Summary
Intercellular signaling is important for accurate circadian rhythms. In Drosophila, the small ventral lateral neurons (s-LNvs) are the dominant pacemaker neurons and set the pace of most other clock neurons in constant darkness. Here we show that two distinct G-protein signaling pathways are required in LNvs for 24hr rhythms. Reducing signaling in LNvs via the G-alpha subunit Gs, which signals via cAMP, or via the G-alpha subunit Go, which we show signals via Phospholipase 21c, lengthens the period of behavioral rhythms. In contrast, constitutive Gs or Go signaling makes most flies arrhythmic. Using dissociated LNvs in culture, we found that Go and the metabotropic GABAB-R3 receptor are required for the inhibitory effects of GABA on LNvs and that reduced GABAB-R3 expression in vivo lengthens period. Although no clock neurons produce GABA, hyper-exciting GABAergic neurons disrupts behavioral rhythms and s-LNv molecular clocks. Therefore, s-LNvs require GABAergic inputs for 24hr rhythms.
Introduction
Circadian rhythms are found in organisms as diverse as plants and humans. In all organisms so far studied, these ~24hr rhythms are driven by intracellular molecular clocks that consist of transcriptional/translational feedback loops with additional post-translational regulation. The importance of molecular clocks in determining period length is exemplified by the numerous clock gene mutations that alter molecular clock speed and behavioral rhythms (reviewed by Allada et al., 2001). Since many mammalian cells show rhythmic clock gene expression in culture, intracellular clocks are often considered cell-autonomous (Balsalobre et al., 1998; Welsh et al., 1995).
However, intercellular communication is also important for circadian rhythms. For example, signals from master pacemaker neurons in the mammalian suprachiasmatic nucleus (SCN) regulate the phase of clocks in peripheral organs (Reppert and Weaver, 2002). Coupling of pacemaker neurons within the SCN is also important because individual pacemaker neurons from a single animal display a range of periods of electrical rhythms when dispersed in culture, whereas only one period is measured in SCN explants and animals (Herzog et al., 1998; Liu et al., 1997). Similarly, deletions of either mPeriod1 (mPer1) or mCryptochrome1 (mCry1) clock genes dramatically weakened molecular rhythms in individual dissociated SCN cells but had minimal effects on molecular rhythms in SCN explants or animal behavioral rhythms (Liu et al., 2007). Presumably, coupling between cells rescues the genetically weakened individual SCN oscillators and suggests that signaling between clock neurons is essential for robust SCN rhythms.
Further support for SCN intercellular communication comes from VPAC2R−/− mice. VPAC2R encodes a G-protein coupled receptor (GPCR) expressed by many SCN neurons and is activated by the neuropeptides VIP and PACAP (Harmar et al., 1998). VPAC2R−/− mutant mice are behaviorally arrhythmic (Harmar et al., 2002) and most neurons in SCN slices from VPAC2R−/− mutants lose mPer1::luciferase rhythms (Maywood et al., 2006). Thus disrupting a membrane-bound receptor that presumably acts as an input to SCN neurons prevented molecular rhythms even though core clock genes were genetically unaffected.
Drosophila have ~150 clock neurons in discrete clusters in the brain, named after their location: In each hemisphere, there are 4 small and 4 large ventral Lateral Neurons (s- and l-LNvs) that synthesize the key circadian neuropeptide Pigment Dispersing Factor (PDF). There are also: a 5th PDF-negative s-LNv; 6 dorsal Lateral neurons (LNds); 3 Lateral posterior clock neurons (LPNs) and ~50 clock neurons located in three different dorsal clusters (DN1-3). Over-expression of the Shaggy/GSK3 (Sgg) kinase only in s-LNvs speeds up their own molecular clocks and the clocks in most other central brain clock neurons. In contrast, sgg over-expression in all clock neurons except LNvs does not alter the speed of s-LNv or most other molecular clocks (Stoleru et al., 2005). Therefore s-LNvs seem to be the master pacemakers in constant darkness (DD) and set the pace for much of the Drosophila clock network. Although the ability of individual Drosophila clock neurons to generate 24hr rhythms has not been tested in culture, intercellular communication between Drosophila pacemaker neurons could explain how molecular and behavioral rhythms persist in DD in vivo (Lin et al., 2004; Peng et al., 2003; Yoshii et al., 2009). In contrast, oscillations in peripheral clocks, which are not coupled to each other, dampen in DD (Stanewsky et al., 1997).
Although s-LNvs are pacemakers in DD, they require signals from their cell membrane for 24hr rhythms. For example, s-LNv molecular clocks desynchronize and/or run down in DD in Pdf01 null mutant flies (Lin et al., 2004; Peng et al., 2003; Yoshii et al., 2009) and run down when hyperpolarized in DD (Nitabach et al., 2002). Pdf01 and PDF receptor (pdfr) mutant flies are either arrhythmic or have short period behavioral rhythms in DD (Hyun et al., 2005; Lear et al., 2005; Mertens et al., 2005; Renn et al., 1999). Like VPAC2R, the PDFR is a GPCR which signals via cAMP at least in vitro (Hyun et al., 2005; Mertens et al., 2005) and LNvs also seem to respond to PDF (Im and Taghert, 2010; Shafer et al., 2008).
LNvs also respond to neurotransmitters from other neurons. The Hofbauer-Buchner eyelet photoreceptor cells project to LNvs and produce acetylcholine (ACh) and histamine (Pollack and Hofbauer, 1991; Yasuyama and Meinertzhagen, 1999). Serotonergic neurons project to adult LNvs and modulate light entrainment via the metabotropic 5-HT1B receptor in LNvs (Yuan et al., 2005) and l-LNvs respond to GABA via the ionotropic GABAA receptor, RDL, to regulate sleep and arousal (Chung et al., 2009; Parisky et al., 2008). Larval LNvs, which become the adult s-LNvs, respond directly to ACh, GABA and glutamate in vitro and produce glutamate and GABA metabotropic receptors (Hamasaka et al., 2007; Hamasaka et al., 2005; Wegener et al., 2004). Although glutamatergic and GABAergic neurons project to larval and adult LNvs (Hamasaka et al., 2007; Hamasaka et al., 2005), their role in circadian rhythms is largely unknown.
We first addressed the role of GPCRs in s-LNvs by manipulating G-protein signaling. We focused on two G-protein alpha subunits: G-sα60A (Gs), which activates Adenylate cyclase to produce cAMP; and G-oα47A (Go), whose signaling mechanism was unclear previous to this study, but likely cAMP-independent (Ferris et al., 2006). We found that reduced signaling via either Gs or Go in LNvs lengthened the period of behavioral rhythms, while constitutively activating Gs or Go made flies largely arrhythmic. In epistasis experiments, expressing the cAMP phosphodiesterase dunce (dnc) rescued the arrhythmicity of constitutively active Gs and lengthened period when expressed alone, showing that Gs signals via cAMP to regulate period length in LNvs. Similarly, reducing Phospholipase C 21C (Plc21C) expression rescued arrhythmicity induced by a constitutively active Go transgene and lengthened behavioral period on its own, indicating that PLC21C lies downstream of Go in LNvs. Given this previously unsuspected role of Go signaling in maintaining 24hr rhythms in LNvs, we sought to identify potential receptors and ligands that signal via Go.
We measured Ca2+ responses of dissociated LNvs in culture and found that inhibiting Go made LNvs unresponsive to GABA but did not affect LNv responses to ACh or glutamate. We found that GABA likely signals via the metabotropic GABA receptor GABAB-R3 in LNvs since knocking down its expression strongly reduced the response to GABA in culture and lengthened behavioral rhythms in adult flies. Although LNvs do not produce GABA themselves (Hamasaka et al., 2005), we found that hyper-exciting GABAergic neurons disrupts animal behavioral rhythms and s-LNv molecular clocks. Therefore s-LNvs integrate signals from GABAergic neurons as part of a network that generates 24hr rhythms.
Results
Gs-mediated signaling in LNvs is required for 24hr rhythms
The neuropeptide PDF is the only described signal that s-LNvs require to maintain synchronous molecular oscillations (Lin et al., 2004; Peng et al., 2003). s-LNvs likely use PDF to signal to each other since they express PDFR (Im and Taghert, 2010), a GPCR that likely couples to Gs (Hyun et al., 2005; Mertens et al., 2005; Shafer et al., 2008). To test how important Gs signaling in LNvs is for circadian rhythms, we assayed the behavior of flies with altered Gs expression or activity in LNvs.
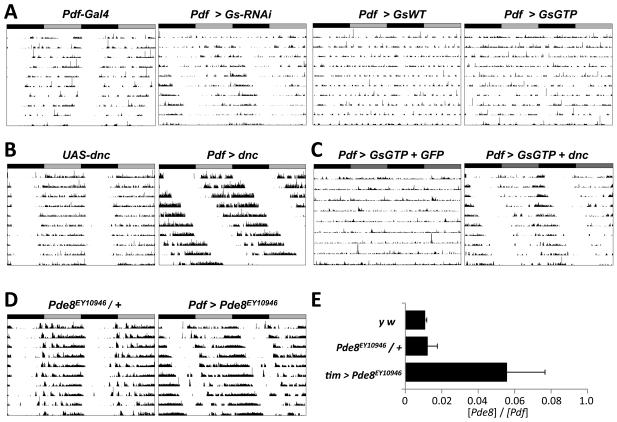
We first measured the locomotor activity in DD of flies in which Gs RNA levels were reduced specifically in LNvs using two copies of the Pdf-Gal4 driver to express a previously described UAS-Gs-RNAi transgene (Ueno et al., 2006). The average period of these Pdf > Gs-RNAi flies was 25.0hr, significantly longer than the 24.0hr or 23.8hr periods of control flies with Pdf-Gal4 or UAS-Gs-RNAi alone (p<.001, Figure 1A and Table1). Next we assayed flies expressing either wild-type Gs or Gs carrying a point mutation that eliminates its intrinsic GTPase activity and makes Gs constitutively active (UAS-Gs and UAS-Gs-GTP respectively, (Wolfgang et al., 1996). We found that more than 60% of Pdf > Gs and Pdf > Gs-GTP flies were arrhythmic while the rhythmic flies had normal periods but were only weakly rhythmic – shown by a lower rhythm power than control flies (Figure 1A, Table1). We checked for the presence of s-LNvs in Pdf > Gs-GTP flies, using antibodies to PDF and found that s-LNvs project normally to the dorsal brain and also express the clock proteins Vrille (VRI) and PER at ZT20 (ZT: Zeitgeber time, time in a 12:12 LD cycle; Figure S1). Therefore the arrhythmicity of many Pdf > Gs-GTP flies is not due to loss of s-LNvs or to visible developmental defects.
Figure 1. Normal Gs signaling in LNvs is required for 24hr locomotor rhythms.
A-D: Representative actograms showing locomotor activity in DD of flies with manipulations to Gs signaling in LNvs. Actograms are double-plotted with gray and black bars indicating prior LD cycles.
(A) Left: Control fly homozygous for two Pdf-Gal4 transgenes with 24.0hr period. The next three panels (from left to right) show activity of flies with Pdf-Gal4 expressing transgenes with RNAi directed against Gs (Pdf > Gs-RNAi), wild type Gs (Pdf > Gs) or constitutively active Gs (Pdf > Gs-GTP). Pdf > Gs-RNAi flies had longer period rhythms (25.0hr) than controls (p<.0001 vs PdfGal4 and UAS-Gs-RNAi / + flies). Pdf > Gs and Pdf > Gs-GTP flies were either arrhythmic or had weaker rhythms than controls.
(B) Left: fly with a UAS-dnc transgene (UAS-dnc, 24.0hr). Right: fly with UAS-dnc expressed in LNvs (Pdf > dnc, 26.6hr). Pdf > dnc flies have longer periods than controls (p<.001).
(C) Flies with Pdf-Gal4 expressing UAS-Gs-GTP and either a UAS-CD8::GFP transgene (Pdf > GsGTP + GFP, left) or a UAS-dnc transgene (Pdf > GsGTP + dnc, right).
(D) Left: Fly heterozygous for a P-element in Pde8 (Pde8EY10946 / +, 24.2hr). Right: Fly with Pde8 mis-expressed in LNvs (Pdf > Pde8EY10946, 25.0hr, right). Pdf > Pde8EY10946 flies have longer periods than controls (p<.001).
(E) RNA was isolated from heads of y w and Pde8EY10946 / + control flies and from flies with tim(UAS)-Gal4 mis-expressing Pde8EY10946 (tim > Pde8EY10946). Levels of Pde8 and Pdf RNA were measured by qPCR and the relative level (Pde8 : Pdf) plotted, with error bars showing SEM. Since tim > Pde8EY10946 flies have more Pde8 RNA than controls (p<0.01), Gal4-activated Pde8EY10946 mis-expresses Pde8.
Table 1. Locomotor rhythms in DD for flies with altered Gs and Go signaling in LNvs.
The nomenclature Pdf > X indicates that two copies of Pdf0.5-Gal4 (Park et al., 2000) were used to express UAS-X. Pdf > X + Y indicates simultaneous expression of UAS-X and UAS-Y. n: number of flies assayed. % ar: percentage of arrhythmic flies. Period is shown in hr for rhythmic flies with standard error of the mean (SEM). Power indicates rhythm strength with its SEM.
| Genotype | n | % ar | Period (hr) ± SEM | Power ± SEM |
|---|---|---|---|---|
| Pdf-Gal4 | 15 | 0 | 24.0 ± 0.1 | 102 ± 23.8 |
| Pdf > Gs-RNAi | 31 | 0 | 25.0 ± 0.1 | 113.8 ± 13.0 |
| Pdf > Gs | 20 | 65 | 24.2 ± 0.2 | 36.8 ± 9.3 |
| Pdf > Gs-GTP | 26 | 62 | 24.0 ± 0.1 | 14.5 ± 2.6 |
| Pdf > dnc | 38 | 0 | 26.6 ± 0.1 | 317.1 ± 26.5 |
| Pdf > Pde8EY10946 | 16 | 6 | 25.0 ± 0.1 | 200.3 ± 22.1 |
| UAS-Gs-RNAi / + | 27 | 4 | 23.8 ± 0.1 | 75.4 ± 6.8 |
| UAS-Gs / + | 19 | 5 | 24.5± 0.1 | 76.2 ± 10.9 |
| UAS-Gs-GTP / + | 16 | 6 | 24.1 ± 0.1 | 77.3 ± 16.0 |
| UAS-dnc / + | 28 | 0 | 24.0 ± 0.1 | 221.8 ± 18.5 |
| Pde8EY10946 / + | 15 | 0 | 24.2 ± 0.1 | 170.8 ± 17.9 |
| Pdf > Gs-GTP + dnc | 20 | 0 | 24.7 ± 0.1 | 197.1 ± 24.7 |
| Pdf > Gs-GTP + CD8::GFP | 19 | 21 | 23.4 ± 0.3 | 74.1 ± 10.3 |
| Pdf > Ptx | 36 | 0 | 25.2 ± 0.1 | 67.8 ± 6.6 |
| Pdf > Go-GDP | 49 | 4 | 25.0 ± 0.1 | 100.8 ± 10.1 |
| Pdf > Go-GTP | 26 | 46 | 22.3 ± 0.3 | 24.8 ± 2.3 |
| UAS-Ptx / + | 17 | 12 | 23.8 ± 0.1 | 66.9 ± 12.7 |
| UAS-Go-GTP / + | 16 | 0 | 23.6 ± 0.1 | 130.1 ± 16.7 |
| UAS-Go-GDP / + | 12 | 0 | 23.8 ± 0.1 | 136.5 ± 33.1 |
| Pdf > Go-GTP + CD8::GFP | 11 | 55 | 22.6 ± 0.2 | 39.7 ± 10.1 |
| Pdf > Go-GTP + Plc21C-RNAi | 22 | 0 | 24.7 ± 0.1 | 199.7 ± 17.9 |
| Pdf > Go-GTP + Gnf1-RNAi | 12 | 17 | 22.8 ± 0.3 | 61.4 ± 11.4 |
| Pdf > Go-GTP + dnc | 13 | 23 | 21.6 ± 0.1 | 48.5 ± 7.3 |
| Pdf > dnc + Ptx | 26 | 8 | 28.4 ± 0.1 | 104.4 ± 14.3 |
| Pdf > Plc21C-RNAi | 29 | 0 | 25.1 ± 0.1 | 195.6 ± 17.3 |
| Pdf > Gnf1-RNAi | 14 | 7 | 24.3 ± 0.1 | 118.2 ± 10.4 |
| Pdf > GABAB-R2-RNAi (1784) | 30 | 0 | 24.3 ± 0.1 | 160.5 ± 10.9 |
| Pdf > GABAB-R2-RNAi (1785) | 22 | 0 | 24.2 ± 0.1 | 78.6 ± 8.4 |
| Pdf > GABAB-R3-RNAi | 21 | 0 | 24.9 ± 0.1 | 92.5 ± 14.6 |
| UAS-GABAB-R3-RNAi / + | 14 | 0 | 23.8 ± 0.1 | 56.7 ± 11.7 |
| UAS-NaChBac2 / + | 16 | 6 | 24.1 ± 0.1 | 112.5 ± 18.4 |
| Gad-Gal4 / + | 15 | 7 | 24.0 ± 0.2 | 119.4 ± 19.9 |
| Gad > NaChBac2 | 31 | 39 | 24.3 ± 0.3 | 38.6 ± 5.2 |
cAMP phosphodiesterases, such as DNC, reduce Gs-mediated signaling by hydrolyzing cAMP to AMP. Therefore, we used a UAS-dnc transgene (Cheung et al., 1999) to decrease cAMP levels as an independent way to alter Gs signaling activity in LNvs. We found that Pdf > dnc flies had much longer periods than control UAS-dnc flies (26.6hr vs. 24.0hr respectively, p<.001, Figure 1B and Table 1). We also found that the arrhythmicity caused by expressing GsGTP in LNvs could be completely rescued by co-expression of UAS-dnc but not by co-expressing a control transgene, UAS-CD8::GFP (Figure 1C, Table 1). Thus the effects of Gs on circadian rhythms are mediated via cAMP rather than direct effects of activated Gs on ion channels. We also noticed that Pdf > Gs-GTP + dnc flies have ~2hr shorter period lengths than Pdf > dnc flies, suggesting that cAMP levels help determine period length as in mammals (O'Neill et al., 2008). Similarly, flies with only one copy of Pdf-Gal4 expressing UAS-dnc have shorter periods (25.5hr) than flies with two copies of Pdf-Gal4 (26.6hr) - presumably the latter have higher DNC levels and lower cAMP levels.
We also tested whether period length could be altered by mis-expressing PDE8, another cAMP-specific phosphodiesterase (Day et al., 2005). We found that Pdf > Pde8EY10946 flies have longer period rhythms than control flies (25.0hr, Figure 1D, Table 1). We confirmed that this previously uncharacterized Pde8 P-element insertion that contains binding sites for Gal4 increases Pde8 RNA levels when activated by a Gal4 driver (Figure 1E). Overall our data show that Gs signaling and cAMP levels in LNvs are important determinants of period length. Although previous studies in Drosophila implicated cAMP-mediated signaling in circadian rhythms (Levine et al., 1994; Majercak et al., 1997), our data point to the LNvs as the relevant cellular substrate.
Normal Go activity in LNvs is required for 24hr rhythms
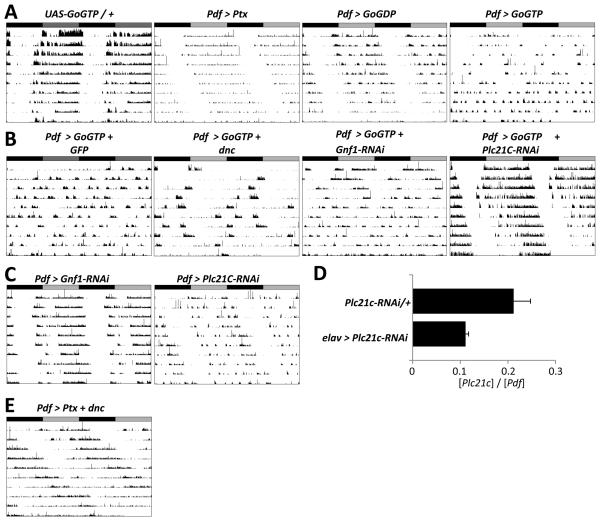
Next we tested if other G-alpha proteins are also important for circadian behavior. One major class of G-proteins is sensitive to Pertussis toxin (Ptx), which specifically inhibits Go signaling in flies (Ferris et al., 2006; Katanaev et al., 2005). Since a Gal4 enhancer trap in the Go locus gave expression in s-LNvs (data not shown), we tested if Go signaling regulates circadian rhythms by expressing a UAS-Ptx transgene (Ferris et al., 2006) in LNvs. The average behavioral period of Pdf > Ptx flies in DD is 25.2hr, significantly longer than control flies (p<.001, Figure 2A, Table 1). We also used a UAS-Go transgene in which a single amino acid change (G203T) reduces Go affinity for GTP and decreases endogenous Go signaling in a dominant-negative manner (Go-GDP, Katanaev et al., 2005). Pdf > Go-GDP flies also had ~1hr longer period than control flies (p<.001, Figure 2A, Table 1). Therefore, inhibiting endogenous Go activity in LNvs using two independent transgenes lengthened period.
Figure 2. Go signaling in LNvs requires Plc21C for 24hr behavioral rhythms.
Representative actograms for flies in DD as in Figure 1.
(A) Left: A control fly heterozygous for a constitutively active Go transgene (UAS-Go-GTP / +, 23.6 hr). The next panels (left to right) show activity of flies with Pdf-Gal4 expressing transgenes with Pertussis toxin (Pdf > Ptx, 25.2hr), constitutively inactive Go (Pdf > Go-GDP, 25.0hr) or constitutively active Go (Pdf > Go-GTP). Pdf > Ptx and Pdf > Go-GDP flies had significantly longer rhythms than controls (p<.001 vs Pdf-Gal4). Pdf > Go-GTP flies either became arrhythmic during DD or had shorter rhythms than control flies (22.3hr, p<.001 vs Pdf-Gal4 and UAS-Go-GTP / + flies).
(B) Pdf > Go-GTP flies expressing a third transgene. From left to right: UAS-CD8::GFP control (Pdf > Go-GTP + GFP), UAS-dnc (Pdf > Go-GTP + dnc), control RNAi to Gnf1 (Pdf > Go-GTP + Gnf1-RNAi) and RNAi to Plc21C (Pdf > Go-GTP + Plc21C-RNAi). Pdf > Go-GTP + Plc21CRNAi flies had stronger rhythms than Pdf > Go-GTP + Gnf1-RNAi flies (p <.001).
(C) Flies with a control RNAi (Pdf > Gnf1-RNAi, 24.3hr, left) or RNAi against Plc21C (Pdf > Plc21C, 25.1hr, right). Pdf > Plc21C-RNAi flies have longer periods than control flies (p<.001 vs Pdf > Gnf1).
(D) qPCR on RNA isolated from adult fly heads from either UAS-Plc21C-RNAi / + heterozygotes or flies with elav-Gal4 expressing UAS-Plc21C-RNAi in post-mitotic neurons (elav > Plc21CRNAi). The relative levels of Plc21C and Pdf RNA are plotted. elav > Plc21C-RNAi flies have less Plc21C RNA than controls (p=0.05).
(E) Locomotor activity of a fly co-expressing UAS-dnc and UAS-Ptx in LNvs (Pdf > dnc + Ptx). Pdf > dnc + Ptx flies had longer periods (28.4hr) than Pdf > dnc and Pdf > Ptx flies (p <.001).
We also assayed behavioral rhythms of flies expressing a constitutively active Go transgene (Go-GTP, Katanaev et al., 2005) and found that nearly 50% of Pdf > Go-GTP flies were arrhythmic. The remaining rhythmic flies had short period rhythms (22.3hr), although with a weak power (Figure 2A, Table 1). Thus reduced signaling via Go in LNvs lengthens period, while increased Go signaling leads to arrhythmicity or short periods.
Plc21C lies downstream of Go in LNvs
Unlike Gs, the signaling pathway downstream of Go in Drosophila is unclear. One well-studied pathway downstream of G-proteins involves PLC enzymes, which cleave the phospholipid PIP2 into second messengers. Plc21C is widely expressed in the brain and co-localizes with Go at low resolution (Shortridge et al., 1991).
We hypothesized that the arrhythmicity of Pdf > Go-GTP flies should be rescued by reducing downstream signaling as observed with co-expression of UAS-dnc with UAS-GsGTP (Figure 1C). Therefore, we measured locomotor rhythms of flies expressing UAS-Go-GTP in LNvs along with either UAS-dnc or with an RNAi transgene that targets Plc21C. To control for transgene expression levels, we also assayed flies with Go-GTP co-expressed with GFP or RNAi to Germ line transcription factor 1 (Gnf1), which is unlikely to be expressed in LNvs. The results in Figure 2B and Table 1 show that Pdf > Go-GTP + GFP and Pdf > Go-GTP + Gnf1-RNAi flies were either arrhythmic or had weak rhythms. Pdf > Go-GTP + dnc flies had low power short-period rhythms, indicating that Go is unlikely to signal by increasing cAMP in LNvs. This is consistent with Go acting independently of the cAMP pathway in mushroom bodies (Ferris et al., 2006). Strikingly, all Pdf > Go-GTP + Plc21C-RNAi flies had strong rhythms. Since Plc21C-RNAi suppressed the Go-GTP phenotype, we conclude that Plc21C lies downstream of Go in LNvs.
We noticed that Pdf > Go-GTP + Plc21C-RNAi flies had slightly longer period rhythms (24.7hr) than control flies. Pdf > Plc-21C-RNAi flies without Go-GTP also have a longer period (25.1hr) than control Pdf > Gnf1-RNAi flies (Figure 2C and Table1), consistent with the long periods seen when Go activity is reduced in LNvs. We confirmed that the UAS-Plc21c-RNAi transgene reduced Plc21C RNA levels when expressed pan-neuronally (Figure 2D). Thus Go / PLC21C signaling is a novel pathway that helps LNvs drive 24hr behavioral rhythms
These data indicate that Go and Gs activate distinct signaling pathways. Since combining two mutations that affect different steps in the molecular clock affects period length additively (Lakin-Thomas and Brody, 1985; Rothenfluh et al., 2000), we tested if Go and Gs pathways act in parallel by measuring the period lengths of flies with both pathways inhibited simultaneously. We found that flies co-expressing dnc and Ptx in LNvs have an average period of 28.4hr, significantly longer than either Pdf > dnc or Pdf > Ptx flies alone (p<0.001, Figure 2D and Table 1). Therefore we conclude that Gs and Go signaling act in parallel in LNvs to promote 24hr rhythms.
Inhibiting Go slows the molecular clock
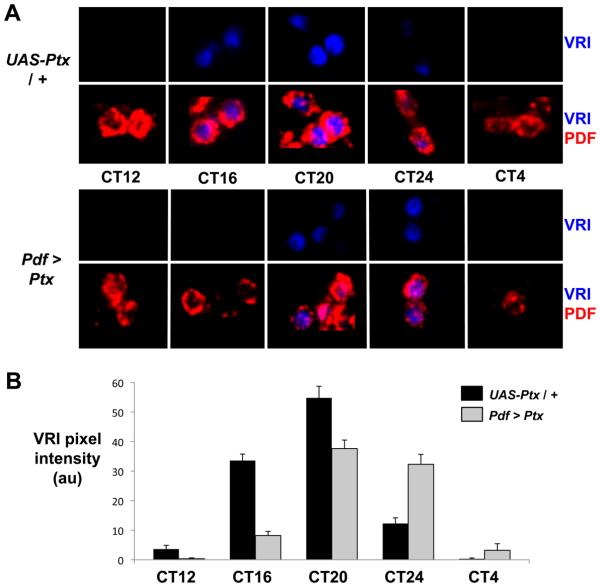
Altered period behavioral rhythms are typically associated with changes in the s-LNv molecular clock. To test this for Go signaling, we assayed molecular clock oscillations in Pdf > Ptx flies. We dissected adult flies on the third day of DD so that the 1.3hr difference in period length per day would result in a ~4hr shift. We stained fly brains at 4hr intervals for the rhythmically produced clock protein VRI, which is detected during subjective night in wild type flies (Cyran et al., 2003; Glossop et al., 2003).
VRI staining was first detected in control s-LNvs at CT16, reached peak levels at CT20 and then became almost undetectable by CT24. In contrast, VRI was not clearly detectable in s-LNvs of Pdf > Ptx flies until CT20 and remained at high levels at CT24, before disappearing by CT4 on the next day (Figure 3). Therefore, the s-LNv molecular clock in Pdf > Ptx flies is rhythmic, but its phase is delayed, consistent with long period behavioral rhythms. Thus GoPLC21C signaling interacts with core clock components to regulate molecular clock speed.
Figure 3. Molecular clock progression is delayed by inhibiting Go signaling.
(A) Representative confocal images of s-LNvs on days 3-4 in DD stained for VRI (blue) and PDF (red) in UAS-Ptx / + control flies (top panels) and Pdf > Ptx flies (bottom). Data is quantified in (B) for UAS-Ptx / + controls (black) and Pdf > Ptx (gray) with error bars showing SEM.
Go is required for GABA to inhibit LNv neuronal activity in vitro
Although LNvs are usually considered cell-autonomous oscillators, the involvement of Gs and Go signaling suggested that they normally receive inputs to generate 24hr rhythms. Given that Go signaling was previously unstudied in fly circadian rhythms, we sought to identify ligands and GPCRs which signal via Go in LNvs as the first step towards identifying the neurons which help set molecular clock speed.
For this, we modified the assay of Wegener et al. (2004) that measures Ca2+ responses in dissociated larval LNvs. We used larval LNvs to be able to use the strong Pdf-Gal4 driver without needing to distinguish s- and l-LNvs by size. Studying larval LNvs is relevant because they become the adult s-LNvs (Kaneko et al., 1997) and gene expression patterns remain similar between larval LNvs and adult s-LNvs (Nagoshi et al., 2010).
Instead of measuring Ca2+ changes via Fura-2, we used the genetically encoded Ca2+ indicator G-CaMP1.6 (Reiff et al., 2005). We found that larval LNvs expressing G-CaMP fluoresce more reliably and strongly than after Fura-2 loading. 10μM ACh robustly increased fluorescence (25-80% of baseline) in >90% G-CaMP+ dissociated LNvs as previously described (Wegener et al., 2004). Fluorescence did not run down during an experiment (Figure 4A, S2-3) allowing a single G-CaMP+ neuron to be recorded for >30 min.
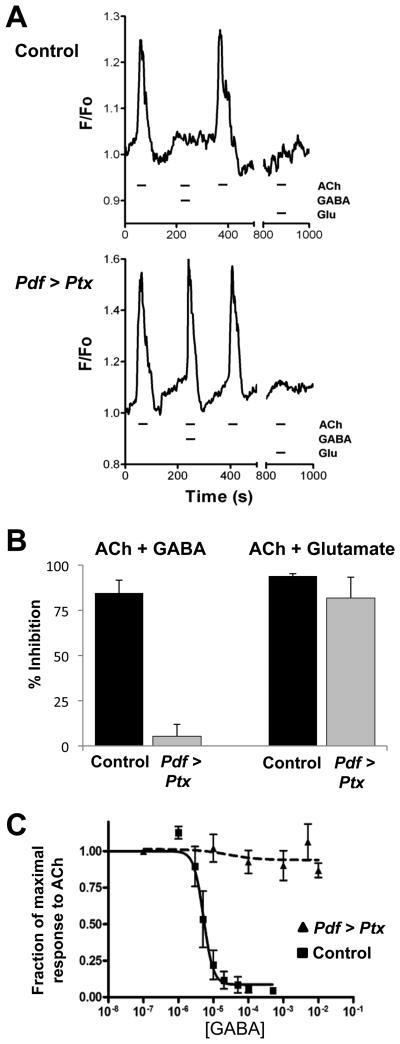
Figure 4. Go is required for the inhibitory effects of GABA on larval LNvs.
(A) Representative fluorescence measurements from dissociated larval LNvs when 10μM acetylcholine (ACh) was applied alone or together with 100μM GABA or glutamate (Glu). Upper traces are LNvs with Pdf-Gal4 expressing UAS-G-CaMP (Control). Lower traces are LNvs with Pdf-Gal4 expressing UAS-G-CaMP and UAS-Ptx (Pdf > Ptx).
(B) Quantitation of inhibition by GABA (left) or glutamate (right) of 10μM ACh-induced Ca2=-responses for Control (black) and Pdf > Ptx (gray) LNvs. Ptx prevented GABA-mediated inhibition (p<.001 vs Pdf > G-CaMP, n=9) but had no effect on glutamate inhibition (p>.2, n=5). (C) Dose-response curve for GABA on Control (squares, solid line) and Pdf > Ptx (triangles, dashed line) LNvs. IC50 for control LNvs is 3.8μM but was unmeasurable for Pdf > Ptx LNvs.
We found a similar concentration-response relationship for ACh in G-CaMP+ neurons as Wegener et al (2004) had found for Fura-2 loaded LNvs (Figure 4C). These Ca2+ increases require nicotinic ACh receptor (nAChR) activation since 10μM nicotine also increased G-CaMP fluorescence (Figure S2A), as reported by Wegener et al. (2004). Furthermore, applying the nAChR antagonist α-Bungarotoxin (α-Btx) prevented intracellular Ca2+ increases when co-applied with ACh (Figure S2A and M. Vömel & C. Wegener, personal communication). α-Btx inhibition on LNvs was reversible (Figure S2A) as previously described in insect cells (Albert and Lingle, 1993).
In agreement with Wegener et al. (2004), we also found ACh-induced intracellular Ca2+ increases require extracellular Ca2+ (Figure S2B) and are blocked by 30μM extracellular Cd2+, which inhibits voltage-gated Ca2+ channels (VGCC, Figure S2C). Raising extracellular K+ to depolarize LNvs also increased G-CaMP fluorescence to similar levels as ACh (EC50 for K+, 30.8 ± 1.2 mM, Figure S3A and data not shown). These data indicate that ACh binds to nAChRs that activate VGCC to allow extracellular Ca2+ to enter LNvs. For this, nAChRs and VGCCs must be in close proximity at least in dissociated larval LNvs and this system offers a powerful way to study the direct effects of ligands on LNvs.
We tested a number of other neurotransmitters, but found no change in G-CaMP-fluorescence in LNvs either during or after applications of 100μM GABA, glutamate, glycine, histamine, 5-HT, NMDA or octopamine (data not shown). These high neurotransmitter doses would be expected to give responses if LNvs possess the appropriate receptors. However, it was previously reported that glutamate and GABA decrease Ca2+ levels via metabotropic glutamate and GABA receptors (GABAB-Rs) in Fura-2 loaded LNvs (Hamasaka et al., 2007; Hamasaka et al., 2005). These receptors are candidates for coupling to Go. Since we could not detect spontaneous activity with GABA and glutamate in dissociated LNvs, we co-applied them with ACh to attempt to detect inhibition. We found that co-applying 100μM GABA or glutamate almost completely inhibited the Ca2+ response to 10μM ACh (Figure 4A, 4B).
To test if Go is required for inhibition by GABA and/or glutamate, we compared the responses of control Pdf > G-CaMP larval LNvs with LNvs also expressing UAS-Ptx (Pdf > G-CaMP + Ptx). Both sets of cells responded similarly to ACh applied alone (Figure 4A, B). When ACh was co-applied with 100μM GABA, the response of control Pdf > G-CaMP LNvs was inhibited by 85%. However, the inhibition by GABA was only 5% in Pdf > G-CaMP + Ptx cells (Figure 4A, B). We estimated the GABA IC50 for control LNvs as 3.8μM whereas GABA did not inhibit the ACh response of Pdf > G-CaMP + Ptx LNvs even at 10mM (Figure 4C). Co-applying glutamate with ACh inhibited the ACh-induced Ca2+ response in both sets of cells (Figure 4A, B), indicating that Ptx specifically blocks GABA-mediated inhibition. Since Ptx prevents the Go-α/β/γ heterotrimer from coupling to activated GPCRs, we propose that metabotropic GABAB-Rs normally signal via Go in LNvs. Similar conclusions were made in Drosophila olfactory receptor neurons using UAS-Ptx (Olsen and Wilson, 2008).
Adult l-LNvs have functional GABAA/RDL receptors that are sensitive to picrotoxin and implicated in arousal (Chung et al., 2009; Parisky et al., 2008). However, we found that 4μM picrotoxin, which blocks the open channel of GABAA/RDL receptors, did not alter GABA-inhibition of ACh-induced Ca2+ responses in larval LNvs (Figure S3C). Thus the GABA-mediated inhibition observed here is independent of GABAA/RDL receptors. The general K+ channel inhibitors TEA or Cs+ (Hille, 2001) did not affect GABA-mediated inhibition (Figure S3C). Although the sequence of 1 of the 3 Drosophila G-protein coupled Inward Rectifier K+ channel suggests that it may not be blocked by TEA (Kavanaugh et al., 1991; McCormack, 2003), the lack of an effect of Cs+ is consistent with GABA inhibiting LNvs independently of K+ channels.
GABA signals via GABAB-R3 receptors in LNvs
Next we sought to identify the GABAB receptor(s) in LNvs responsible for GABA-inhibition of ACh. The Drosophila genome has three annotated GABAB-R genes (Mezler et al., 2001): GABAB-R1 and GABAB-R2 form a functional heterodimer, while GABAB-R3 does not dimerize with GABAB-R1 or R2 and likely interacts with an as yet unidentified additional GABABR (Kaupmann et al., 1998; Mezler et al., 2001).
To identify which GABAB receptor(s) is required in LNvs, we first used 3-APMPA, an agonist for Drosophila GABAB-R1/2 (Hamasaka et al., 2005; Mezler et al., 2001). 3-APMPA did not alter LNv Ca2+ responses when co-applied with ACh, even at 20μM (data not shown), which is more than twice the 3-APMPA reported EC50 (Hamasaka et al 2005). GABA also inhibited LNv Ca2+ responses to ACh when LNvs expressed a UAS-GABAB-R2-RNAi transgene (Figure 5A) even though this GABAB-R2-RNAi transgene potently reduces GABAB-R2 expression (Figure 5B). Since GABAB-R1 and GABAB-R2 seem to function together, we conclude that GABAB-R1/2 is not involved in GABA-mediated inhibition of ACh responses in LNvs.
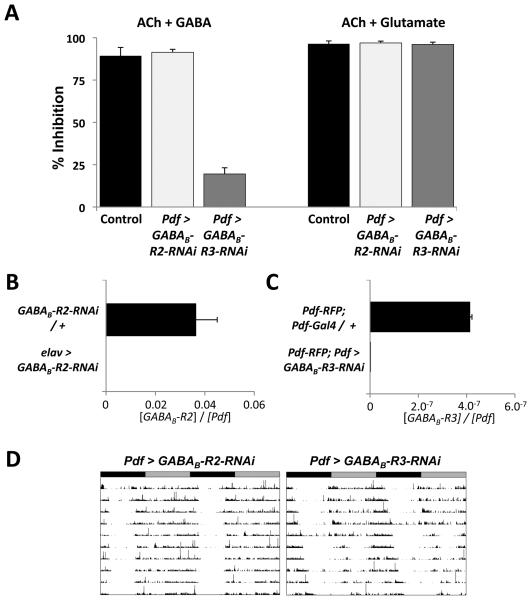
Figure 5. RNAi to the metabotropic GABA receptor GABAB-R3 subunit reduces inhibition by GABA and lengthens period of adult locomotor rhythms.
(A) Quantification of inhibition of Ca2=-responses for ACh + GABA (left) or ACh + glutamate (right) compared to ACh. LNvs from larvae with Pdf-Gal4 and UAS-G-CaMP and either no other transgenes (Control, black bars, 89% inhibition), UAS-GABAB-R2-RNAi on Chr. III (Pdf > GABAB-R2-RNAi, light gray, 91% inhibition, p>.5 vs Control) or UAS-GABAB-R3-RNAi (Pdf > GABAB-R3-RNAi, dark gray, 19.5% inhibition, p<.001 vs Control).
(B) Adult head RNA levels of GABAB-R2 and Pdf were measured as in Figure 2 from either UAS-GABAB-R2-RNAi heterozygotes (GABAB-R2-RNAi / +) or flies with elav-Gal4 expressing UAS-GABAB-R2-RNAi (elav > GABAB-R2-RNAi). GABAB-R2 RNA levels were lower in elav > GABAB-R2-RNAi flies (p<0.01).
(C) GABAB-R3 and Pdf RNA levels measured by qPCR on amplified RNA from adult LNvs isolated from Pdf-RFP; Pdf-Gal4 / + controls or flies which also had UAS-GABAB-R3-RNAi (PdfRFP; Pdf > GABAB-R3-RNAi). GABAB-R3 RNA levels were reduced in Pdf-RFP; Pdf > GABABR3-RNAi flies (p<0.02). Pdf RNA levels were ~2.5-fold higher with GABAB-R3-RNAi flies and so the extent of knockdown with this RNAi transgene (>400-fold) may be over-estimated.
(D) Representative actograms of adult flies expressing either GABAB-R2-RNAi (Pdf > GABABR2-RNAi, left) or GABAB-R3-RNAi (Pdf > GABAB-R3-RNAi, right) in LNvs. Pdf > GABAB-R3-RNAi flies have longer period rhythms than Pdf > GABAB-R2-RNAi flies (24.9hr vs 24.3hr, p <.001).
GABAB-R3 expression is enriched ~20-fold in adult s-LNvs at ZT12 compared to other differentiated neurons (Kula-Eversole et al., 2010). To test a role for GABAB-R3 in mediating GABA responses in LNvs, we used a UAS-GABAB-R3-RNAi transgene since there are no pharmacological agents that target GABAB-R3. We compared the responses of LNvs from control Pdf > G-CaMP larvae and from larvae expressing both G-CaMP and GABAB-R3-RNAi transgenes. The results in Figure 5A, S3A and S3B show normal activation by ACh and inhibition by glutamate. However, while GABA inhibited the response of control Pdf > G-CaMP LNvs to ACh by 89%, Pdf > G-CaMP + R3-RNAi LNvs showed only 20% inhibition. We could not detect a significant change in GABAB-R3 expression in RNA from whole fly heads when GABABR3-RNAi was expressed with elav-Gal4. Instead, we measured GABAB-R3 RNA levels from adult LNvs purified via flow cytometry using a Pdf-RFP transgene (Blanchard et al., 2010) to sort LNvs. This technique reliably reports rhythmic clock gene expression in LNvs (MR, M. Drapeau & JB, manuscript in preparation). The RNA analyzed is likely from a mixture of s- and l-LNvs. The results in Figure 5C show that GABAB-R3 RNA levels are significantly higher in LNvs isolated from Pdf-RFP control flies than in LNvs from Pdf-RFP; Pdf > GABAB-R3-RNAi flies. Therefore, the GABAB-R3-RNAi transgene reduces GABAB-R3 expression. Overall, our data indicate that GABAB-R3 is the relevant GABAB receptor that mediates the inhibitory effects of GABA on LNv Ca2+ responses.
To test whether reducing expression of GABAB-R3 alters adult behavioral rhythms, we assayed the locomotor activity of flies expressing RNAi against GABAB-R3 in adult LNvs and compared them to flies expressing GABAB-R2-RNAi. The results in Figure 5D and Table 1 show that Pdf > GABAB-R3-RNAi flies had longer periods than Pdf > GABAB-R2-RNAi flies (24.9hr vs 24.3hr respectively, p<.001). The long period is consistent with reduced signaling via Go in Pdf > Ptx and Pdf > Go-GDP flies (Table1). Taken together our results indicate that signaling via GABAB-R3 in s-LNvs helps to generate 24hr rhythms and raise the possibility that GABAergic neurons are part of the circadian network.
Hyper-exciting GABAergic neurons disrupts circadian rhythms
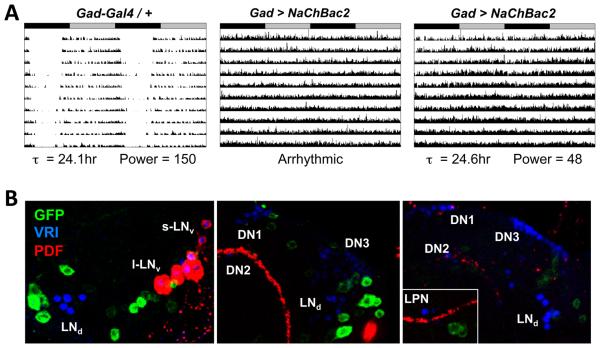
Given the presence of functional GABAB-R3 receptors in LNvs, we asked whether manipulating GABAergic neurons themselves also affects circadian rhythms. We used two Gal4 drivers with regulatory regions from Glutamate decarboxylase 1 (Gad1), which encodes the enzyme that converts Glutamate into GABA, and one with the regulatory region of the vesicular GABA transporter (vGAT, Fei et al., 2010). However, these GABAergic neuron drivers were mainly lethal when crossed to transgenes that ablate, hyperpolarize or hyper-excite neurons or block synaptic release. However, healthy offspring were obtained by crossing Gad-Gal4 (Mehren and Griffith, 2006) to UAS-NaChBac2, which encodes a voltage-gated bacterial Na+ channel that hyper-excites neurons (Nitabach et al., 2006; Sheeba et al., 2008). The results in Figure 6A and Table 1 show that Gad > NaChBac2 flies were either arrhythmic or had significantly weaker rhythms than control flies.
Figure 6. Hyper-exciting GABAergic neurons causes behavioral arrhythmicity.
(A) Representative actograms of heterozygous control Gad-Gal4 / + fly (left) and two flies with Gad-Gal4 expressing UAS-NaChBac2 (Gad > NaChBac2, center and right). ClockLab marked the Gad > NaChBac2 center fly as arrhythmic while the fly on the right as having a period of 24.6hr and a low power rhythm. Rhythmic Gad > NaChBac2 flies had weaker power rhythms then control flies (p<.001).
(B) Localization of Gad-Gal4 expression using a UAS-CD8::GFP reporter in three individual brains stained at ZT20 with antibodies to GFP (green), VRI (blue, marks all clock neurons) and PDF (red). Although some GFP+ neurons are close to VRI+ clock neurons, there is no obvious co-localization of VRI and GFP. Images are representative of 13 brain lobes.
To test if these behavioral phenotypes are due to expression in clock neurons, we crossed Gad-Gal4 with GFP reporter flies. We could not detect GFP expression in any clock neurons (Figure 6B), consistent with no GABA immunoreactivity in clock neurons (Hamasaka et al., 2005). Therefore the disrupted locomotor rhythms in Gad > NaChBac2 flies are due to hyper-exciting Gad-Gal4 expressing cells that are not canonical clock neurons.
Altering GABAergic neuron activity changes Drosophila sleep levels in LD cycles (Agosto et al., 2008; Parisky et al., 2008). Therefore the altered behavioral rhythms of Gad > NaChBac2 flies could result from altered arousal mediated via GABAergic neurons. In this scenario, the s-LNv molecular clocks would be expected to run normally in Gad > NaChBac2 flies. Alternatively, the weak behavioral rhythms of Gad > NaChBac2 flies could be at least partly due to increased GABAergic signaling that alters s-LNv molecular clocks.
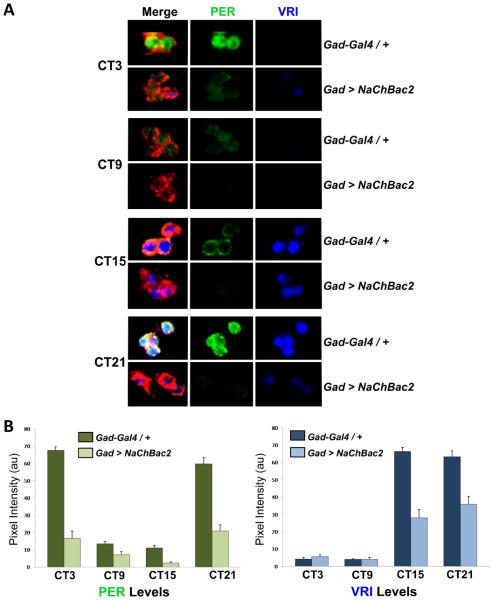
To assay the s-LNv molecular clock, we compared levels of the clock proteins VRI and PER in s-LNvs of Gad-Gal4 control flies and Gad > NaChBac2 flies at 4 different time points on day 3 in DD. Figure 7 shows that overall VRI and PER levels are lower in Gad > NaChBac2 flies than in control flies. Furthermore, VRI and PER levels are variable between s-LNvs in the same cluster in Gad > NaChBac2 brains. For example, VRI was detected in only 2 of the 4 s-LNvs in the Gad > NaChBac2 fly shown at CT3 (Figure 7A). We also measured VRI and PER levels in LD cycles and found no significant difference between control and experimental flies (data not shown), indicating that s-LNvs entrain properly and that light over-rides the effect of hyper-exciting GABAergic neurons. Since molecular rhythms in s-LNvs are disrupted by hyper-exciting GABAergic neurons, we conclude that normal levels of signaling from GABAergic neurons are necessary for 24hr molecular clock rhythms in s-LNvs in DD.
Figure 7. Hyper-exciting GABAergic neurons disrupts s-LNv molecular rhythms.
(A) Representative confocal images of s-LNvs at 4 timepoints on day 3 in DD using antibodies to PER (green), VRI (blue), and PDF (red) in Gad-Gal4 / + control flies (top panels) and Gad > NaChBac2 flies (bottom panels). Data are quantified in (B) for Gad-Gal4 / + controls (dark green and dark blue bars for PER and VRI respectively) and for Gad > NaChBac2 (light green and light blue) with SEM shown. Experiments were carried out on 3 separate days with at least 12 brains and 30 clearly identifiable s-LNvs measured in total.
Discussion
G-protein signaling in LNvs
Here we demonstrate the importance of G-protein signaling in s-LNvs for 24hr rhythms in DD. We show that Gs and Go pathways act in parallel to regulate the s-LNv molecular clock since simultaneously reducing signaling via Gs and Go lengthens rhythms by more than 4hr. Therefore LNvs normally require an appropriate level of signaling via Gs and Go to generate 24hr rhythms in DD. Since activation of Gs and Go generates intracellular second messengers, our work adds to the evidence implicating small molecules in regulating molecular clocks across species (Dodd et al., 2007; Harrisingh et al., 2007; O'Neill et al., 2008).
Signaling via Gs in LNvs
The long-periods we observed with reduced Gs signaling are consistent with four other manipulations of cAMP levels or PKA activity that alter fly circadian behavior. First, long-period rhythms with dnc over-expression complement the short periods of dnc hypomorphs (Levine et al., 1994) and suggest that the latter are due to loss of dnc from LNvs. dnc mutants also increase phase shifts to light in the early evening. However, we found no difference in phase delays or advances between Pdf > dnc and control flies (data not shown), suggesting that altered light-responses of dnc hypomorphs are due to dnc acting in other clock neurons. The period-altering effects we see when manipulating cAMP levels are also consistent with data from Shafer et al. (2008) who found that expressing the cAMP-binding domain of mammalian Epac1 in LNvs lengthens period. This Epac1 domain likely reduces free cAMP levels in LNvs, although presumably not as potently as UAS-dnc. Third, mutations in PKA catalytic or regulatory subunits that affect the whole fly disrupt circadian behavior (Levine et al., 1994; Majercak et al., 1997; Park et al., 2000). Fourth, over-expressing a PKA catalytic subunit in LNvs rescues the period-altering effect of a UAS-shibire transgene that alters vesicle recycling, although the PKA catalytic subunit had no effect by itself (Kilman et al., 2009). The long periods we observed with reduced Gs signaling in LNvs also parallel mammalian studies in which pharmacologically reducing Adenylate cyclase activity lengthened period in SCN explants and mice (O'Neill et al., 2008).
G-proteins typically transduce extracellular signals. What signals could activate Gs in s-LNvs? PDF is one possibility since PDFR induces cAMP signaling in response to PDF in vitro, indicating that it likely couples to Gs (Hyun et al., 2005; Lear et al., 2005). PDF could signal in an autocrine manner since PDFR is present in LNvs (Im and Taghert, 2010). However, the long-periods we observed with reduced Gs signaling differ from the short-period and arrhythmic phenotypes of Pdf and pdfr mutants. The likeliest explanation for these differences is that the altered behavior of Pdf and pdfr mutants results from effects of PDF signaling over the entire circadian circuit (Peng et al., 2003; Shafer et al., 2008), whereas our manipulations specifically targeted LNvs. Indeed, LNvs are not responsible for the short-period rhythms in Pdf01 null mutant flies (Yoshii et al., 2009). Other possible explanations for the differences between the long-period rhythms with decreased Gs signaling in LNvs and the short-period rhythms of Pdf and pdfr mutants are that additional GPCRs couple to Gs in s-LNvs and influence molecular clock speed and that our manipulations decrease rather than abolish reception of PDF. In summary, our data shows that Gs signaling via cAMP in s-LNvs modulates period length.
Go and GABAergic signaling help generate 24hr periods
Go signaling via PLC21C constitutes a novel pathway that regulates the s-LNv molecular clock. We found that Go and the metabotropic GABAB-R3 receptor are required for the inhibitory effects of GABA on larval LNvs, which develop into adult s-LNvs (Kaneko et al., 1997). The same genetic manipulations that block GABA inhibition of LNvs in culture (expression of Ptx or GABAB-R3-RNAi) lengthened the period of adult locomotor rhythms. Furthermore, the molecular clock in s-LNvs is disrupted when a subset of GABAergic neurons are hyper-excited. Since the LNvs do not produce GABA themselves (Hamasaka et al., 2005 and Fig. 6B), s-LNvs require GABAergic inputs to generate 24hr rhythms. Thus s-LNvs are less autonomous for determining period length in DD than previously anticipated (Stoleru et al., 2005).
Activation of G-proteins can have both short- and long-term effects on a cell. With Go signaling blocked by Ptx, we detected short-term effects on LNv responses to excitatory ACh and longer-term effects on the molecular clock. The latter are presumably explained by PLC activation since the behavioral phenotypes of Pdf > GoGTP flies were rescued by reducing Plc21C expression.
Since s-LNv clocks were unchanged even when the speed of all non-LNv clock neurons were genetically manipulated (Stoleru et al., 2005), it is surprising to find s-LNv clocks altered by signaling from GABAergic non-clock neurons. Why would LNvs need inputs from non-clock neurons to generate 24hr rhythms? One possibility is that LNvs receive multiple inputs which either accelerate or slow down the pace of their molecular clock but overall balance each other to achieve 24hr rhythms in DD. Since reducing signaling by Gs and Go lengthens period, these pathways normally accelerate the molecular clock. According to this model, there are unidentified inputs to LNvs which delay the clock. Identifying additional receptors in LNvs would allow this idea to be tested.
Previous work showed that GABAergic neurons project to LNvs (Hamasaka et al., 2005) and that GABAA receptors in l-LNvs regulate sleep (Chung et al., 2009; Parisky et al., 2008). Our data show that constitutive activation of Go signaling dramatically alters behavioral rhythms, suggesting that LNvs normally receive rhythmic GABAergic inputs. But how can s-LNvs integrate temporal information from non clock-containing GABAergic neurons? s-LNvs could respond rhythmically to a constant GABAergic tone by controlling GABAB-R3 activity. Indeed, a recent study found that GABAB-R3 RNA levels in s-LNvs are much higher at ZT12 than at ZT0 (KulaEversole et al., 2010). Strikingly, this rhythm in GABAB-R3 expression is in antiphase to LNv neuronal activity (Cao and Nitabach, 2008; Sheeba et al., 2008). Thus regulated perception of inhibitory GABAergic inputs could at least partly underlie rhythmic LNv excitability. GABAergic inputs could also help synchronize LNvs as in the cockroach circadian system (Schneider and Stengl, 2005). Thus GABA's short-term effects on LNv excitability, likely mediated by Gβ/γ, and GABA's longer-term effects on the molecular clock via Go may both contribute to robust rhythms.
Conclusion
Our work adds to the growing network view of circadian rhythms in Drosophila where LNvs integrate information to set period for the rest of the clock network in DD. The period-altering effects of decreased G-protein signaling in LNvs point to a less hierarchical and more distributed network than previously envisioned. Since our data strongly suggests that GABA inputs are novel regulators of 24hr rhythms, the GABAergic neurons that fine-tune the s-LNv clock should be considered part of the circadian network.
Experimental Procedures
Behavioral Analysis
Fly strains used are described in Supplementary Experimental procedures. For circadian locomotor behavior, flies were loaded into Trikinetics activity monitors and entrained to LD cycles for at least 3 days before transfer to DD. Data were analyzed by ClockLab in conjunction with MatLab. Period length was determined using the Lomb-Scargle algorithm and power determined by measuring the height of the peak at this period. Flies with a power >11 had significant rhythms (p <.025) while flies with power <11 were deemed arrhythmic. A two-tailed student t-test with equal variance was used to test differences in period length.
Immunostaining
Antibodies against PDF (1:50; DSHB), VRI (1:3000; from Paul Hardin), PER (1:5000; from Jeff Hall) and GFP (1:500; AbDSerotec) were used on adult brains as described (Collins et al., 2006). Three experiments were performed on different days with ≥8 brains and ≥16 clearly identifiable s-LNvs analyzed per timepoint. Images were obtained on a Leica SP5 Confocal and Image J used to quantify pixel intensity. Since background staining of VRI and PER was <0.2 au for each condition, background was not subtracted from raw values (range: 2-75), which were pooled and averaged across experiments.
Live-cell Ca2+ imaging of dissociated Drosophila clock neurons
For each experiment, 30-60 3rd instar wandering larvae were collected at ZT9 and rinsed in dPBS. Larval brains were dissected, cleaned, rinsed and placed into dPBS on ice. After two brief rinses in dPBS and gentle centrifugation at 0.2 rcf, brains were re-suspended in 400μl of 1:1 mixture of dPBS: Schneider's S2 medium (Invitrogen) containing 2 Units/mL Dispase II (Roche). After 2hr at room temp, brains were washed twice in dPBS, re-suspended in 200μl S2 medium and dissociated by pipetting up and down 100x through a 200μl pipette tip. 50-65μl aliquots of the cell suspension were placed onto rectangular coverglass (Warner Instruments) and kept in a humidified dark chamber. Cells were allowed at least 2hr to attach to the coverglass before imaging and were discarded 12 hr after dissection.
The coverglass was mounted on an inverted Nikon TE2000U epifluorescence microscope via a small volume recording chamber (Warner Instruments). Neurons were illuminated at 480nm, 6nm bandpass using a monochromator (Photon Technology International) and fluorescent emission viewed through a 40x/0.60 Plan Fluor oil-immersion objective (Nikon) and standard FITC filter set (Chroma Technology). Cells were continuously superfused at 2 ml/min with standard saline (Jan and Jan, 1976) using a gravity-driven system. Chemicals were diluted in standard saline and a manual switching system used for solution exchange without interrupting flow to cells. Un-stimulated G-CaMP expressing neurons were located by their green emission and subsequent fluorescence summed over 2 second intervals was captured at 12-bit resolution via a CoolsnapFx CCD camera (Roper Scientific). Images were acquired and stored using Imaging Workbench 5.2 (INDEC Biosystems). Ovoid regions of interest were defined entirely within the fluorescent signal within each neuron tested and time-lapse numerical data obtained during post-hoc playback exported for further analysis in PRISM 4 (Graphpad). Physiology-grade salts and chemicals were from Fisher Scientific or Sigma Aldrich except for α-bungarotoxin from Tocris.
RNA analysis
Quantitative real time RT-PCR (qPCR) was used on RNA isolated at ZT9 from either whole heads or purified adult LNvs. RNA was extracted using standard procedures for whole fly heads. To isolate RNA from adult LNvs, brains were dissected starting at ZT9 from adult flies with Pdf-RFP, Pdf-Gal4 and, for experimental flies, the UAS-GABAB-R3:RNAi transgene. Brains were dissociated into a single-cell suspension as for Ca2+ imaging above and a 35μm nylon mesh filter (BD Falcon) used to remove cell clusters to minimize contamination by RFP- cells attached to RFP+ cells. Cells in Schneider's medium / 10% FBS were kept on ice for transport to NYU School of Medicine FACS center for flow cytometry. We typically obtained ~300 RFP+ cells from ~50 adult brains, which were sorted directly into 500μl PicoPure Extraction Buffer and purified using the PicoPure RNA Isolation Kit (Molecular Devices). mRNA was amplified using the Nugen WT-Ovation™ Pico System to generate ~5μg single stranded amplified unlabeled cDNA product.
For qPCR reactions, 100ng of adult head RNA or 20ng LNv cDNA was amplified in a Roche LightCycler using the Roche 2-step RT PCR RNA Master HybProbe Kit. RNA levels were determined by comparing when the reaction moved into detectable exponential phase with standard curves for each primer set constructed by re-amplifying known quantities of PCR products. We normalized the absolute level of each gene in an experiment to RNA levels of Pdf (a non-cycling transcript) in that experiment. Three biological replicates were averaged for RNA from whole heads and two for LNv RNA. Primer and probe sequences are listed in Supplemental experimental procedures.
Supplementary Material
Acknowledgements
We thank Harold Atwood, Mike Forte, Leslie Griffith, Paul Hardin, Dierk Reiff, Andrew Tomlinson, Gregg Roman, Julie Simpson, Bloomington Stock Center, DSHB and the VDRC for flies and antibodies. We thank Chris Wegener, Gregg Roman and Mike Nitabach for advice suggestions. We also thank Karim Baroudy, Taniya Kaur and Alyson Knowles for help in initial stages of this project and Ben Collins for help with dissections. We thank Matthieu Cavey, Ben Collins, Alex Keene, Dogukan Mizrak, Afroditi Petsakou and Daniel Vasiliauskas for comments on the manuscript. Confocal images were obtained in the NYU Center for Genomics & Systems Biology. The investigation was largely conducted in a facility constructed with support from Research Facilities Improvement Grant Number C06 RR-15518-01 from NCRR, NIH. The work was supported by NIH grants NS030808 (MA) and GM063911 (JB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of GABAA receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert JL, Lingle CJ. Activation of nicotinic acetylcholine receptors on cultured Drosophila and other insect neurones. J Physiol. 1993;463:605–630. doi: 10.1113/jphysiol.1993.sp019613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Emery P, Takahashi JS, Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Blanchard FJ, Collins B, Cyran SA, Hancock DH, Taylor MV, Blau J. The transcription factor Mef2 is required for normal circadian behavior in Drosophila. J Neurosci. 2010;30:5855–5865. doi: 10.1523/JNEUROSCI.2688-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci. 2008;28:6493–6501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung US, Shayan AJ, Boulianne GL, Atwood HL. Drosophila larval neuromuscular junction's responses to reduction of cAMP in the nervous system. J Neurobiol. 1999;40:1–13. doi: 10.1002/(sici)1097-4695(199907)40:1<1::aid-neu1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABAA receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol. 2009;19:386–390. doi: 10.1016/j.cub.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Mazzoni EO, Stanewsky R, Blau J. Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr Biol. 2006;16:441–449. doi: 10.1016/j.cub.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, Hardin PE, Young MW, Storti RV, Blau J. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell. 2003;112:329–341. doi: 10.1016/s0092-8674(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Day JP, Dow JA, Houslay MD, Davies SA. Cyclic nucleotide phosphodiesterases in Drosophila melanogaster. Biochem J. 2005;388:333–342. doi: 10.1042/BJ20050057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Gardner MJ, Hotta CT, Hubbard KE, Dalchau N, Love J, Assie JM, Robertson FC, Jakobsen MK, Goncalves J, et al. The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science. 2007;318:1789–1792. doi: 10.1126/science.1146757. [DOI] [PubMed] [Google Scholar]
- Fei H, Chow DM, Chen A, Romero-Calderon R, Ong WS, Ackerson LC, Maidment NT, Simpson JH, Frye MA, Krantz DE. Mutation of the Drosophila vesicular GABA transporter disrupts visual figure detection. J Exp Biol. 2010;213:1717–1730. doi: 10.1242/jeb.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris J, Ge H, Liu L, Roman G. G(o) signaling is required for Drosophila associative learning. Nat Neurosci. 2006;9:1036–1040. doi: 10.1038/nn1738. [DOI] [PubMed] [Google Scholar]
- Glossop NR, Houl JH, Zheng H, Ng FS, Dudek SM, Hardin PE. VRILLE feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron. 2003;37:249–261. doi: 10.1016/s0896-6273(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Rieger D, Parmentier ML, Grau Y, Helfrich-Forster C, Nassel DR. Glutamate and its metabotropic receptor in Drosophila clock neuron circuits. J Comp Neurol. 2007;505:32–45. doi: 10.1002/cne.21471. [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Wegener C, Nassel DR. GABA modulates Drosophila circadian clock neurons via GABAB receptors and decreases in calcium. J Neurobiol. 2005;65:225–240. doi: 10.1002/neu.20184. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase-Activating Polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, et al. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Wu Y, Lnenicka GA, Nitabach MN. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J Neurosci. 2007;27:12489–12499. doi: 10.1523/JNEUROSCI.3680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. 3rd edn Sinauer Associates; Sunderland, Mass.: 2001. [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, et al. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J Comp Neurol. 2010;518:1925–1945. doi: 10.1002/cne.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Helfrich-Forster C, Hall JC. Spatial and temporal expression of the period and timeless genes in the developing nervous system of Drosophila: newly identified pacemaker candidates and novel features of clock gene product cycling. J Neurosci. 1997;17:6745–6760. doi: 10.1523/JNEUROSCI.17-17-06745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Kavanaugh MP, Varnum MD, Osborne PB, Christie MJ, Busch AE, Adelman JP, North RA. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991;266:7583–7587. [PubMed] [Google Scholar]
- Kilman VL, Zhang L, Meissner RA, Burg E, Allada R. Perturbing dynamin reveals potent effects on the Drosophila circadian clock. PLoS ONE. 2009;4:e5235. doi: 10.1371/journal.pone.0005235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kula-Eversole E, Nagoshi E, Shang Y, Rodriguez J, Allada R, Rosbash M. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc Natl Acad Sci U S A. 2010;107:13497–13502. doi: 10.1073/pnas.1002081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin-Thomas PL, Brody S. Circadian rhythms in Neurospora crassa: interactions between clock mutations. Genetics. 1985;109:49–66. doi: 10.1093/genetics/109.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Levine JD, Casey CI, Kalderon DD, Jackson FR. Altered circadian pacemaker functions and cyclic AMP rhythms in the Drosophila learning mutant dunce. Neuron. 1994;13:967–974. doi: 10.1016/0896-6273(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide Pigment-Dispersing Factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Majercak J, Kalderon D, Edery I. Drosophila melanogaster deficient in Protein Kinase A manifests behavior-specific arrhythmia but normal clock function. Mol Cell Biol. 1997;17:5915–5922. doi: 10.1128/mcb.17.10.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- McCormack TJ. Comparison of K+-channel genes within the genomes of Anopheles gambiae and Drosophila melanogaster. Genome Biol. 2003;4:R58. doi: 10.1186/gb-2003-4-9-r58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehren JE, Griffith LC. Cholinergic neurons mediate CaMKII-dependent enhancement of courtship suppression. Learn Mem. 2006;13:686–689. doi: 10.1101/lm.317806. [DOI] [PubMed] [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Mezler M, Muller T, Raming K. Cloning and functional expression of GABAB receptors from Drosophila. Eur J Neurosci. 2001;13:477–486. doi: 10.1046/j.1460-9568.2001.01410.x. [DOI] [PubMed] [Google Scholar]
- Nagoshi E, Sugino K, Kula E, Okazaki E, Tachibana T, Nelson S, Rosbash M. Dissecting differential gene expression within the circadian neuronal circuit of Drosophila. Nat Neurosci. 2010;13:60–8. doi: 10.1038/nn.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Sedore SA, Cronmiller C, Hirsh J. Type II cAMP-dependent protein kinase-deficient Drosophila are viable but show developmental, circadian, and drug response phenotypes. J Biol Chem. 2000;275:20588–20596. doi: 10.1074/jbc.M002460200. [DOI] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- Reiff DF, Ihring A, Guerrero G, Isacoff EY, Joesch M, Nakai J, Borst A. In vivo performance of genetically encoded indicators of neural activity in flies. J Neurosci. 2005;25:4766–4778. doi: 10.1523/JNEUROSCI.4900-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Abodeely M, Young MW. Short-period mutations of per affect a double-time-dependent step in the Drosophila circadian clock. Curr Biol. 2000;10:1399–1402. doi: 10.1016/s0960-9822(00)00786-7. [DOI] [PubMed] [Google Scholar]
- Schneider NL, Stengl M. Pigment-dispersing factor and GABA synchronize cells of the isolated circadian clock of the cockroach Leucophaea maderae. J Neurosci. 2005;25:5138–5147. doi: 10.1523/JNEUROSCI.5138-A-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol. 2008;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortridge RD, Yoon J, Lending CR, Bloomquist BT, Perdew MH, Pak WL. A Drosophila phospholipase C gene that is expressed in the central nervous system. J Biol Chem. 1991;266:12474–12480. [PubMed] [Google Scholar]
- Stanewsky R, Jamison CF, Plautz JD, Kay SA, Hall JC. Multiple circadian-regulated elements contribute to cycling period gene expression in Drosophila. EMBO J. 1997;16:5006–5018. doi: 10.1093/emboj/16.16.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Ueno K, Kohatsu S, Clay C, Forte M, Isono K, Kidokoro Y. Gsα is involved in sugar perception in Drosophila melanogaster. J Neurosci. 2006;26:6143–6152. doi: 10.1523/JNEUROSCI.0857-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener C, Hamasaka Y, Nassel DR. Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J Neurophysiol. 2004;91:912–923. doi: 10.1152/jn.00678.2003. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Wolfgang WJ, Roberts IJ, Quan F, O'Kane C, Forte M. Activation of protein kinase A-independent pathways by Gsα in Drosophila. Proc Natl Acad Sci U S A. 1996;93:14542–14547. doi: 10.1073/pnas.93.25.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA. Extraretinal photoreceptors at the compound eye's posterior margin in Drosophila melanogaster. J Comp Neurol. 1999;412:193–202. doi: 10.1002/(sici)1096-9861(19990920)412:2<193::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Yoshii T, Wulbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, HelfrichForster C. The neuropeptide Pigment-Dispersing Factor adjusts period and phase of Drosophila's clock. J Neurosci. 2009;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.