Abstract
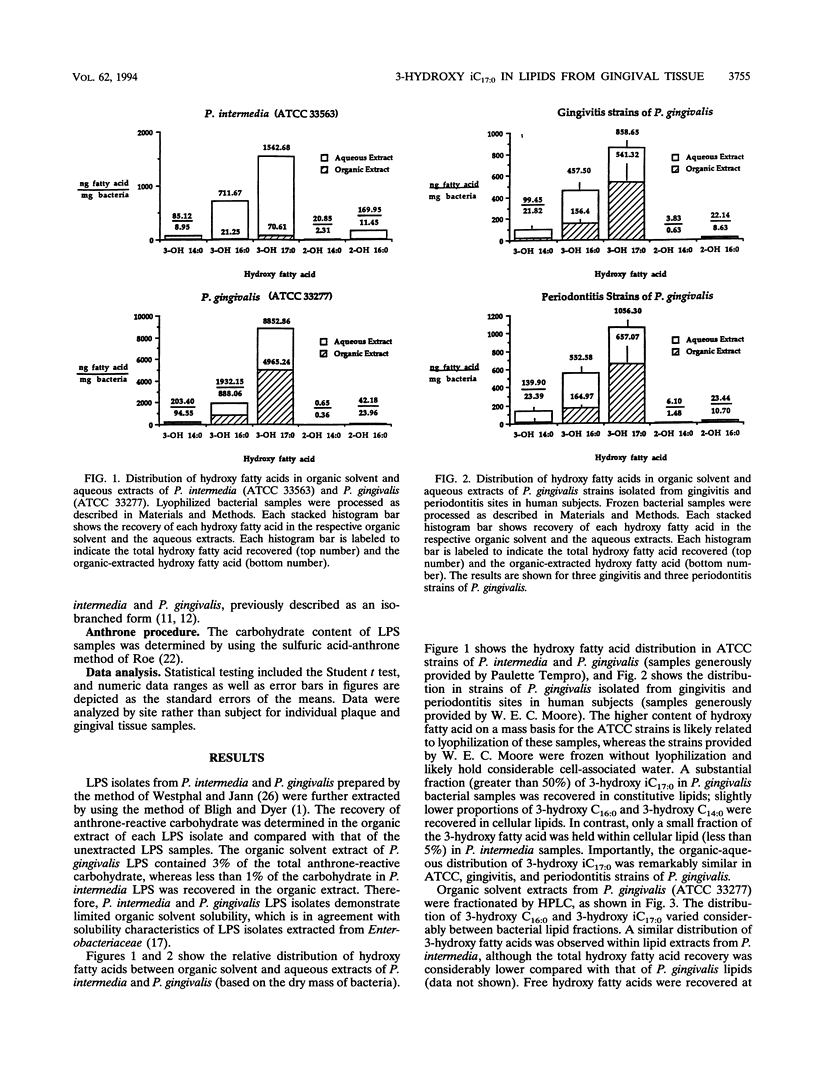
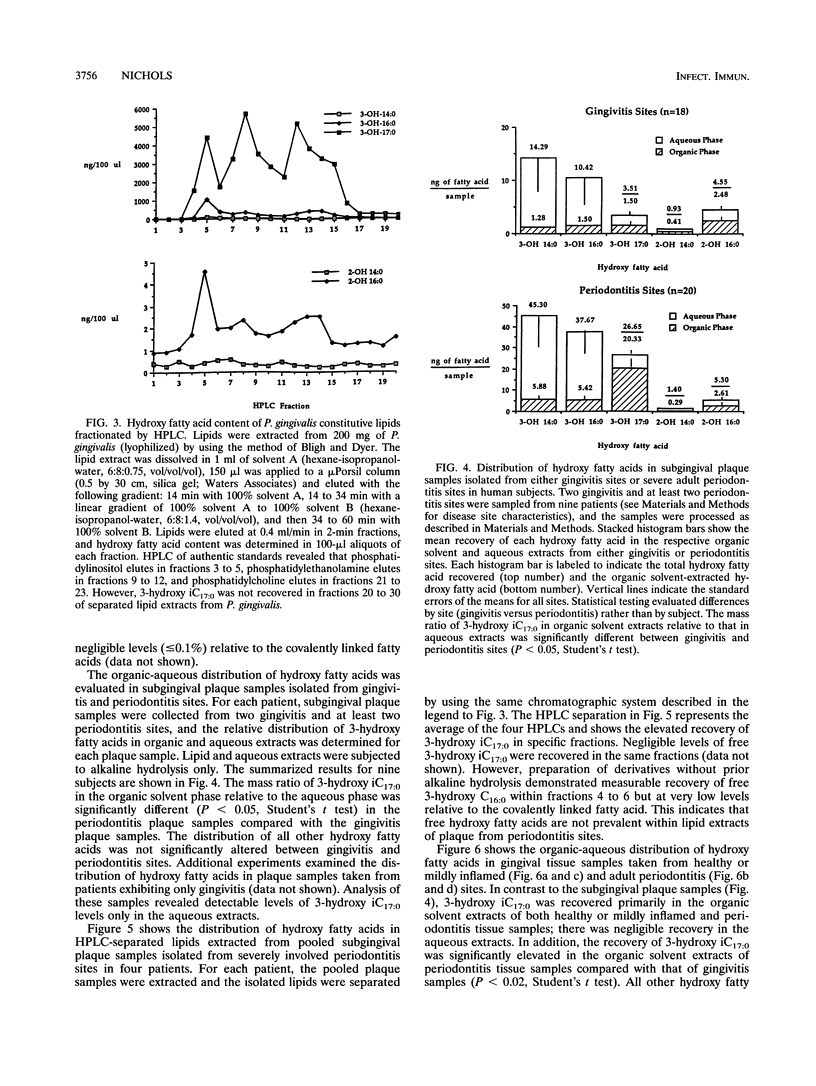
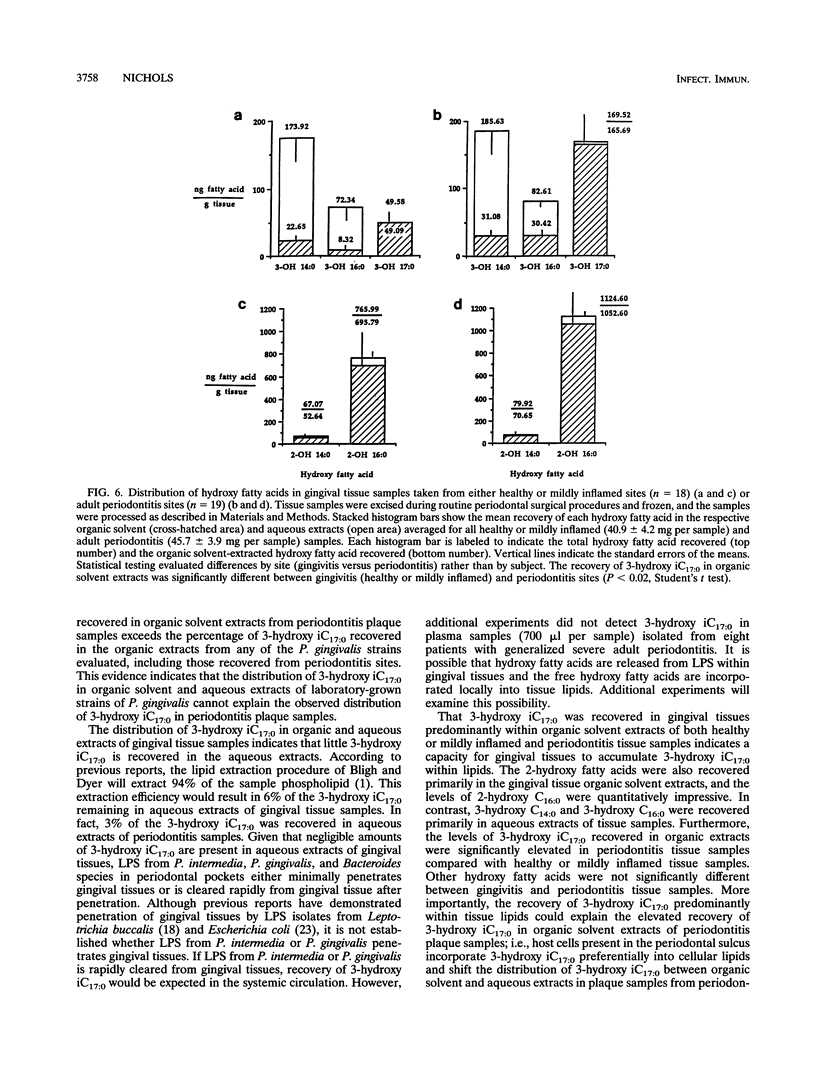
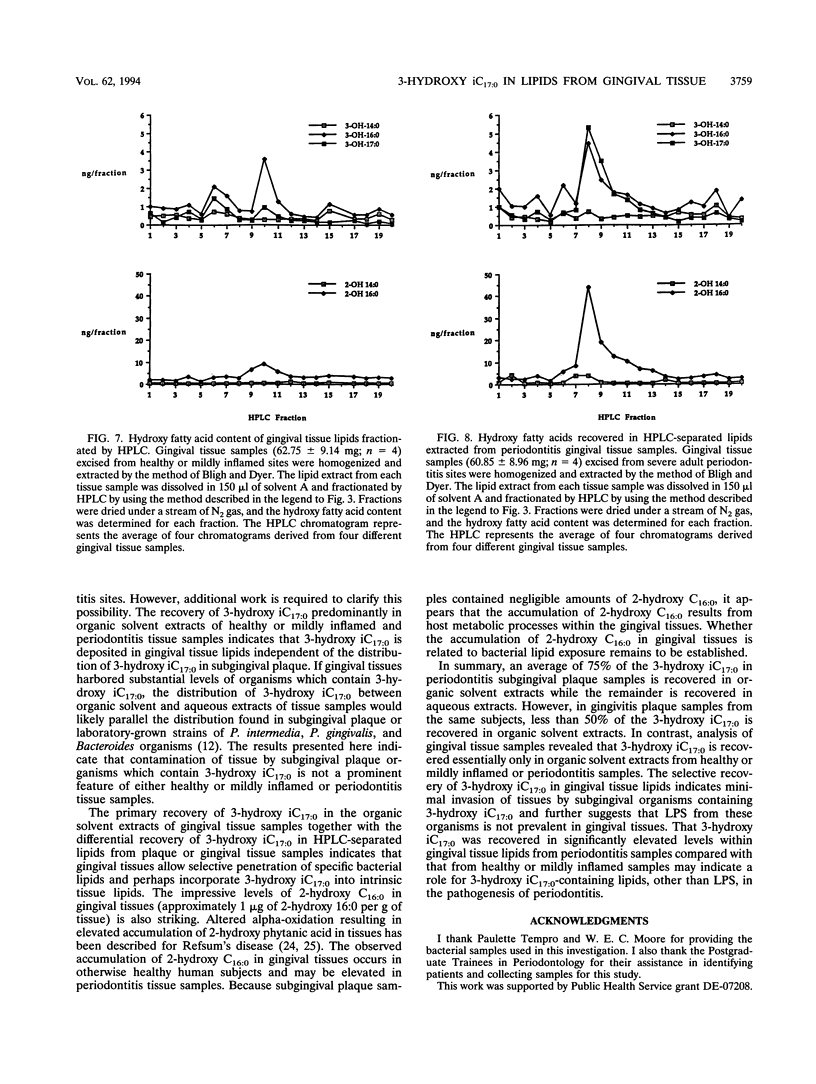
Gram-negative organisms incorporate hydroxy fatty acids into the lipid A moiety of lipopolysaccharide (LPS), and in the case of some members of the family Enterobacteriaceae, hydroxy fatty acids are incorporated exclusively into lipid A. However, a limited number of Bacteroides species have been shown to incorporate several classes of 3-hydroxy fatty acids, particularly 3-hydroxy iC17:0, into constitutive lipids as well as LPS. The present study examined the distribution of hydroxy fatty acids in two periodontal pathogens, Prevotella intermedia and Porphyromonas gingivalis, by employing a phospholipid extraction procedure (E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol. 37:911-917, 1959) which partitioned constitutive lipids into the organic solvent phase and LPS into the aqueous phase. The distribution of hydroxy fatty acids within organic solvent and aqueous extracts of these bacterial species was then compared with the distribution in subgingival plaque samples isolated from either gingivitis or severe periodontitis sites as well as the distribution in gingival tissue samples. The organic solvent and aqueous extracts were hydrolyzed under strong alkaline conditions, and the free fatty acids were treated to form pentafluorobenzyl-ester, trimethylsilyl-ether derivatives. Hydroxy fatty acid levels were quantified by using gas chromatography-negative-ion chemical ionization-mass spectrometry. By using this approach, the mean values of the 3-hydroxy iC17:0 recovered within organic solvent extracts of P. gingivalis strains ranged from 56 to 63% of total 3-hydroxy iC17:0. Substantially less 3-hydroxy iC17:0 (< 5%) was recovered in organic solvent extracts of P. intermedia. By comparison, 75% of the 3-hydroxy iC17:0 in periodontitis subgingival plaque samples was recovered in organic solvent extracts, while only 43% of the 3-hydroxy iC17:0 in gingivitis plaque samples from the same patients was recovered in organic solvent extracts. However, 3-hydroxy iC17:0 was recovered essentially only in organic solvent extracts of both healthy or mildly inflamed and periodontitis gingival tissue samples. The preferential recovery of 3-hydroxy iC17:0 in tissue lipids indicates that gingival tissues do not harbor significant levels of subgingival plaque organisms which contain 3-hydroxy iC17:0. Furthermore, these results indicate that LPS from these organisms is not prevalent in gingival tissues. Finally, these results indicate either selective penetration of certain bacterial lipids into gingival tissues or that 3-hydroxy iC17:0 is metabolically transferred from bacterial lipids into gingival tissue lipids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brondz I., Olsen I. Chemical differences in lipopolysaccharides from Actinobacillus (Haemophilus) actinomycetemcomitans and Haemophilus aphrophilus: clues to differences in periodontopathogenic potential and taxonomic distinction. Infect Immun. 1989 Oct;57(10):3106–3109. doi: 10.1128/iai.57.10.3106-3109.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARBUS J., DELUCA H. F., LOOMANS M. E., STRONG F. M. The rapid incorporation of phosphate into mitochondrial lipids. J Biol Chem. 1963 Jan;238:59–63. [PubMed] [Google Scholar]
- Haeffner N., Chaby R., Szabó L. Identification of 2-methyl-3-hydroxydecanoic and 2-methyl-3-hydroxytetradecanoic acids in the 'lipid X' fraction of the Bordetella pertussis endotoxin. Eur J Biochem. 1977 Aug 1;77(3):535–544. doi: 10.1111/j.1432-1033.1977.tb11696.x. [DOI] [PubMed] [Google Scholar]
- Hax W. M., van Kessel W. S. High-performance liquid chromatographic separation and photometric detection of phospholipids. J Chromatogr. 1977 Nov 11;142:735–741. doi: 10.1016/s0021-9673(01)92081-3. [DOI] [PubMed] [Google Scholar]
- Johne B., Bryn K. Chemical composition and biological properties of a lipopolysaccharide from Bacteroides intermedius. Acta Pathol Microbiol Immunol Scand B. 1986 Aug;94(4):265–271. doi: 10.1111/j.1699-0463.1986.tb03051.x. [DOI] [PubMed] [Google Scholar]
- Johne B., Olsen I., Bryn K. Fatty acids and sugars in lipoplysaccharides from Bacteroides intermedius. Bacteroides gingivalis and Bacteroides loescheii. Oral Microbiol Immunol. 1988 Mar;3(1):22–27. doi: 10.1111/j.1399-302x.1988.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Mashimo J., Yoshida M., Ikeuchi K., Hata S., Arata S., Kasai N., Okuda K., Takazoe I. Fatty acid composition and Shwartzman activity of lipopolysaccharides from oral bacteria. Microbiol Immunol. 1985;29(5):395–403. doi: 10.1111/j.1348-0421.1985.tb00840.x. [DOI] [PubMed] [Google Scholar]
- Mayberry W. R. Cellular distribution and linkage of D-(-)-3-hydroxy fatty acids in Bacteroides species. J Bacteriol. 1980 Oct;144(1):200–204. doi: 10.1128/jb.144.1.200-204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberry W. R. Hydroxy fatty acids in Bacteroides species: D-(--)-3-hydroxy-15-methylhexadecanoate and its homologs. J Bacteriol. 1980 Aug;143(2):582–587. doi: 10.1128/jb.143.2.582-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E. Microbiology of periodontal disease. J Periodontal Res. 1987 Sep;22(5):335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Nair B. C., Mayberry W. R., Dziak R., Chen P. B., Levine M. J., Hausmann E. Biological effects of a purified lipopolysaccharide from Bacteroides gingivalis. J Periodontal Res. 1983 Jan;18(1):40–49. doi: 10.1111/j.1600-0765.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Nichols F. C., Peluso J. F., Tempro P. J., Garrison S. W., Payne J. B. Prostaglandin E release from human monocytes treated with lipopolysaccharides isolated from Bacteroides intermedius and Salmonella typhimurium: potentiation by gamma interferon. Infect Immun. 1991 Jan;59(1):398–406. doi: 10.1128/iai.59.1.398-406.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Ranney R. R., Montgomery E. H. Vascular leakage resulting from topical application of endotoxin to the gingiva of the beagle dog. Arch Oral Biol. 1973 Aug;18(8):963–970. doi: 10.1016/0003-9969(73)90177-5. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Lüderitz O., Volk W. A. Nature, type of linkage, and absolute configuration of (hydroxy) fatty acids in lipopolysaccharides from Xanthomonas sinensis and related strains. J Bacteriol. 1975 Jun;122(3):1180–1188. doi: 10.1128/jb.122.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Stinson F. L., Parker R. B. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J Periodontol. 1972 May;43(5):270–276. doi: 10.1902/jop.1972.43.5.270. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Herndon J. H., Jr, Uhlendorf B. W., Mize C. E., Avigan J., Milne G. W. Refsum's disease: nature of the enzyme defect. Science. 1967 Jun 30;156(3783):1740–1742. doi: 10.1126/science.156.3783.1740. [DOI] [PubMed] [Google Scholar]
- Van Kessel W. S., Hax W. M., Demel R. A., De Gier J. High performance liquid chromatographic separation and direct ultraviolet detection of phospholipids. Biochim Biophys Acta. 1977 Mar 25;486(3):524–530. doi: 10.1016/0005-2760(77)90102-3. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Rietschel E. T., Hofstad T., Weintraub A., Lindberg A. A. Nature, type of linkage, quantity, and absolute configuration of (3-hydroxy) fatty acids in lipopolysaccharides from Bacteroides fragilis NCTC 9343 and related strains. J Bacteriol. 1980 Dec;144(3):898–903. doi: 10.1128/jb.144.3.898-903.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brink H. J., Schor D. S., Kok R. M., Poll-The B. T., Wanders R. J., Jakobs C. Phytanic acid alpha-oxidation: accumulation of 2-hydroxyphytanic acid and absence of 2-oxophytanic acid in plasma from patients with peroxisomal disorders. J Lipid Res. 1992 Oct;33(10):1449–1457. [PubMed] [Google Scholar]


