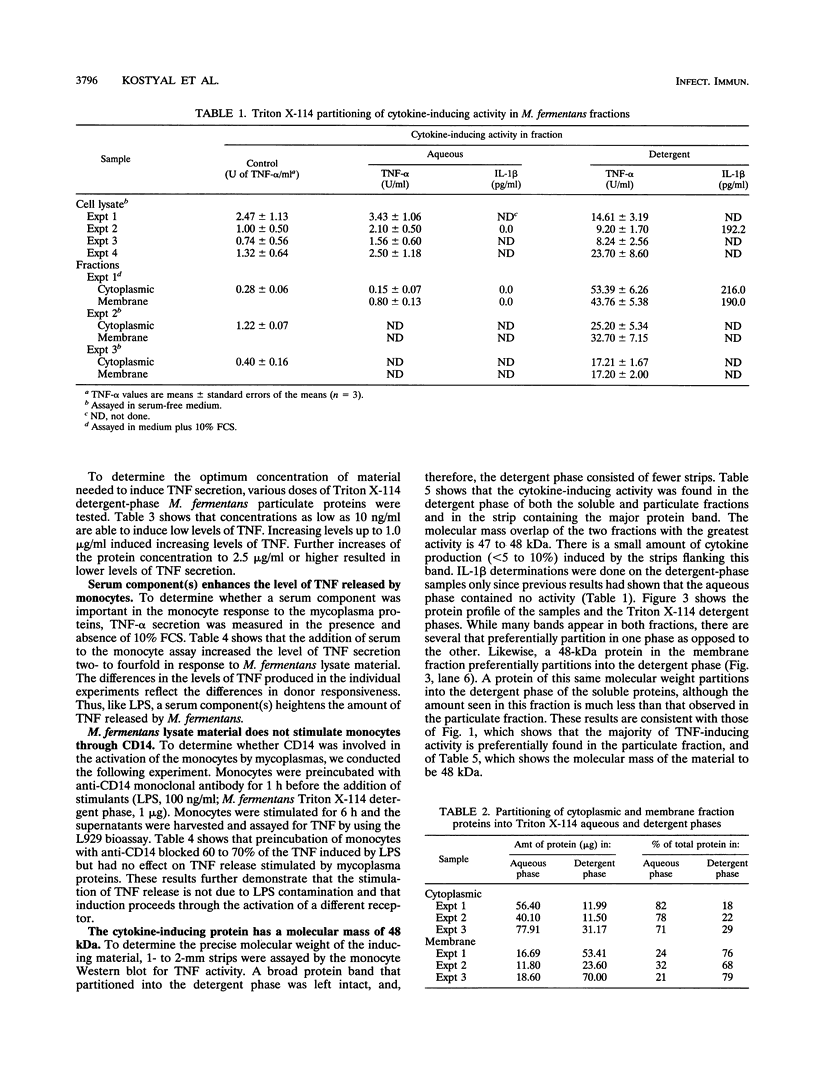
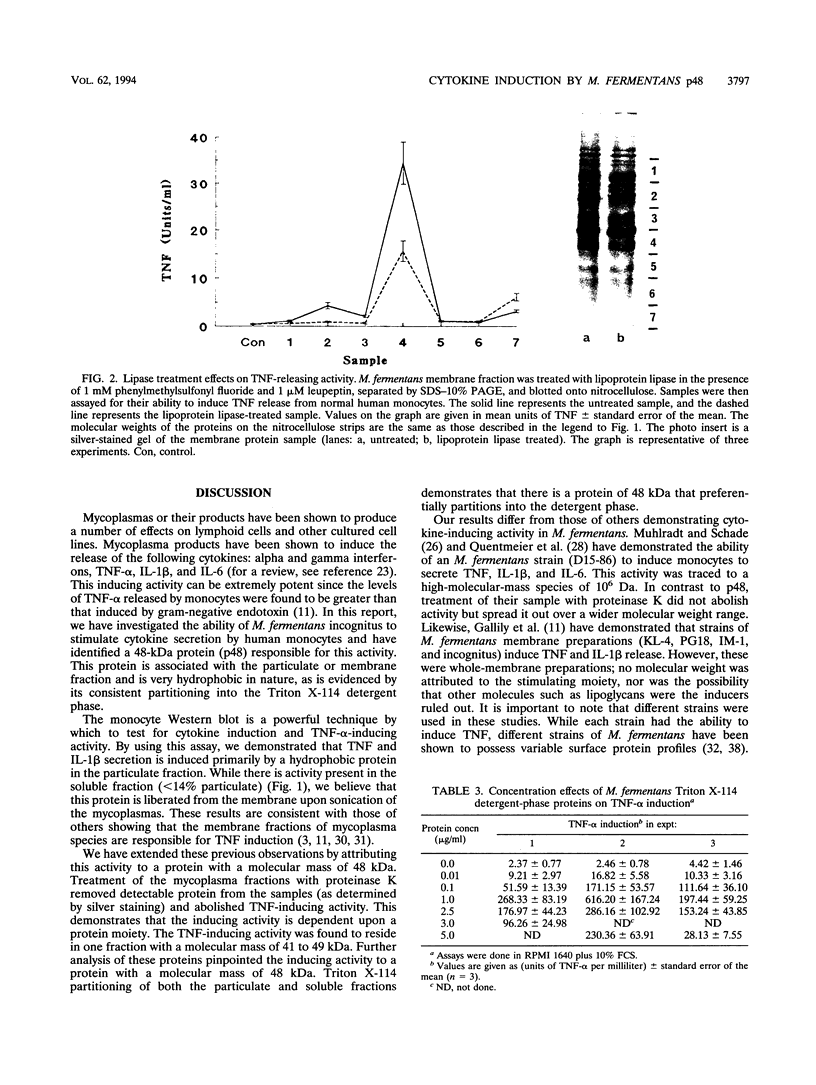
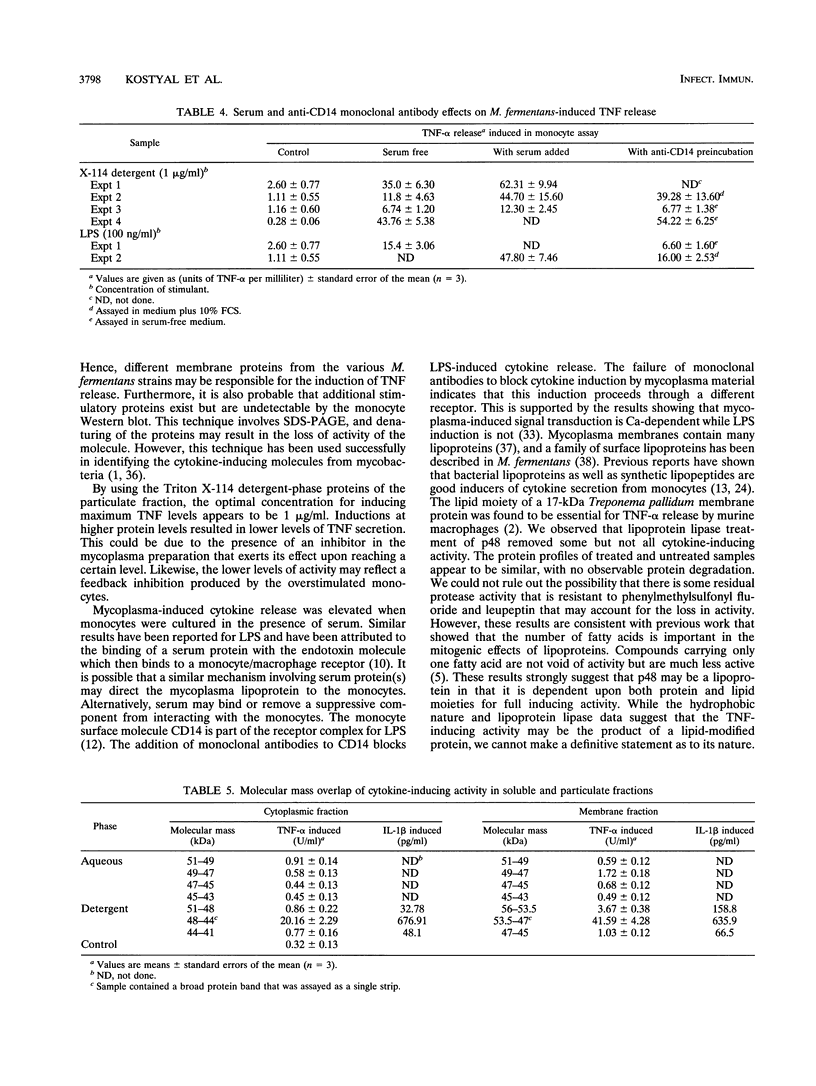
Abstract
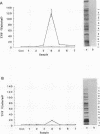
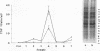
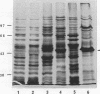
Mycoplasma fermentans is one of several Mycoplasma species that have been reported to stimulate tumor necrosis factor (TNF) secretion from monocytes. This activity has been associated primarily with the mycoplasma membrane fraction. In this article, we have characterized a membrane protein that stimulates TNF and interleukin 1 beta secretion. The TNF-releasing activity partitioned into the Triton X-114 detergent phase, suggesting that the molecules is hydrophobic. The secretion of TNF is elevated in the presence of serum, which suggests that a serum component may play a role in the interaction between this mycoplasma protein and monocytes. Treatment of monocytes with monoclonal anti-CD14 antibody had no effect on the levels of TNF-releasing activity. By using the monocyte Western blot (immunoblot) technique, we have determined the molecular mass of the active molecule to be 48 kDa. This molecule appears to be distinct from the recently described family of variable lipoproteins of M. fermentans. Mycoplasma particulate material treated with proteinase K lost all inducing activity, whereas lipoprotein lipase-treated samples retained some level of activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Akins D. R., Purcell B. K., Mitra M. M., Norgard M. V., Radolf J. D. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect Immun. 1993 Apr;61(4):1202–1210. doi: 10.1128/iai.61.4.1202-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S., Furukawa M., Munakata T., Kuwano K., Inoue H., Miyazaki T. Enhancement of cytotoxicity of active macrophages by mycoplasma: role of mycoplasma-associated induction of tumor necrosis factor-alpha (TNF-alpha) in macrophages. Microbiol Immunol. 1990;34(3):231–243. doi: 10.1111/j.1348-0421.1990.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Beezhold D. H., Leftwich J. A., Hall R. E. P48 induces tumor necrosis factor and IL-1 secretion by human monocytes. J Immunol. 1989 Nov 15;143(10):3217–3221. [PubMed] [Google Scholar]
- Bessler W. G., Simon E., Rotering H. Mitogenicity of a lipid-deficient lipoprotein from a mutant Escherichia coli strain. Infect Immun. 1980 Jun;28(3):818–823. doi: 10.1128/iai.28.3.818-823.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Butler G. H., Kotani H., Kong L., Frick M., Evancho S., Stanbridge E. J., McGarrity G. J. Identification and characterization of proteinase K-resistant proteins in members of the class Mollicutes. Infect Immun. 1991 Mar;59(3):1037–1042. doi: 10.1128/iai.59.3.1037-1042.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I. H., Munakata T., Koyanagi Y., Kobayashi S., Arai S., Yamamoto N. Mycoplasma can enhance HIV replication in vitro: a possible cofactor responsible for the progression of AIDS. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1365–1370. doi: 10.1016/0006-291x(90)90545-x. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Atkin C. L. The Mycoplasma arthritidis T-cell mitogen, MAM: a model superantigen. Immunol Today. 1991 Aug;12(8):271–276. doi: 10.1016/0167-5699(91)90125-D. [DOI] [PubMed] [Google Scholar]
- Corradin S. B., Mauël J., Gallay P., Heumann D., Ulevitch R. J., Tobias P. S. Enhancement of murine macrophage binding of and response to bacterial lipopolysaccharide (LPS) by LPS-binding protein. J Leukoc Biol. 1992 Oct;52(4):363–368. doi: 10.1002/jlb.52.4.363. [DOI] [PubMed] [Google Scholar]
- Gallily R., Salman M., Tarshis M., Rottem S. Mycoplasma fermentans (incognitus strain) induces TNF alpha and IL-1 production by human monocytes and murine macrophages. Immunol Lett. 1992 Sep;34(1):27–30. doi: 10.1016/0165-2478(92)90023-h. [DOI] [PubMed] [Google Scholar]
- Heumann D., Gallay P., Barras C., Zaech P., Ulevitch R. J., Tobias P. S., Glauser M. P., Baumgartner J. D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992 Jun 1;148(11):3505–3512. [PubMed] [Google Scholar]
- Hoffmann P., Wiesmüller K. H., Metzger J., Jung G., Bessler W. G. Induction of tumor cytotoxicity in murine bone marrow-derived macrophages by two synthetic lipopeptide analogues. Biol Chem Hoppe Seyler. 1989 Jun;370(6):575–582. doi: 10.1515/bchm3.1989.370.1.575. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Brehm G., Nicklas W., Beck R., Herbst F. Biochemical characterization of the T-cell mitogen derived from Mycoplasma arthritidis. Scand J Immunol. 1986 Sep;24(3):245–249. doi: 10.1111/j.1365-3083.1986.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Kostyal D. A., Beezhold D. H., Hall R. E. Differentiation-inducing cytokine P48 exists in a membrane-associated form. J Immunol. 1991 Aug 1;147(3):893–898. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaître M., Henin Y., Destouesse F., Ferrieux C., Montagnier L., Blanchard A. Role of mycoplasma infection in the cytopathic effect induced by human immunodeficiency virus type 1 in infected cell lines. Infect Immun. 1992 Mar;60(3):742–748. doi: 10.1128/iai.60.3.742-748.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S. C., Dawson M. S., Newton P. B., 3rd, Sonoda M. A., Shih J. W., Engler W. F., Wang R. Y., Wear D. J. Association of the virus-like infectious agent originally reported in patients with AIDS with acute fatal disease in previously healthy non-AIDS patients. Am J Trop Med Hyg. 1989 Sep;41(3):364–376. [PubMed] [Google Scholar]
- Lo S. C., Tsai S., Benish J. R., Shih J. W., Wear D. J., Wong D. M. Enhancement of HIV-1 cytocidal effects in CD4+ lymphocytes by the AIDS-associated mycoplasma. Science. 1991 Mar 1;251(4997):1074–1076. doi: 10.1126/science.1705362. [DOI] [PubMed] [Google Scholar]
- Macon W. R., Lo S. C., Poiesz B. J., Montefiori D. C., Dawson M. S., Mason T. E., Michael P. R., Cohen A. G., Davis C. M., Cousar J. B. Acquired immunodeficiency syndrome-like illness associated with systemic Mycoplasma fermentans infection in a human immunodeficiency virus-negative homosexual man. Hum Pathol. 1993 May;24(5):554–558. doi: 10.1016/0046-8177(93)90169-h. [DOI] [PubMed] [Google Scholar]
- Marshall A., Miles R. J., Richards L. Contrasting effects of Mycoplasma fermentans and M. felis on the viability and chemiluminescence response of human polymorphonuclear leukocytes. FEMS Microbiol Lett. 1993 May 15;109(2-3):167–171. doi: 10.1111/j.1574-6968.1993.tb06162.x. [DOI] [PubMed] [Google Scholar]
- Metzger J., Jung G., Bessler W. G., Hoffmann P., Strecker M., Lieberknecht A., Schmidt U. Lipopeptides containing 2-(palmitoylamino)-6,7-bis(palmitoyloxy) heptanoic acid: synthesis, stereospecific stimulation of B-lymphocytes and macrophages, and adjuvanticity in vivo and in vitro. J Med Chem. 1991 Jul;34(7):1969–1974. doi: 10.1021/jm00111a008. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Takaku H., Umeda M., Fujita T., Huang W. D., Kimura T., Yamashita J., Horio T. Potent growth inhibition of human tumor cells in culture by arginine deiminase purified from a culture medium of a Mycoplasma-infected cell line. Cancer Res. 1990 Aug 1;50(15):4522–4527. [PubMed] [Google Scholar]
- Mühlradt P. F., Schade U. MDHM, a macrophage-stimulatory product of Mycoplasma fermentans, leads to in vitro interleukin-1 (IL-1), IL-6, tumor necrosis factor, and prostaglandin production and is pyrogenic in rabbits. Infect Immun. 1991 Nov;59(11):3969–3974. doi: 10.1128/iai.59.11.3969-3974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust J. J., Buchholz M. A., Nordin A. A. A "lymphokine-like" soluble product that induces proliferation and maturation of B cells appears in the serum-free supernatant of a T cell hybridoma as a consequence of mycoplasmal contamination. J Immunol. 1985 Jan;134(1):390–396. [PubMed] [Google Scholar]
- Quentmeier H., Schmitt E., Kirchhoff H., Grote W., Mühlradt P. F. Mycoplasma fermentans-derived high-molecular-weight material induces interleukin-6 release in cultures of murine macrophages and human monocytes. Infect Immun. 1990 May;58(5):1273–1280. doi: 10.1128/iai.58.5.1273-1280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuth E., Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989 Dec;112:133–160. doi: 10.1111/j.1600-065x.1989.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Sher T., Rottem S., Gallily R. Mycoplasma capricolum membranes induce tumor necrosis factor alpha by a mechanism different from that of lipopolysaccharide. Cancer Immunol Immunother. 1990;31(2):86–92. doi: 10.1007/BF01742371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher T., Yamin A., Matzliach M., Rottem S., Gallily R. Partial biochemical characterization of spiroplasma membrane component inducing tumor necrosis factor alpha. Anticancer Drugs. 1990 Oct;1(1):83–87. doi: 10.1097/00001813-199010000-00014. [DOI] [PubMed] [Google Scholar]
- Städtlander C. T., Zuhua C., Watson H. L., Cassell G. H. Protein and antigen heterogeneity among strains of Mycoplasma fermentans. Infect Immun. 1991 Sep;59(9):3319–3322. doi: 10.1128/iai.59.9.3319-3322.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama K., Kuwano K., Furukawa M., Himeno Y., Satoh T., Arai S. Mycoplasmas induce transcription and production of tumor necrosis factor in a monocytic cell line, THP-1, by a protein kinase C-independent pathway. Infect Immun. 1990 Nov;58(11):3564–3567. doi: 10.1128/iai.58.11.3564-3567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaku H., Takase M., Abe S., Hayashi H., Miyazaki K. In vivo anti-tumor activity of arginine deiminase purified from Mycoplasma arginini. Int J Cancer. 1992 May 8;51(2):244–249. doi: 10.1002/ijc.2910510213. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Amir-Tahmasseb M., Ellner J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990 May;87(9):3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F., Theiss P. M., Lo S. C. A family of strain-variant surface lipoproteins of Mycoplasma fermentans. Infect Immun. 1993 Aug;61(8):3327–3333. doi: 10.1128/iai.61.8.3327-3333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]