Abstract
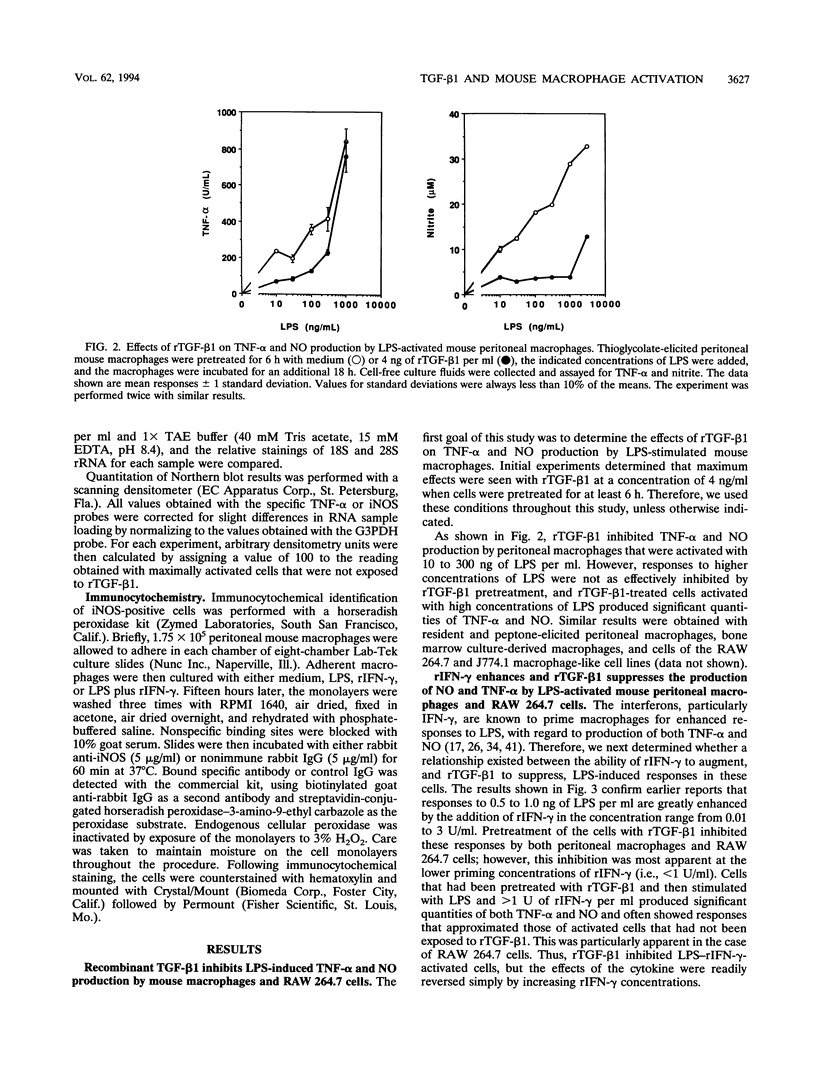
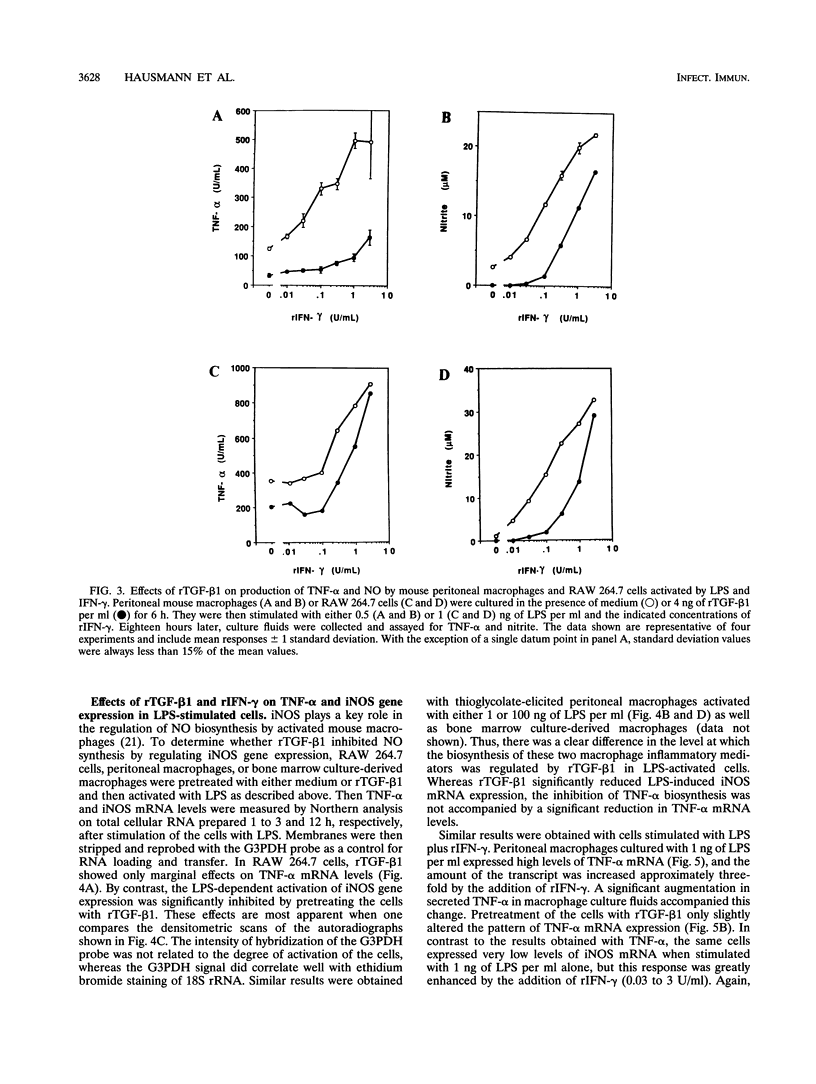
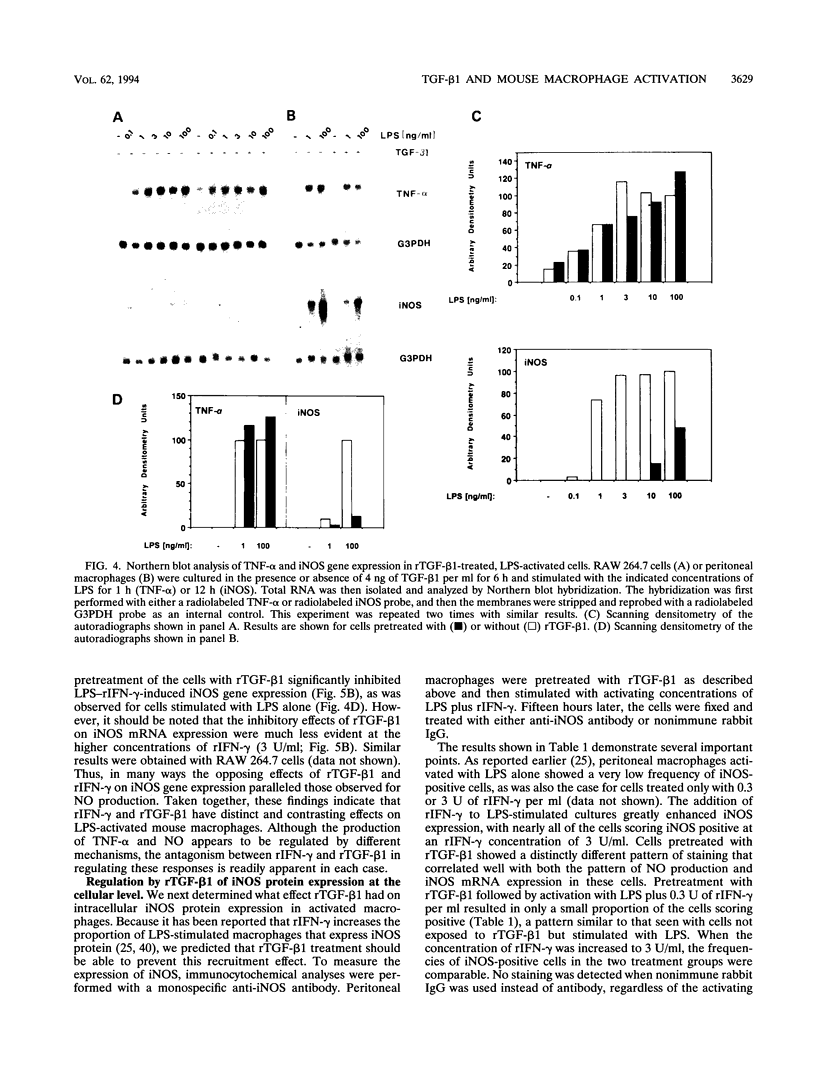
Bacterial lipopolysaccharides (LPS) are potent inducers of macrophage activation, leading to the production of a number of proinflammatory mediators. Although several cytokines that prime macrophages for enhanced LPS-triggered responses have been identified, far less is known regarding the role that cytokines play in down-regulating macrophage responses to LPS. This study was designed to determine the effects of recombinant transforming growth factor beta 1 (rTGF-beta 1) on macrophage activation by LPS. Pretreatment of either mouse peritoneal macrophages or cells of the RAW 264.7 macrophage-like cell line with rTGF-beta 1 inhibited their ability to produce both tumor necrosis factor alpha (TNF-alpha) and nitric oxide (NO) in response to LPS. These inhibitory effects were reversed by increasing the concentration of LPS or by priming cells with optimal concentrations of recombinant gamma interferon (rIFN-gamma). Pretreatment of cells with rTGF-beta 1 had only a modest inhibitory effect on the expression of TNF-alpha mRNA. By contrast, the expression of mRNA for the inducible form of nitric oxide synthase (iNOS), which is responsible for NO production in activated macrophages, was significantly inhibited by rTGF-beta 1 pretreatment. Thus, rTGF-beta 1-dependent suppression of macrophage TNF-alpha biosynthesis was manifest at a posttranscriptional level, whereas the inhibition of NO production correlated with a direct effect on iNOS gene expression. Importantly, both of these suppressive effects of rTGF-beta 1 were reversed by exposing the cells to priming concentrations of rIFN-gamma. As with NO production, immunocytochemical analysis of iNOS expression in LPS-stimulated macrophages revealed that rIFN-gamma and rTGF-beta 1 had antagonistic effects, with the former increasing, and the latter reducing, the number of iNOS-expressing cells induced by LPS. These data suggest that a balance between the priming effects of IFN-gamma and the inhibitory effects of TGF-beta 1 can determine the overall level of macrophage activation induced by LPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez L. E. Production of transforming growth factor-beta by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-gamma. J Immunol. 1993 Mar 1;150(5):1838–1845. [PubMed] [Google Scholar]
- Bogdan C., Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci. 1993 Jun 23;685:713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Paik J., Vodovotz Y., Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992 Nov 15;267(32):23301–23308. [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Paik J., Xie Q. W., Nathan C. Traces of bacterial lipopolysaccharide suppress IFN-gamma-induced nitric oxide synthase gene expression in primary mouse macrophages. J Immunol. 1993 Jul 1;151(1):301–309. [PubMed] [Google Scholar]
- Chantry D., Turner M., Abney E., Feldmann M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989 Jun 15;142(12):4295–4300. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Shalaby M. R., Lackides G. A., Lewis G. D., Shepard H. M., Palladino M. A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987 Aug 1;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Oswald I. P., Hieny S., James S. L., Sher A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur J Immunol. 1992 Oct;22(10):2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- Gilbert R. S., Herschman H. R. Transforming growth factor beta differentially modulates the inducible nitric oxide synthase gene in distinct cell types. Biochem Biophys Res Commun. 1993 Aug 31;195(1):380–384. doi: 10.1006/bbrc.1993.2054. [DOI] [PubMed] [Google Scholar]
- Haak-Frendscho M., Wynn T. A., Czuprynski C. J., Paulnock D. Transforming growth factor-beta 1 inhibits activation of macrophage cell line RAW 264.7 for cell killing. Clin Exp Immunol. 1990 Nov;82(2):404–410. doi: 10.1111/j.1365-2249.1990.tb05461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo R., Takeda K., Nagumo M., Konno K. Effects of combinations of transforming growth factor-beta 1 and tumor necrosis factor on induction of differentiation of human myelogenous leukemic cell lines. J Immunol. 1990 Feb 15;144(4):1311–1316. [PubMed] [Google Scholar]
- Keller R., Keist R., van der Meide P. H. Modulation of tumoricidal activity, induced in bone-marrow-derived mononuclear phagocytes by interferon gamma or Corynebacterium parvum, by interferon beta, tumor necrosis factor, prostaglandin E2, and transforming growth factor beta. Int J Cancer. 1991 Nov 11;49(5):796–800. doi: 10.1002/ijc.2910490526. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsbach R. B., Murphy W. J., Lowenstein C. J., Snyder S. H., Russell S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993 Jan 25;268(3):1908–1913. [PubMed] [Google Scholar]
- Lowenstein C. J., Glatt C. S., Bredt D. S., Snyder S. H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nelson B. J., Ralph P., Green S. J., Nacy C. A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J Immunol. 1991 Mar 15;146(6):1849–1857. [PubMed] [Google Scholar]
- Noble P. W., Henson P. M., Lucas C., Mora-Worms M., Carré P. C., Riches D. W. Transforming growth factor-beta primes macrophages to express inflammatory gene products in response to particulate stimuli by an autocrine/paracrine mechanism. J Immunol. 1993 Jul 15;151(2):979–989. [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., LeBlanc P. A., Murasko D. M. Comparative effects of various classes of mouse interferons on macrophage activation for tumor cell killing. J Immunol. 1985 Feb;134(2):977–981. [PubMed] [Google Scholar]
- Parmely M. J., Zhou W. W., Edwards C. K., 3rd, Borcherding D. R., Silverstein R., Morrison D. C. Adenosine and a related carbocyclic nucleoside analogue selectively inhibit tumor necrosis factor-alpha production and protect mice against endotoxin challenge. J Immunol. 1993 Jul 1;151(1):389–396. [PubMed] [Google Scholar]
- Parmely M., Gale A., Clabaugh M., Horvat R., Zhou W. W. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun. 1990 Sep;58(9):3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson D. M., LeClaire R. D., Lorsbach R. B., Parmely M. J., Russell S. W. Regulation by transforming growth factor-beta 1 of expression and function of the receptor for IFN-gamma on mouse macrophages. J Immunol. 1992 Sep 15;149(6):2028–2034. [PubMed] [Google Scholar]
- Ranges G. E., Figari I. S., Espevik T., Palladino M. A., Jr Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987 Oct 1;166(4):991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F., Varesio L., Ochoa A., Ortaldo J. Pleiotropic effects of transforming growth factor-beta on cells of the immune system. Ann N Y Acad Sci. 1993 Jun 23;685:488–500. doi: 10.1111/j.1749-6632.1993.tb35911.x. [DOI] [PubMed] [Google Scholar]
- Santambrogio L., Hochwald G. M., Saxena B., Leu C. H., Martz J. E., Carlino J. A., Ruddle N. H., Palladino M. A., Gold L. I., Thorbecke G. J. Studies on the mechanisms by which transforming growth factor-beta (TGF-beta) protects against allergic encephalomyelitis. Antagonism between TGF-beta and tumor necrosis factor. J Immunol. 1993 Jul 15;151(2):1116–1127. [PubMed] [Google Scholar]
- Sessa W. C., Harrison J. K., Barber C. M., Zeng D., Durieux M. E., D'Angelo D. D., Lynch K. R., Peach M. J. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem. 1992 Aug 5;267(22):15274–15276. [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. S., Twardzik D. R., Reed S. G. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-beta). J Exp Med. 1991 Sep 1;174(3):539–545. doi: 10.1084/jem.174.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Ding A., Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988 Jul 21;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Sporn M., Nathan C. Comparison of transforming growth factor-beta and a macrophage- deactivating polypeptide from tumor cells. Differences in antigenicity and mechanism of action. J Immunol. 1989 May 15;142(10):3462–3468. [PubMed] [Google Scholar]
- Vodovotz Y., Bogdan C., Paik J., Xie Q. W., Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993 Aug 1;178(2):605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Alley E. W., Russell S. W., Morrison D. C. Necessity and sufficiency of beta interferon for nitric oxide production in mouse peritoneal macrophages. Infect Immun. 1994 Jan;62(1):33–40. doi: 10.1128/iai.62.1.33-40.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Morrison D. C. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J Exp Med. 1993 Feb 1;177(2):511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Morrison D. C. Pertussis toxin-sensitive factor differentially regulates lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production in mouse peritoneal macrophages. J Immunol. 1993 Feb 1;150(3):1011–1018. [PubMed] [Google Scholar]