to these direct target genes, PIF1 indirectly regulates some hormone metabolic genes. These analyses suggested that the phytochrome-PIF1 signaling module regulates seed germination by coordinating hormone signaling and cell wall properties in imbibed seeds. Molecular networks that link phytochrome-PIFs modules to other downstream light responses, such as hypocotyl negative gravitropism, are not clearly understood.
Plant gravitropic responses can be divided conceptually into four steps, consisting of gravity sensing, signal generation, signal transmission to the responding tissues, and asymmetric elongation (13). Among these steps, gravity sensing requires starch-filled amyloplasts in root columella cells for root gravitropism or in shoot endodermis for hypocotyl and shoot negative gravitropism (14, 15). Consistent with the critical role of endodermis in shoot gravity sensing, the scarecrow (scr) and the shortroot (shr) mutants, which do not develop an endodermis, do not display shoot negative gravitropism (16, 17). Starch levels in the amyloplasts also affect gravitropism of both the shoot and root, as indicated by the reduced gravitropic responses of a phosphoglucomutase mutant (pgm) that has a reduced level of amyloplast starch, and by the stronger gravitropic responses of starch-excessive mutant (sex1), with higher levels of amyloplast starch (18–22). A series of shoot gravitropic mutants named shoot gravitropism (sgr1–sgr5, sgr7) further demonstrate the importance of endodermis, endodermal vacuole biogenesis, and vacuolar membrane dynamics in shoot gravity sensing (13, 23–26). Mislocalization and altered movement of amyloplasts in these sgr mutants have been implicated in reduced gravity sensing. Once gravity is sensed by amyloplasts, the biophysical signal is presumed to be converted to a biochemical signal that is then transmitted to responding tissues for asymmetric elongation partly through auxin signaling (13, 27, 28).
The inhibition of hypocotyl negative gravitropism by light is one of the less-understood light responses. Hypocotyl negative gravitropism, which assists seedlings during their emergence from the soil, could hinder their growth toward light, particularly in the complex environment at the soil surface. Accordingly, plants may have adopted phytochrome signaling to inhibit hypocotyl negative gravitropism in light. In support of this inference, seedlings display stronger phototropic responses to directional blue light when negative gravitropism is inhibited (29). Under more natural conditions, the inhibition of hypocotyl negative gravitropism by light increases survival fitness when seeds are covered with mulch (30). It is not also clear how phytochromes inhibit hypocotyl negative gravitropism. The pifQ mutant displays completely disrupted hypocotyl negative gravitropism in the dark, indicating that phytochromes inhibit hypocotyl negative gravitropism by inhibiting PIFs (6). In this article, we investigated how phytochromes inhibit hypocotyl negative gravitropism through PIFs. We show that phytochromes promote the conversion of endodermal amyloplasts to plastids with etioplastic or chloroplastic features by inhibiting PIFs. We further demonstrate that the specific expression of PIF1 in the endodermis of the dark-grown pifQ mutant is sufficient to inhibit the conversion of endodermal amyloplasts to etioplasts, thereby enabling hypocotyl negative gravitropism in this mutant.
Results
PIFs Inhibit the Conversion of Endodermal Amyloplasts to Etioplasts.
Phytochromes inhibit hypocotyl negative gravitropism by inhibiting PIFs in red or far-red light (SI Text and Figs. S1 and S2). We investigated how phytochromes and PIFs regulate hypocotyl negative gravitropism. To simplify our analysis, we focused mainly on the inhibition of hypocotyl negative gravitropism by red light, as follows.
Inhibition of hypocotyl negative gravitropism could be caused by the disruption of gravity sensing, subsequent signaling, or the asymmetric elongation steps. We examined whether gravity sensing is disrupted by red light or by the pifQ mutation. In the analysis, we grew seedlings on vertical plates for 2 d either in the dark or in red light, and then incubated them for 2 more days under the same light conditions after changing the direction of gravity by 90°. Dark-grown wild-type hypocotyls curved against the direction of gravity upon alteration of the gravity vector, whereas red light-grown hypocotyls continued to grow in the same direction (Fig. 1). In contrast to hypocotyls, roots of both dark-grown and red light-grown seedlings curved toward the direction of gravity, indicating that red light inhibits hypocotyl negative gravitropism but does not inhibit root positive gravitropism. The pifQ mutant behaved like red light-grown wild-type seedlings. Irrespective of light conditions, hypocotyls of the pifQ mutant did not respond to the change in direction of gravity, whereas roots of the mutant curved toward the direction of gravity. The curving of roots suggested that both the light-grown wild-type and the pifQ mutant possess the ability to elongate asymmetrically. This finding was further supported by hypocotyl phototropism toward blue light both in the wild-type and the pifQ mutant (Fig. S1C). Taken together, our results indicated that hypocotyls of the pifQ mutant, like those of the light-grown wild-type, cannot sense gravity or cannot process subsequent gravity signaling. However, they do possess the ability to elongate asymmetrically.
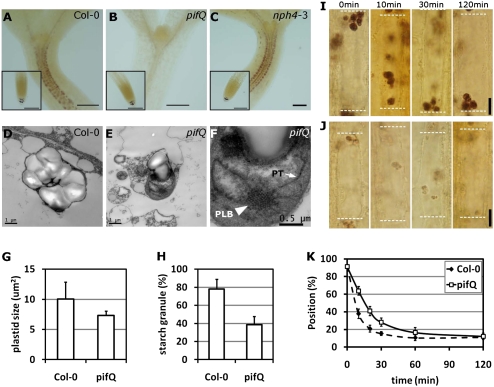
Fig. 1.
Hypocotyls of pifQ mutant seedlings do not respond to changes in the direction of gravity. The direction of gravity was altered by turning plates 90° after the wild-type (Col-0) and the pif quadruple mutant (pifQ) were grown for 2 d either in the dark or in continuous red/far-red light on vertical agar plates. The plates were incubated for another 2 d under the same light conditions. Dc, Rc, and FRc indicate continuous dark, continuous red light (20 μmol·m−2·s−1), and continuous far-red light (2.4 μmol·m−2·s−1) conditions.
Gravity sensing or subsequent signaling could be disrupted by altered expression of previously reported gravitropic genes, such as SHOOT GRAVITROPISM genes (SGR1–SGR5, SGR7), ALTERED RESPONSE TO GRAVITY1 (ARG1) and its related genes (ARL1, ARL2), phosphoglucomutase (PGM), STARCH EXCESS1, -4 (SEX1, SEX4), GRAVITROPIC IN THE LIGHT1 (GIL1), and PROTEIN KINASE SUBSTRATE1, -4 (PKS1, PKS4) (13, 30–33). Among these gravitropic genes, GIL1, SEX1, SEX4, and PKS1, PKS4 are negatively acting gravitropic genes, whereas other genes are positively acting genes for shoot or hypocotyl negative gravitropism. We examined two previously reported pifQ microarray datasets to determine if the expression of these gravitropic genes is altered by the pifQ mutation in both microarray datasets (5, 6). The positively acting gravitropic genes are expressed similarly in dark-grown wild-type and in the pifQ mutant when a 1.5-fold criterion [>1.5-fold, false-discovery rate (FDR) < 0.05) is applied (Fig. S3). The negatively acting gravitropic genes are also expressed similarly. Our results suggest that agravitropism of the pifQ mutant is not caused by severe repression or activation of these previously identified shoot gravitropic genes, with potential exceptions of PKS1 and -4 that show higher expression in the pifQ in one set of microarray data.
Gravity sensing could be compromised by a defect in the development of endodermal amyloplasts. Amyloplasts filled with starch granules are required to sense gravity both in endodermis and in columella cells of root caps. Because PIFs have been shown to regulate chloroplast development (6, 34), we investigated whether PIFs regulate hypocotyl negative gravitropism by modulating the development of amyloplasts in the endodermis. We first examined endodermal amyloplasts by staining them with I2-KI. In dark-grown wild-type seedlings, amyloplasts in the endodermis of the hypocotyl elongation zone and in the columella cells of root cap were stained dark by I2-KI (Fig. 2A). In the dark-grown pifQ mutant, however, no amyloplasts could be detected by I2-KI staining in the endodermis of the hypocotyl elongation zone, but amyloplasts were still detected in the columella cells of the root cap (Fig. 2B). The nph4-3 mutant, which is caused by a mutation in the AUXIN-RESPONSE FACTOR 7 (ARF7) gene, also displayed hypocotyl agravitropism in the dark (35). Unlike the pifQ mutant, the nph4-3 mutant was stained strongly by I2-KI (Fig. 2C), indicating that the lack of amyloplast staining by I2-KI is not a common feature of all agravitropic mutants and that I2-KI can be used to distinguish different defects in gravity sensing and response.
Fig. 2.
Amyloplasts are partially converted to etioplasts in the endodermis of the dark-grown pif quadruple mutant (pifQ). (A–C) I2-KI staining patterns of the wild-type (Col-0) (A), the pifQ mutant (B), and the nph4-3 mutant (C). (Scale bars, 100 μm.). (D and E) TEM images of endodermal plastids of the wild-type (D) and the pifQ (E). (F) A magnified image of a pifQ plastid shows the prolamellar body (PLB) and prothylakoids (PT). (G) Endodermal plastid sizes of wild-type and pifQ mutant. Data are mean with 95% confidence intervals indicated; n = 10. (H) Plastid areas occupied by starch granules in wild-type and pifQ mutant. Data are mean with 95% confidence intervals indicated; n = 10. (I and J) Sedimentation of wild-type amyloplasts (I) and mutant plastids (J) in response to the changing gravity vector. Time indicates minutes after changing the gravity vector by 180°. White broken lines indicate the top and bottom of each endodermal cell. (Scale bars, 10 μm.) (K) Quantification of plastid sedimentation in response to the changing gravity vector. Data are mean with 95% confidence intervals indicated; n = 100.
To further examine the status of endodermal amyloplasts in the dark-grown pifQ mutant, we performed transmission electron microscopy (TEM) of endodermal amyloplasts. Wild-type endodermal amyloplasts were filled with large starch granules (Fig. 2D). In contrast, endodermal plastids of the pifQ mutant contained only small starch granules (Fig. 2E). In addition, they also contained a prolamellar body and prothylakoids (Fig. 2F), which are characteristics of etioplasts. Quantification further showed that plastids sizes are not significantly different between wild-type and the pifQ, but starch granules occupy less area in the pifQ (Fig. 2 G and H). The results indicate that endodermal amyloplasts are converted to plastids with etioplastic features in the dark-grown pifQ mutant, and that the hypocotyls of pifQ mutants are agravitropic because of the conversion of gravity-sensing amyloplasts to other forms of plastids in the endodermis.
We determined if the endodermal plastids of wild-type and pifQ settle equally well in response to changing gravity vector. For this experiment, we first settled plastids of pifQ by reorienting and incubating dark-grown pifQ seedlings vertically. After the plastids settled, we rotated the seedlings 180° and then determined the plastid movement in response to this changed gravity vector. At 10 min after changing the gravity vector, amyloplasts of wild-type moved more than half a cell length (62%), but plastids of pifQ only moved an average of 35% of the cell length (Fig. 2 I–K). Two hours after rotation, plastids of both wild-type and pifQ had moved to the new cell bottoms (89 and 88%, respectively). The results indicate that plastids of pifQ mutant settle more slowly to changing gravity vector.
The conversion of endodermal amyloplasts to other forms of plastids with etioplastic or chloroplastic features and the concomitant reduction of starch granules were also observed in light-grown wild-type seedlings. When wild-type seedlings were grown in monochromatic light, red light-grown endodermal plastids contained small starch granules and developed thylakoids (Fig. 3A). Similarly, far-red light-grown endodermal plastids also contained small starch granules, a prolamellar body, and prothylakoids. Overall, negatively gravitropic dark-grown wild-type seedlings possessed endodermal amyloplasts that were characterized by large starch granules and the lack of thylakoids or prolamellar bodies, whereas seedlings that exhibited agravitropism possessed endodermal plastids that were characterized by relatively small starch granules and the presence of thylakoids or a prolamellar body. During seedling development, I2-KI stains both wild-type and pifQ equally well, irrespective light condition until 36 h after germination induction. At 48 h after the germination induction, however, dark-grown wild-type are stained darker than light-grown wild-type and dark-grown pifQ seedlings, and at 60 h, only dark-grown wild-type seedlings are stained darkly (Fig. S4). The results imply that PIFs inhibit the conversion of amyloplasts to other plastids in the dark.
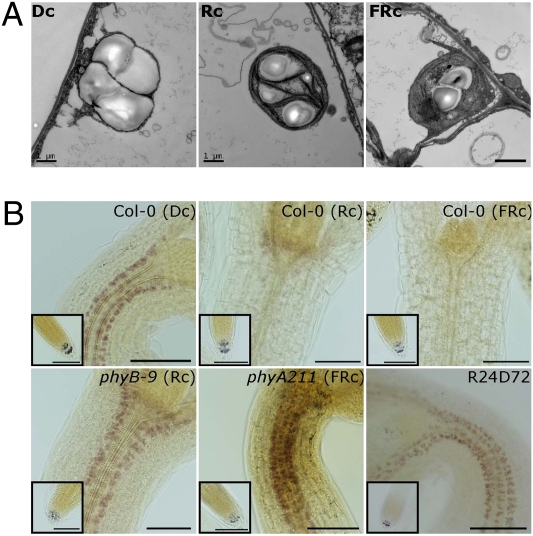
Fig. 3.
Phytochromes promote the conversion of amyloplasts to other forms of plastids in the endodermis. (A) TEM images of endodermal plastids of the wild-type grown in the dark (Dc), in continuous red light (Rc), and in continuous far-red light (FRc). (Scale bars, 1 μm.) (B) I2-KI staining of the wild-type (Col-0), the phyA mutant (phyA-211), and the phyB mutant (phyB-9) grown in the dark (Dc), in continuous red light (Rc), or in continuous far-red light (FRc). R24D72 indicates I2-KI staining of wild-type seedlings grown in red light for 24 h and transferred to the dark for 72 h. (Scale bars, 100 μm.)
The reduction in the size of endodermal starch granules by light was dependent on phytochrome signaling. When the I2-KI solution was used to stain endodermal starch granules in seedlings, I2-KI strongly stained endodermal starch granules in dark-grown seedlings, but not in red or far-red light-grown seedlings (Fig. 3B). These results were consistent with the TEM images of endodermal starch granules of seedlings grown under different light conditions (Fig. 3A). In the phyA mutant, I2-KI stained endodermal starch granules not only in dark-grown seedlings but also in far-red light-grown seedlings. In contrast, in the phyB mutant, I2-KI stained both the dark-grown and red light-grown seedlings (Fig. 3B and Fig. S5). These findings indicated that phytochromes mediate red or far-red light reduction of endodermal starch granule size.
When etiolated seedlings were transferred to red light, seedlings showed slightly weaker staining at 6 h after the transfer, had noticeably fainter staining at 9 h, and exhibited no staining at 12 h after the transfer (Fig. S6). The results indicate that amyloplasts are converted to other plastids rather slowly and further suggest that seedlings should lose their ability to sense gravity rather slowly when transferred to red light. To investigate how long it takes for red light-transferred seedlings to lose the ability to sense gravity, we transferred etiolated seedlings to red light for various times and then transferred them back to dark, and changing the gravity vector by 90°. The ability to sense gravity decreased gradually with increased exposure to red light; if seedlings were incubated more than 9 h, they did not respond to the changing gravity (Fig. S6). The slow loss of gravity sensing is consistent with the slow conversion of amyloplasts under red light. The ability of red light-transferred seedlings to sense gravity soon after transfer was also previously observed (33).
The irreversible inhibition of hypocotyl gravitropism by light (Fig. S2) was associated with irreversible reduction of endodermal starch granules by light. When seedlings were grown in red light for 24 h and transferred to the dark for 3 d, endodermal starch granules were strongly stained by I2-KI, indicating that the first 24 h of red light were not effective in reducing endodermal starch granules (Fig. 3B). However, when seedlings were grown in red light for 36 h and transferred to the dark for 60 h, they were stained only very weakly by the I2-KI solution, indicating that treatment with red light for 36 h causes an irreversible reduction in the size of starch granules and irreversible inhibition of hypocotyl negative gravitropism (Fig. S5). Taken together, our results suggest that the conversion of starch-filled endodermal amyloplasts to other forms of plastids by phytochromes is one of the main light responses associated with the inhibition of hypocotyl negative gravitropism.
Endodermis-Specific PIF1 Is Sufficient to Inhibit the Conversion of Amyloplasts to Etioplast and Hypocotyl Negative Gravitropism.
Because light and the pifQ mutation have been shown to disrupt the development of endodermal amyloplasts, we investigated whether hypocotyl negative gravitropism in the pifQ mutant could be rescued by endodermis-specific PIFs. PIF1 was expressed under the endodermis-specific SCR promoter in the pifQ mutant background (Fig. 4A). The SCR promoter has been shown to be active in the endodermis of roots, hypocotyls, and cotyledons (36). Two independent homozygous lines were established and used for further analysis (SCRpro::PIF1/pifQ #1, #2).
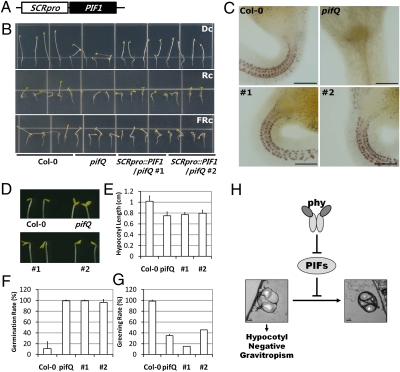
Fig. 4.
Endodermis-specific expression of PIF1 restores hypocotyl negative gravitropism in the pifQ mutant. (A) Schematic diagram showing the SCRpro::PIF1 construct. (B) Restoration of hypocotyl negative gravitropism by endodermal PIF1. The wild-type (Col-0), the pifQ mutant, and two transgenic lines (SCRpro::PIF1/pifQ #1, #2) were grown in the dark (Dc), in red light (Rc), and in far-red light (FRc) on vertical agar plates. (Scale bars, 100 μm.) (C) I2-KI staining of the dark-grown wild-type, the pifQ mutant, and two transgenic lines (#1, #2). (D) Partial restoration of hook formation and cotyledon opening in the dark-grown SCRpro::PIF1/pifQ transgenic lines (#1, #2). (E–G) No restoration of hypocotyl length in the dark (E), germination frequency in the dark (F), and greening during the dark-to-light transition (G) in two SCRpro::PIF1/pifQ transgenic lines (#1, #2). (H) A diagram summarizing the role of PIFs on hypocotyl negative gravitropism. PIFs suppress the conversion of starch-filled endodermal amyloplasts to plastids with small starch granules and more developed thylakoids in the dark. In the light, phytochromes inhibit PIFs by targeting them for protein degradation, which in turn promotes the conversion of gravity sensing amyloplasts to other plastid forms.
Expression of PIF1 by the SCR promoter restored hypocotyl negative gravitropism of the pifQ mutant in the dark. Two SCRpro::PIF1/pifQ lines displayed wild-type–like hypocotyl negative gravitropism in the dark, and also displayed wild-type–like hypocotyl agravitropism in red and far-red light (Fig. 4B). The restoration of hypocotyl negative gravitropism by endodermis-specific expression of PIF1 was accompanied by the restoration of endodermal amyloplasts (Fig. 4C). Taken together, the results indicate that endodermis-specific PIF1 is sufficient to inhibit the conversion of endodermal amyloplast to etioplasts and subsequent hypocotyl negative gravitropism in the dark.
We also investigated whether endodermis-specific PIF1 can rescue other phenotypes associated with the pifQ mutant. Among light responses, SCRpro::PIF1 partially rescued hook formation and cotyledon-opening phenotypes of the pifQ mutant in the dark (Fig. 4D), indicating that endodermal PIFs contribute to hook formation and cotyledon closure in the dark. Unlike hook and cotyledon phenotypes, the SCRpro::PIF1 lines still had short hypocotyls in the dark (Fig. 4E). These lines also exhibited high germination frequency in the dark (Fig. 4F), as well as a higher degree of bleaching during the dark-to-light transition (Fig. 4G). These results indicated that endodermis-specific PIF1 can rescue some pifQ mutant phenotypes either fully (e.g., hypocotyl gravitropism) or partially (e.g., hook formation and cotyledon opening), but cannot rescue others (e.g., hypocotyl elongation, bleaching during the dark-to-light transition, and germination in the dark). It should be noted, however, that, although the SCR promoter was shown to be specific to the endodermis, one cannot completely rule out the possibility of weak expression of PIF1 in other tissues.
Discussion
In previous work, we reported that pif1 and pif3 single mutants and their double mutants have reduced levels of hypocotyl negative gravitropism in the dark, whereas the pifQ mutant is completely agravitropic in the dark. This finding led us to conclude that phytochromes inhibit hypocotyl negative gravitropism by inhibiting these four PIFs. Other studies have shown that phytochromes inhibit PIFs by destabilizing PIF proteins. It is unclear, however, how PIFs promote hypocotyl negative gravitropism. In this report, we show that the pifQ mutant possesses endodermal plastids with small starch granules and etioplastic features, such as a prolamellar body and prothylakoids, instead of starch-filled amyloplasts, indicating that PIFs are necessary for the development of endodermal amyloplasts for the sensing of gravity in the dark. We also show that phytochromes promote the partial conversion of endodermal amyloplasts to other forms of plastids with small starch granules and etioplastic or chloroplastic features in far-red or red light conditions, indicating that endodermal amyloplasts are also targets of phytochrome action during seedling development. By analyzing transgenic plants expressing PIF1 under the SCR promoter, we further demonstrated that endodermal PIF1 is sufficient to restore endodermal amyloplasts and hypocotyl negative gravitropism in the pifQ mutant. Our results link hypocotyl negative gravitropism to endodermal plastid development and suggest that the molecular mechanisms regulating these two seemingly distinct processes are closely related (Fig. 4H).
The conversion of endodermal amyloplasts to other forms of plastids by light or by mutations in PIF genes might be partially associated with changes in the expression of various genes, such as starch metabolic enzyme genes, chlorophyll biosynthetic genes, and photosynthetic genes. As shown in Figs. 2 and 3, dark-grown endodermal amyloplasts are characterized by large starch granules and the lack of a noticeable prolamellar body and prothylakoids. In contrast, endodermal plastids in the dark-grown pifQ mutant and the far-red light-grown wild-type are characterized by small starch granules and conspicuous prolamellar bodies and prothylakoids, whereas endodermal plastids in the red light-grown wild-type are characterized by small starch granules and well-developed thylakoids. Consistent with these morphological changes in plastids, previous microarray data indicate that both red light-grown wild-type and the dark-grown pifQ mutant seedlings express higher levels of chlorophyll biosynthetic genes and photosynthetic genes compared with dark-grown wild-type seedlings (5, 6). Expression patterns of starch metabolic genes are also consistent with the reduced levels of starch in the pifQ mutant (Fig. S7). Among starch metabolic genes, some starch degrading enzymes genes, including the debranching enzyme gene (LDA1) and glucan phosphorylase (PHS1) are expressed higher (criterion: >1.5-fold, FDR < 0.05) in the dark-grown pifQ mutant seedlings in two independent microarray data (5, 6), indicating that smaller starch granules in the pifQ seedlings are associated with higher expression of starch-degrading enzyme genes. Whether the up-regulation of these starch metabolic genes is sufficient to reduce the size of starch granules in endodermal amyloplsts needs further testing.
Our data do not exclude the possibility that PIFs regulate hypocotyl negative gravitropism also partly through auxin. Auxin is responsible for the tropic elongation downstream of gravity sensing or photosensing, as shown by altered gravitropism or phototropism of various auxin-related mutants, including nph4, pgp19, shy2-1D, pin2, pin3, and aux1 (28, 37–44). Phytochromes have been shown to regulate auxin biosynthetic genes, transport genes, and signaling genes either at the transcriptional level or at the protein level (37, 45–47), suggesting that phytochromes may regulate some of these genes through PIFs. Consistent with this idea, an analysis of two previously reported microarray datasets (5, 6) indicate that PIFs regulate an auxin transport gene (PGP4) and two auxin signaling genes (ARF18, IAA29) (criterion: >1.5-fold, FDR < 0.05) in two independent microarray data (Table S1). In imbibed seeds, PIF1 was shown to directly bind to promoters of ARF18 and PGP4 (12), indicating that PIFs may regulate ARF18 and PGP4 by directly binding to their promoters also in seedlings. Currently, it is not clear if these auxin-related genes regulate the hypocotyl negative gravitropism. It should be also noted that the criterion we applied is an arbitrary criterion; thus, genes that do not pass the criterion might still play important roles in the hypocotyl negative gravitropism. In regard to this theory, it is noteworthy that the expression of PGP19 that was shown to regulate tropic elongation downstream of phytochromes (37) is only mildly altered in the microarray data. Thus, further analyses are required to determine if PIFs regulate hypocotyl negative gravitropism also through changes in auxin levels or signaling.
Materials and Methods
Plant Materials and Growth Conditions.
Arabidopsis thaliana plants were grown in a growth room with a 16-h light/8-h dark cycle at 22 to 24 °C for general growth and seed harvesting. To express PIF1 under endodermis-specific SCR promoter, a SCR promoter (∼1.7 kb) and PIF1 cDNA were amplified with specific primer sets (SCRpro LP: 5′-GAGAA AGCTT CTATT CAAAT ATGGA CTTGG AGAA-3′, SCRpro RP: 5′-GAGAT CTAGA GGAGA TTGAA GGGTT GTTG-3′, PIF1 LP: 5′-GAGAC CTAGG ATGCA TCATT TTGTC CC T-3′ PIF1 RP: 5′-GAGAT GATCA AACCT GTTGT GTGGT TTCCG TGA-3′) and cloned into pCAMBIA1300 vector to generate a SCRpro::PIF1 vector. The SCRpro::PIF1 vector was then transformed into the pifQ mutant and two independent homozygous lines were subsequently selected for the analysis. All Arabidopsis plants used in the experiments (Col-0, phyA-211, phyB-9, pifQ, SCRpro::PIF1/pifQ #1, #2) are Col-0 ecotype.
Hypocotyl Negative Gravitropism, Photobleaching, and Germination Assays.
For hypocotyl negative gravitropism assay and photobleaching assay, surface-sterilized seeds were plated on MS agar (1/2 MS, 0.8% phytoagar, and 0.05% Mes, pH 5.7), imbibed for 3 d at 4 °C in the dark, and irradiated with white light (100 μmol·m−2·s−1) for 3 h for the induction of germination. For the hypocotyl negative gravitropism assay, plates were incubated vertically for 3 d in the dark, continuous red light (20 μmol·m−2·s−1), or continuous far-red light (2.4 μmol·m−2·s−1) at 22 °C. Growth orientations of hypocotyls were determined by the degrees from vertical axis. For the dark-to-red or red-to-dark transfer experiments, seedlings were incubated either in the dark or red light for various hours and transfered to either red light or dark, respectively. Total incubation time after the induction of germination was 96 h. To investigate if seedlings can respond to the changing direction of gravity, seedlings were incubated vertically for 2 d either in the dark or red light, turned by 90° counterclockwise, and incubated 2 more days in the same light conditions. Photobleaching assay and germination assay were performed as described previously (6, 48).
Visualization of Endodermal Amyloplasts.
To visualize endodermal amyloplasts by iodine staining, seedlings were fixed in FAA (5% Formaldehyde, 45% Ethanol, 5% Acetic acid) solution for 24 h at 4 °C. After fixation, seedlings were rinsed in 50% (vol/vol) ethanol once and stained in I2-KI solution [2% (wt/vol) iodine, 5% (wt/vol) potassium iodine and 20% (wt/vol) chloral hydrate] for 1 min. Samples were de-stained in 1:1:1 trichloroacetic acid: phenol: lactic acid = 1:1:1) for 5 min and carefully mounted on slides with a drop of destaining solution for the light microscopic observation.
To visualize endodermal amyloplasts by EM, seedlings were fixed with 3% glutaraldehyde in PBS for 2 h at room temperature. After fixation, seedlings were washed five times with 0.1 M cacodylate buffer (pH 7.2) containing 0.1% CaCl2 at 4 °C and then postfixed with 1% OsO4 in 0.1 M cacodylate buffer (pH 7.2) containing 0.1% CaCl2 for 3 h at 4 °C. After rinsing with cold distilled water, samples were dehydrated slowly with an ethanol series and propylene oxide at 4 °C. The samples were embedded in Spurr's epoxy resin. After the polymerization of the resin at 70 °C for 36 h, serial sections were made with a diamond knife and mounted on formvar-coated slot grids. Sections were stained with 4% uranyl acetate for 10 min and lead citrate for 7 min. Samples were observed by a Tecnai G2 Spirit Twin transmission electron microscope (FEI Company) and JEM ARM 1300S high-voltage electron microscope (JEOL).
To determine the sedimentation speed, both wild-type and pifQ mutants were grown in the dark on vertical agar plates. Two-d-old dark-grown pifQ seedlings were then reoriented vertically and incubated 12 more hours in the dark to settle down plastids to the bottom. Plates were turned upside down to change the gravity vector by 180°. At various times after the changing gravity vector, seedlings were transferred to FAA solution and stained using I2-KI solution. The relative positions of plastids were determined by assigning the original bottom of a cell to 0 and the top of the cell to 1.
Microarray Analysis.
Microarray data from Shin et al. were analyzed as reported previously (6). For reanalysis of microarray data from Leivar et al. (5), RMA normalized data were downloaded from the National Center for Biotechnology Information GEO database http://www.ncbi.nlm.nih.gov/geo/query/browse.cgi (accession no. GSE17159) and analyzed in R/Bioconductor [R Development Core Team, 2010 (49)] using the packages GEOquerry and limma (50–52). We contrasted expression values for wild-type and pif quadruple mutant seedlings grown for 2 d in dark conditions. Genes with a FDR < 0.05 and a fold-change > 1.5 in both microarray data were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Patricia Müller-Moulé for helpful suggestions. This work was supported in part by Grants 2010-0018926 and 2010-0000291 from the National Research Foundation of Korea (to G.C.) and Grant IOS-0820854 from the National Science Foundation (to J.N.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011066108/-/DCSupplemental.
References
- 1.Bae G, Choi G. Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol. 2008;59:281–311. doi: 10.1146/annurev.arplant.59.032607.092859. [DOI] [PubMed] [Google Scholar]
- 2.Franklin KA, Quail PH. Phytochrome functions in Arabidopsis development. J Exp Bot. 2009;61:11–24. doi: 10.1093/jxb/erp304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huq E. Degradation of negative regulators: A common theme in hormone and light signaling networks? Trends Plant Sci. 2006;11:4–7. doi: 10.1016/j.tplants.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Leivar P, et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol. 2008;18:1815–1823. doi: 10.1016/j.cub.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leivar P, et al. Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell. 2009;21:3535–3553. doi: 10.1105/tpc.109.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin J, et al. Phytochromes promote seedling light responses by inhibiting four negatively-acting phytochrome-interacting factors. Proc Natl Acad Sci USA. 2009;106:7660–7665. doi: 10.1073/pnas.0812219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorrain S, Trevisan M, Pradervand S, Fankhauser C. Phytochrome interacting factors 4 and 5 redundantly limit seedling de-etiolation in continuous far-red light. Plant J. 2009;60:449–461. doi: 10.1111/j.1365-313X.2009.03971.x. [DOI] [PubMed] [Google Scholar]
- 8.Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-García JF, Huq E, Quail PH. Direct targeting of light signals to a promoter element-bound transcription factor. Science. 2000;288:859–863. doi: 10.1126/science.288.5467.859. [DOI] [PubMed] [Google Scholar]
- 10.Huq E, et al. Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science. 2004;305:1937–1941. doi: 10.1126/science.1099728. [DOI] [PubMed] [Google Scholar]
- 11.Shin J, Park E, Choi G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 2007;49:981–994. doi: 10.1111/j.1365-313X.2006.03021.x. [DOI] [PubMed] [Google Scholar]
- 12.Oh E, et al. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell. 2009;21:403–419. doi: 10.1105/tpc.108.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita MT, Tasaka M. Gravity sensing and signaling. Curr Opin Plant Biol. 2004;7:712–718. doi: 10.1016/j.pbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Sack FD. Plastids and gravitropic sensing. Planta. 1997;203(Suppl 1):S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- 15.Stanga JP, Boonsirichai K, Sedbrook JC, Otegui MS, Masson PH. A role for the TOC complex in Arabidopsis root gravitropism. Plant Physiol. 2009;149:1896–1905. doi: 10.1104/pp.109.135301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukaki H, et al. Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J. 1998;14:425–430. doi: 10.1046/j.1365-313x.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- 17.Benfey PN, et al. Root development in Arabidopsis: Four mutants with dramatically altered root morphogenesis. Development. 1993;119:57–70. doi: 10.1242/dev.119.Supplement.57. [DOI] [PubMed] [Google Scholar]
- 18.Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. [PubMed] [Google Scholar]
- 19.Weise SE, Kiss JZ. Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. Int J Plant Sci. 1999;160:521–527. doi: 10.1086/314142. [DOI] [PubMed] [Google Scholar]
- 20.Tanimoto M, Tremblay R, Colasanti J. Altered gravitropic response, amyloplast sedimentation and circumnutation in the Arabidopsis shoot gravitropism 5 mutant are associated with reduced starch levels. Plant Mol Biol. 2008;67:57–69. doi: 10.1007/s11103-008-9301-0. [DOI] [PubMed] [Google Scholar]
- 21.Kiss JZ, Guisinger MM, Miller AJ, Stackhouse KS. Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol. 1997;38:518–525. doi: 10.1093/oxfordjournals.pcp.a029199. [DOI] [PubMed] [Google Scholar]
- 22.Vitha S, Yang M, Sack FD, Kiss JZ. Gravitropism in the starch excess mutant of Arabidopsis thaliana. Am J Bot. 2007;94:590–598. doi: 10.3732/ajb.94.4.590. [DOI] [PubMed] [Google Scholar]
- 23.Kato T, et al. SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell. 2002;14:33–46. doi: 10.1105/tpc.010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita MT, et al. A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J. 2006;47:619–628. doi: 10.1111/j.1365-313X.2006.02807.x. [DOI] [PubMed] [Google Scholar]
- 25.Saito C, Morita MT, Kato T, Tasaka M. Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell. 2005;17:548–558. doi: 10.1105/tpc.104.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano D, et al. A SNARE complex containing SGR3/AtVAM3 and ZIG/VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc Natl Acad Sci USA. 2003;100:8589–8594. doi: 10.1073/pnas.1430749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi H, Miyazawa Y, Fujii N. Hormonal interactions during root tropic growth: Hydrotropism versus gravitropism. Plant Mol Biol. 2009;69:489–502. doi: 10.1007/s11103-008-9438-x. [DOI] [PubMed] [Google Scholar]
- 28.Esmon CA, Pedmale UV, Liscum E. Plant tropisms: Providing the power of movement to a sessile organism. Int J Dev Biol. 2005;49:665–674. doi: 10.1387/ijdb.052028ce. [DOI] [PubMed] [Google Scholar]
- 29.Lariguet P, Fankhauser C. Hypocotyl growth orientation in blue light is determined by phytochrome A inhibition of gravitropism and phototropin promotion of phototropism. Plant J. 2004;40:826–834. doi: 10.1111/j.1365-313X.2004.02256.x. [DOI] [PubMed] [Google Scholar]
- 30.Allen T, Ingles PJ, Praekelt U, Smith H, Whitelam GC. Phytochrome-mediated agravitropism in Arabidopsis hypocotyls requires GIL1 and confers a fitness advantage. Plant J. 2006;46:641–648. doi: 10.1111/j.1365-313X.2006.02727.x. [DOI] [PubMed] [Google Scholar]
- 31.Boonsirichai K, Sedbrook JC, Chen R, Gilroy S, Masson PH. ALTERED RESPONSE TO GRAVITY is a peripheral membrane protein that modulates gravity-induced cytoplasmic alkalinization and lateral auxin transport in plant statocytes. Plant Cell. 2003;15:2612–2625. doi: 10.1105/tpc.015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan C, Rosen ES, Boonsirichai K, Poff KL, Masson PH. The ARG1-LIKE2 gene of Arabidopsis functions in a gravity signal transduction pathway that is genetically distinct from the PGM pathway. Plant Physiol. 2003;133:100–112. doi: 10.1104/pp.103.023358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schepens I, Boccalandro HE, Kami C, Casal JJ, Fankhauser C. PHYTOCHROME KINASE SUBSTRATE4 modulates phytochrome-mediated control of hypocotyl growth orientation. Plant Physiol. 2008;147:661–671. doi: 10.1104/pp.108.118166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephenson PG, Fankhauser C, Terry MJ. PIF3 is a repressor of chloroplast development. Proc Natl Acad Sci USA. 2009;106:7654–7659. doi: 10.1073/pnas.0811684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper RM, et al. The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell. 2000;12:757–770. doi: 10.1105/tpc.12.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN. Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development. 2000;127:595–603. doi: 10.1242/dev.127.3.595. [DOI] [PubMed] [Google Scholar]
- 37.Nagashima A, et al. Phytochromes and cryptochromes regulate the differential growth of Arabidopsis hypocotyls in both a PGP19-dependent and a PGP19-independent manner. Plant J. 2008;53:516–529. doi: 10.1111/j.1365-313X.2007.03358.x. [DOI] [PubMed] [Google Scholar]
- 38.Moon J, et al. A new CULLIN 1 mutant has altered responses to hormones and light in Arabidopsis. Plant Physiol. 2007;143:684–696. doi: 10.1104/pp.106.091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BC, Soh MS, Hong SH, Furuya M, Nam HG. Photomorphogenic development of the Arabidopsis shy2-1D mutation and its interaction with phytochromes in darkness. Plant J. 1998;15:61–68. doi: 10.1046/j.1365-313x.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- 40.Chen R, et al. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller A, et al. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- 44.Marchant A, et al. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;18:2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carabelli M, et al. Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev. 2007;21:1863–1868. doi: 10.1101/gad.432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colón-Carmona A, Chen DL, Yeh KC, Abel S. Aux/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol. 2000;124:1728–1738. doi: 10.1104/pp.124.4.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh E, et al. PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell. 2004;16:3045–3058. doi: 10.1105/tpc.104.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gentleman RC, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sean D, Meltzer PS. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 51.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3(Article3) doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 52.Smyth GK. Limma: Linear models for microarray data. In: Gentlemen R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.