Abstract
Background:
Quantitative MRI techniques have demonstrated thalamocortical abnormalities in idiopathic generalized epilepsy (IGE). However, there are few studies examining IGE early in its course and the neurodevelopmental course of this region is not adequately defined.
Objective:
We examined the 2-year developmental course of the thalamus and frontal lobes in pediatric new-onset IGE (i.e., within 12 months of diagnosis).
Methods:
We performed whole-brain MRI in 22 patients with new-onset IGE and 36 age-matched healthy controls. MRI was repeated 24 months after baseline MRI. Quantitative volumetrics were used to examine thalamic and frontal lobe volumes.
Results:
The IGE group showed significant differences in thalamic volume within 1 year of seizure onset (baseline) and went on to show thalamic volume loss at a significantly faster rate than healthy control children over the 2-year interval. The control group also showed a significantly greater increase in frontal white matter expansion than the IGE group. In contrast, frontal lobe gray matter volume differences were moderate at baseline and persisted over time, indicating similar developmental trajectories with differences early in the disease process that are maintained.
Conclusions:
Brain tissue abnormalities in thalamic and frontal regions can be identified very early in the course of IGE and an abnormal trajectory of growth continues over a 2-year interval.
Idiopathic generalized epilepsies (IGEs) constitute a heterogeneous group of seizure syndromes usually beginning in childhood and adolescence.1 By definition, individuals with IGEs have normal MRIs upon visual inspection.2 However, quantitative neuroimaging methods (e.g., structural and functional MRI) have identified several regional abnormalities. Since Gloor et al.3 first suggested that an abnormal cortical response to thalamocortical afferents might be responsible for generalized seizures, there is considerable evidence that thalamocortical networks are pathophysiologic in IGE.4–6 Previous imaging studies have shown consistent structural and functional thalamic and cortical abnormalities, particularly in the frontal lobes,7,8 and thalamofrontal white matter connectivity.5 The thalamus and frontal lobes have extensive anatomic interconnectivity with bidirectional pathways9 and both structures are involved in generalized spike wave discharges associated with IGE.10
The timing of the onset, manifestation, and development of thalamofrontal abnormalities in IGE is poorly defined. Most studies examining brain structure have focused on adults with chronic epilepsy, confounding the distinction between the effects of onset and duration. We previously reported thalamic and frontal volumetric changes within 1 year of epilepsy onset in juvenile myoclonic epilepsy (JME) and found that these abnormalities were related to executive dysfunction.11 Due to the cross-sectional nature of previous studies, it is unclear whether these changes early in the course of epilepsy are static and unchanging or whether neurodevelopment is negatively impacted. Thus, the present study reports on longitudinal thalamofrontal neurodevelopment in IGE. The purpose of the current study is to report the developmental trajectories of thalamofrontal structure across a 2-year period in children with new-onset IGE and healthy controls.
METHODS
Subjects.
Research participants included 22 children diagnosed with IGE aged 8–18 years. Subjects were recruited from epilepsy clinics at 2 large Midwestern medical centers. We identified all potential participants from the epilepsy clinic each month who met the inclusion criteria (stated below). Following the recruitment procedures approved by the IRB, we then invited these subjects to participate in the research project. Baseline selection criteria included onset of epilepsy within the past 12 months, absence of other developmental disabilities, no other neurologic disorder, normal neurologic examination, and normal clinical MRI. The IGE group was composed of 16 subjects diagnosed with JME, 3 subjects with juvenile absence epilepsy (JAE), 2 with childhood absence epilepsy (CAE), and 1 unspecified generalized syndrome.
A board-certified pediatric neurologist reviewed all subjects' history, seizure semiology, and laboratory findings (including EEG and MRI) and provided a diagnosis of IGE. MRIs were also reviewed by a board-certified neuroradiologist to ensure that no brain lesions were identifiable on visual inspection. Both procedures were conducted without knowledge of quantitative MRI findings.
Thirty-six control participants were age- and gender-matched first-degree cousins of IGE subjects. Controls were selected to exclude those without a history of any initial precipitating event (e.g., febrile seizures), seizure-like episodes, diagnosed neurologic condition, loss of consciousness due to trauma greater than 5 minutes, or other family history of a first-degree relative with epilepsy or febrile convulsions. First-degree cousins were used as controls to facilitate study participation/retention and because they were more genetically distant than siblings and thus less predisposed than siblings to shared genetic factors that may contribute to anomalies in brain structure.
MRI acquisition.
Images were obtained at the University of Wisconsin Hospital on a 1.5-Tesla GE Signa MRI scanner. Sequences acquired for each subject included the following: 1) T1-weighted (T1), 3-dimensional SPGR acquired with the following parameters: echo time (TE) = 5 msec, repetition time (TR) = 24 msec, flip angle = 40 degrees, number of excitations (NEX) = 2, field of view (FOV) = 26, slice thickness = 1.5 mm, matrix = 256 × 256; and 2) T2-weighted (T2) image and 3) proton density, both with the following parameters: TE = 36 msec (for proton density) or 96 msec (for T2), TR = 3,000 msec, NEX = 1, FOV = 26, slice thickness = 3 mm, matrix = 256 × 192, with echo train length = 8.
MRI processing.
Images were processed using the semi-automated software program BRAINS2 (Brain Research: Analysis of Images, Networks, and Systems).12 As part of the standard workup, T1, T2, and proton density images were realigned to a standard orientation, coregistered, and resampled to 1 mm3 voxels. This process established the horizontal axis of the brain to the anterior commissure–posterior commissure line and allowed for voxel-by-voxel correspondence between the images. A tissue classification algorithm13 was employed to create a continuously segmented image in which voxels were classified as gray matter, white matter, CSF, or blood. Magnetic resonance preprocessing details can be found elsewhere.14,15 All subjects underwent MRI within 12 months of seizure onset and again approximately 24 months later after baseline evaluation.
BRAINS2 automated neural network and additional guidelines established at the University of Iowa were used to guide the thalamus trace.16 A more detailed description of thalamic tracing is available elsewhere.11 Briefly, images were traced in the coronal plane using a color-enhanced T1 with reference to the segmented image and unenhanced T1. An automated thalamic mask was derived from the BRAINS2 software and manually edited slice by slice, beginning at the rostral portion and proceeding caudally. Thalamic volumes were obtained by an automated process that sums the total number of voxels included within the region of interest. An inter-rater reliability of 0.98 (intraclass correlation coefficient) for 4 individuals on a sample of 10 brains was achieved. Given the high inter-rater reliability, each MRI was processed by only one individual. All raters were blinded to demographic and group variables.
Frontal lobe tissue volumes (gray matter and white matter separately) were obtained through an automated process whereby BRAINS2 uses anatomic Talairach-based landmarks to partition the cortex into lobes.
Statistical analyses.
Thalamic and frontal lobe volumes were collected for every subject at baseline and follow-up. Age and intracranial volume (ICV) were statistically corrected by entering them in as covariates in 2-way mixed design analysis of covariance (ANCOVA). Simple effects were examined using one-way ANCOVAs and paired-samples t tests. Because group-by-time interactions were hypothesized, simple effects for all analyses were examined. Statistical analyses were conducted using SPSS 13 and Cohen d was used to report effect sizes. According to standard convention, small effects were denoted as d values 0.20–0.49, medium effect sizes as 0.50–0.79, and large effect sizes as 0.80+.17 Mann-Whitney U tests were used to determine if antiepileptic drugs (AEDs) significantly impacted volumes. Analyses were repeated comparing only the JME participants and controls to determine if findings were consistent within a more homogeneous group of IGE subjects.
Standard protocol approvals, registrations, and patient consents.
The Institutional Review Boards at the University of Wisconsin–Madison Hospital and Rosalind Franklin University of Medicine and Science approved this study. Parents of all participants provided written consent on the day of the study and all participants provided written assent.
RESULTS
Demographic and clinical seizure variables.
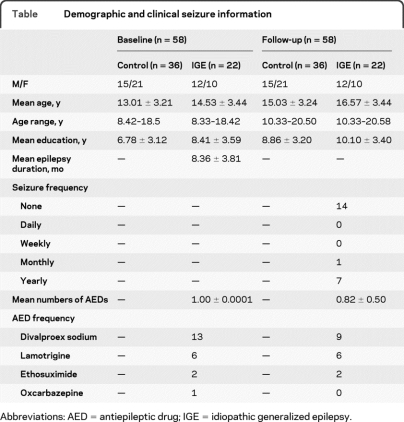
The table shows demographic and clinical seizure information for both groups. No significant differences were found on baseline age (t57 = −1.70, p = 0.09), follow-up age (t57 = −1.72, p = 0.09), baseline education (t57 = −1.82, p = 0.07), follow-up education (t57 = −1.37, p = 0.18), or gender [χ2 (1) = 0.91, p = 0.34]. As expected, age and education were strongly correlated for the entire group (r = 0.99, p < 0.0001). The IGE group did not differ between baseline and follow-up on number of AEDs (t21 = 1.70, p = 0.10). No participants were on polytherapy at baseline, interval, or follow-up.
Table.
Demographic and clinical seizure information
Abbreviations: AED = antiepileptic drug; IGE = idiopathic generalized epilepsy.
Thalamic volumes.
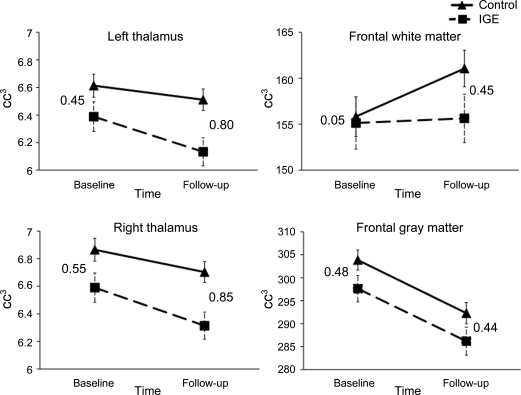
A group-by-time interaction was seen for left thalamic volume (F1,55 = 5.19, p = 0.03). Analysis of simple effects showed that the IGE group had smaller volumes than controls at follow-up (F1,55 = 6.42, p = 0.01), but not at baseline (F1,55 = 2.67, p = 0.11). A simple effect of time also was observed for both controls (t35 = 2.62, p = 0.01) and IGEs (t21 = 4.84, p < 0.001) on left thalamic volume, with both groups showing significant decreases in volume over time. Effect sizes were small at baseline (d = 0.45) and large at follow-up (d = 0.80). Thus, no between-group baseline volume differences were observed, but the IGE group lost left thalamic volume at a faster rate than the control group over 2 years.
The group-by-time interaction for right thalamic volume did not reach significance (F1,55 = 1.74, p = 0.19), but the pattern of development was quite similar to that of the left thalamus. A main effect of group was observed (F1,55 = 7.18, p = 0.01). The IGE group had smaller right thalami at both baseline (F1,55 = 4.09, p = 0.05) and follow-up (F1,55 = 6.96, p = 0.01). Although a significant baseline difference was present, examination of effect sizes shows that the IGE group lost more right thalamic volume over time compared to controls, with a medium effect size at baseline (d = 0.55) and a large effect size at follow-up (d = 0.85). The figure shows thalamic volume plots with effect sizes.
Figure. Plots and effect sizes (Cohen d) of thalamic and frontal volumes.
0.20–0.49 = small; 0.50–0.79 = medium; 0.80+ = large. IGE = idiopathic generalized epilepsy.
Frontal lobe volumes.
Because no lateralized effects were found in initial analyses, findings for total frontal volumes are presented. A group-by-time interaction for frontal white matter was observed (F1,55 = 5.28, p = 0.03), wherein only the control group demonstrated an increase in volume from baseline to follow-up (t35 = −4.07, p < 0.001). In contrast, the IGE group's frontal white volume remained approximately the same across time (t21 = 0.35, p = 0.73). No significant interaction or main effects were observed for frontal gray matter (F1,55 = 3.05, p = 0.09), although moderate gray matter volume differences (as demonstrated by medium effect sizes) were seen at both time points. The figure shows total frontal lobe gray and white matter volume plots with effect sizes.
JME analyses.
Examination of the JME group (n = 16) and controls revealed a nearly identical pattern of findings as found in the IGE–control comparisons. The interaction effect for the left thalamus showed a strong trend toward significance (F1,49 = 3.62, p = 0.06) and follow-up tests revealed the same pattern of findings as in the larger IGE group. Although the frontal lobe white matter interaction did not reach significance (F1,49 = 1.53, p = 0.22), follow-up analyses showed that the control group increased frontal white matter volume over the 2-year interval (t1,35 = −4.07, p = < 0.0001) while the JME group did not (t15 = 0.42, p = 0.68). The pattern of findings and effect sizes for right thalamic and frontal gray volumes were nearly identical to those observed in the larger IGE group. Thus, the overall pattern of findings in the JME group was comparable to that of the larger IGE group.
Antiepileptic drugs.
Because valproate was the most commonly prescribed AED, analyses were conducted to determine if those who had been prescribed valproate at baseline, during the test interval, or at follow-up (n = 14) differed from those who had never been prescribed valproate (n = 8). Mann-Whitney U test showed that the 2 groups did not differ on volumes at either time point (all p > 0.05). In addition, no group differences were found on volumes at either time point (all p > 0.05) for IGE subjects whose first AED was valproate (n = 12) compared to those whose first AED was anything other than valproate (n = 10).
DISCUSSION
Studies of normal brain development have shown gray matter decrease and white matter increase in late childhood to early adolescence.18,19 We employed a longitudinal design to examine changes in these regions in a group with new seizure onset over a 2-year interval and focused on the thalamus and frontal lobes because they have been identified as an important pathophysiologic component of IGE. Our findings indicate that the developmental patterns in these regions are different in IGE than healthy controls. The IGE group showed a steeper decline in thalamic volume and less expansion of frontal white matter volume than healthy controls over a 2-year interval. In contrast, both the IGE and control groups showed a similar degree of frontal gray matter volume loss, although the IGE demonstrated moderate loss within 1 year of seizure onset that was maintained. Thus, brain tissue abnormalities in thalamofrontal regions can be identified very early in the course of IGE with distinct developmental patterns compared to healthy controls. Furthermore, the developmental trajectories for the thalamus and frontal lobes seen here are different from those observed for other nonfrontal cortical lobar regions in a mixed syndrome epilepsy group.20 First, gray matter cortical lobe volumes were not significantly different from controls and only parietal lobe white matter development showed a similar trajectory to frontal white matter development in the mixed epilepsy syndrome group. Thus, the present findings appear to be fairly region specific.
Previous cross-sectional IGE studies examining the frontal lobes and thalamus have produced mixed findings. Our findings are consistent with those of 4 previous studies showing thalamic and frontal volumetric reductions. Increased gray matter frontal cortex volume,21 decreased frontal gray matter,22 and both regional increased and decreased regional white matter volumes8 have all been reported. Thalamic findings in IGE include anterior thalamic gray matter volume reductions,8,21,22 increased anterior thalamic gray matter density,7,23,24 decreased thalamic volume,11 and no thalamic volumetric differences.25–27 Although studies reporting thalamic abnormalities conflict, these studies consistently report that differences lie in the anterior and medial thalamus, a region strongly connected to the prefrontal cortex via the anterior thalamic radiation.28,29 However, a recent EEG-fMRI study found that posterior intralaminar nuclei may be involved in the initiation of epileptic discharges and the anterior nucleus may play a role in seizure maintenance.6 Variable findings are likely due to varying methods of measurement (e.g., voxel-based morphometry, manual volumetrics), combining left and right findings, chronological age, duration of epilepsy at MRI, and heterogeneity of IGE group. In the current study, assessment of volume early in the course was useful in removing the confounding influence of duration of epilepsy.
In the current study, 16 of the 22 IGE subjects were classified as JME. Supplementary analyses indicated that the pattern of findings for the thalamofrontal regions in the JME group was similar to that of the larger IGE group. Nevertheless, there is considerable evidence demonstrating that the pathophysiology of thalamofrontal circuitry is disrupted in several IGE syndromes. Additional study of thalamofrontal circuitry in other IGE syndromes would be helpful, particularly as these findings relate to potentially shared genetic mechanisms.
Structural connectivity between the thalamus and cortical structures is important in understanding the pattern of brain development in IGE. JME subjects show white matter alterations (i.e., decreased fractional anisotropy using diffusion tensor imaging) in the anterior limb of the internal capsule that contains the anterior thalamic radiation. Another recent study examining IGE subjects with generalized tonic-clonic seizures found frontocentral and lateral temporal cortical thinning were strongly correlated with thalamic volume loss,4 suggesting a direct relationship between the thalamus and cortical structure. In addition to connectivity, the impact of differences in thalamofrontal development in IGE may have important consequences for cognitive development. We previously reported that thalamic and frontal abnormalities are related to executive dysfunction in children with new-onset JME.11 Executive functions are generally considered to continue to develop throughout childhood and adolescence and the course of disrupted development in the thalamus and frontal lobes may have important implications for executive function development.
The effects of AEDs on human brain structure and neurodevelopment remain unclear. There is literature suggesting that AEDs in humans can negatively impact gray matter structure, although these reports come from outside the epilepsy literature.30,31 Several animal studies suggest AEDs affect cell proliferation, differentiation, and migration, and other processes such as myelination which may cause neurodegenerative changes in regions such as the thalamus.32 It is unknown if these findings translate to individuals beginning AED treatment in late childhood and adolescence, as there is no longitudinal literature indicating AED-induced gray matter loss in children with postnatal only exposure to AEDs. A study in pediatric bipolar patients found that valproate was actually neuroprotective for cingulate gyrus volume.33 We did not find differences in volumes between those not treated with valproate and those treated with valproate, commonly the first-line treatment of choice in IGE.34 In addition, no volume differences were evident between children whose first medication was valproate and children whose first medication was not valproate. If AED effects were present, they were uniform across subjects and not dependent upon drug type. Additionally, the presence of baseline thalamic and frontal gray matter group differences further supports the notion of minimal AED effects. IGE subjects had a relatively short exposure to AEDs (i.e., 1–12 months) at baseline.
Several limitations of the current study should be noted. First, the IGE sample was relatively small. Statistical power was increased with use of repeated measures design and effect sizes were primarily in the moderate to large range. We examined a heterogeneous group of IGE subjects, which potentially produces variability in volumetric findings. Nevertheless, we found similar results when a homogeneous subset of JME subjects was examined. Second, examination of microstructural integrity (e.g., diffusion tensor imaging) would provide a more comprehensive picture of brain development. Microstructural abnormalities may be detected before volumetric abnormalities and may provide insight into the nature of thalamofrontal dysfunction. Diffusion tensor imaging may prove especially useful in explaining the slowed rate of frontal white matter growth in the context of age-appropriate frontal gray matter volume loss. Third, usage of cousins as control subjects may have underestimated the present findings, as cousins share 1/8 genetic heritability. Use of a completely unrelated control group may yield larger differences with the IGE group. Fourth, this cohort was examined across a 2-year period. The question remains as to the future development of these structures, particularly with seizure remission. It is unknown if the present effects are permanent or if accelerated growth occurs in IGE in later years. We plan to follow up the present study by examining this cohort of children again 5–6 years after seizure onset.
ACKNOWLEDGMENT
The authors thank Leslie Guidotti and Jared Morton at Rosalind Franklin University of Medicine and Science for their assistance in thalamus tracing.
Footnotes
- AED
- antiepileptic drugs
- ANCOVA
- analysis of covariance
- FOV
- field of view
- ICV
- intracranial volume
- IGE
- idiopathic generalized epilepsy
- NEX
- number of excitations
- TE
- echo time
- TR
- repetition time
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Dr. Dalin T. Pulsipher.
DISCLOSURE
Dr. Pulsipher has received predoctoral fellowship support from the Epilepsy Foundation. K. Dabbs and V. Tuchsherer report no disclosures. Dr. Sheth serves on the editorial boards of Epilepsy and Behavior and the Journal of Child Neurology and receives research support from the NIH (NINDS 2RO1 NS44351 [coinvestigator]). Dr. Koehn receives research support from the NIH (NINDS 2RO1 NS44351 [coinvestigator]). Dr. Hermann serves as an Associate Editor for Epilepsia and receives research support from the NIH (NINDS 2RO1 NS44351 [PI], R01 AG027161 [coinvestigator], P50AG3314 [coinvestigator], 1R01NS064034 [coinvestigator], and RO1AG031790 [coinvestigator]). Dr. Seidenberg receives research support from the NIH (NINDS 2ROI NS44351 [coinvestigator] and RO1AG022304 [coinvestigator]).
REFERENCES
- 1. Mattson RH. Overview: idiopathic generalized epilepsies. Epilepsia 2003;44:2–6 [DOI] [PubMed] [Google Scholar]
- 2. Hommet C, Sauerwein HC, De Toffol B, Lassonde M. Idiopathic epileptic syndromes and cognition. Neurosci Biobehav Rev 2006;30:85–96 [DOI] [PubMed] [Google Scholar]
- 3. Gloor P, Pellegrini A, Kostopoulos GK. Effects of changes in cortical excitability upon the epileptic bursts in generalized penicillin epilepsy of the cat. Electroencephalogr Clin Neurophysiol 1979;46:274–289 [DOI] [PubMed] [Google Scholar]
- 4. Bernhardt BC, Rozen DA, Worsley KJ, Evans AC, Bernasconi N, Bernasconi A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage 2009;46:373–381 [DOI] [PubMed] [Google Scholar]
- 5. Deppe M, Kellinghaus C, Duning T, et al. Nerve fiber impairment of anterior thalamocortical circuitry in juvenile myoclonic epilepsy. Neurology 2008;71:1981–1985 [DOI] [PubMed] [Google Scholar]
- 6. Tyvaert L, Chassagnon S, Sadikot A, Levan P, Dubeau F, Gotman J. Thalamic nuclei activity in idiopathic generalized epilepsy: an EEG-fMRI study. Neurology 2009;73:2018–2022 [DOI] [PubMed] [Google Scholar]
- 7. Betting LE, Mory SB, Li LM, et al. Voxel-based morphometry in patients with idiopathic generalized epilepsies. Neuroimage 2006;32:498–502 [DOI] [PubMed] [Google Scholar]
- 8. Chan CH, Briellmann RS, Pell GS, Scheffer IE, Abbott DF, Jackson GD. Thalamic atrophy in childhood absence epilepsy. Epilepsia 2006;47:399–405 [DOI] [PubMed] [Google Scholar]
- 9. McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci 2002;22:8117–8132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moeller F, Siebner HR, Wolff S, et al. Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia 2008;49:1510–1519 [DOI] [PubMed] [Google Scholar]
- 11. Pulsipher DT, Seidenberg M, Guidotti L, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia 2009;50:1210–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph 2002;26:251–264 [DOI] [PubMed] [Google Scholar]
- 13. Harris G, Andreasen NC, Cizadlo T, et al. Improving tissue classification in MRI: a three- dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Topogr 1999;23:144–154 [DOI] [PubMed] [Google Scholar]
- 14. Oyegbile TO, Bhattacharya A, Seidenberg M, Hermann BP. Quantitative MRI biomarkers of cognitive morbidity in temporal lobe epilepsy. Epilepsia 2006;47:143–152 [DOI] [PubMed] [Google Scholar]
- 15. Seidenberg M, Kelly KG, Parrish J, et al. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia 2005;46:420–430 [DOI] [PubMed] [Google Scholar]
- 16. Ooteman W, Crestinger K. Thalamus tracing guidelines. Available at: http://www.psychiatry.uiowa.edu/mhcrc/pdf/papers/thalamus.pdf Accessed March 1, 2004
- 17. Cohen J. A power primer. Psychol Bull 1992;112:155–159 [DOI] [PubMed] [Google Scholar]
- 18. Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol 2002;44:4–16 [DOI] [PubMed] [Google Scholar]
- 19. Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 2005;26:1261–1270 [DOI] [PubMed] [Google Scholar]
- 20. Hermann BP, Dabbs K, Becker T, et al. Brain development in children with new onset epilepsy: a prospective controlled cohort investigation. Epilepsia (in press 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim JH, Lee JK, Koh SB, et al. Regional grey matter abnormalities in juvenile myoclonic epilepsy: a voxel-based morphometry study. Neuroimage 2007;37:1132–1137 [DOI] [PubMed] [Google Scholar]
- 22. Ciumas C, Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology 2006;67:683–686 [DOI] [PubMed] [Google Scholar]
- 23. Betting LE, Mory SB, Lopes-Cendes I, et al. MRI volumetry shows increased anterior thalamic volumes in patients with absence seizures. Epilepsy Behav 2006;8:575–580 [DOI] [PubMed] [Google Scholar]
- 24. Woermann FG, Sisodiya SM, Free SL, Duncan JS. Quantitative MRI in patients with idiopathic generalized epilepsy: evidence of widespread cerebral structural changes. Brain 1998;121:1661–1667 [DOI] [PubMed] [Google Scholar]
- 25. Bernasconi A, Bernasconi N, Natsume J, Antel SB, Andermann F, Arnold DL. Magnetic resonance spectroscopy and imaging of the thalamus in idiopathic generalized epilepsy. Brain 2003;126:2447–2454 [DOI] [PubMed] [Google Scholar]
- 26. Natsume J, Bernasconi N, Andermann F, Bernasconi A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology 2003;60:1296–1300 [DOI] [PubMed] [Google Scholar]
- 27. Seeck M, Dreifuss S, Lantz G, et al. Subcortical nuclei volumetry in idiopathic generalized epilepsy. Epilepsia 2005;46:1642–1645 [DOI] [PubMed] [Google Scholar]
- 28. Fuster JM. Frontal lobe and cognitive development. J Neurocytol 2002;31:373–385 [DOI] [PubMed] [Google Scholar]
- 29. Mori S, Kaufmann WE, Davatzikos C, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med 2002;47:215–223 [DOI] [PubMed] [Google Scholar]
- 30. Hantson P, Duprez T. The value of morphological neuroimaging after acute exposure to toxic substances. Toxicol Rev 2006;25:87–98 [DOI] [PubMed] [Google Scholar]
- 31. Chang K, Barnea-Goraly N, Karchemskiy A, et al. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biol Psychiatry 2005;58:197–203 [DOI] [PubMed] [Google Scholar]
- 32. Ikonomidou C, Turski L. Antiepileptic drugs and brain development. Epilepsy Res 2010;88:11–22 [DOI] [PubMed] [Google Scholar]
- 33. Atmaca M, Ozdemir H, Cetinkaya S, et al. Cingulate gyrus volumetry in drug free bipolar patients and patients treated with valproate or valproate and quetiapine. J Psychiatr Res 2007;41:821–827 [DOI] [PubMed] [Google Scholar]
- 34. Sullivan JE, Dlugos DJ. Idiopathic generalized epilepsy. Curr Treat Options Neurol 2004;6:231–242 [DOI] [PubMed] [Google Scholar]