Abstract
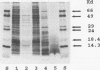
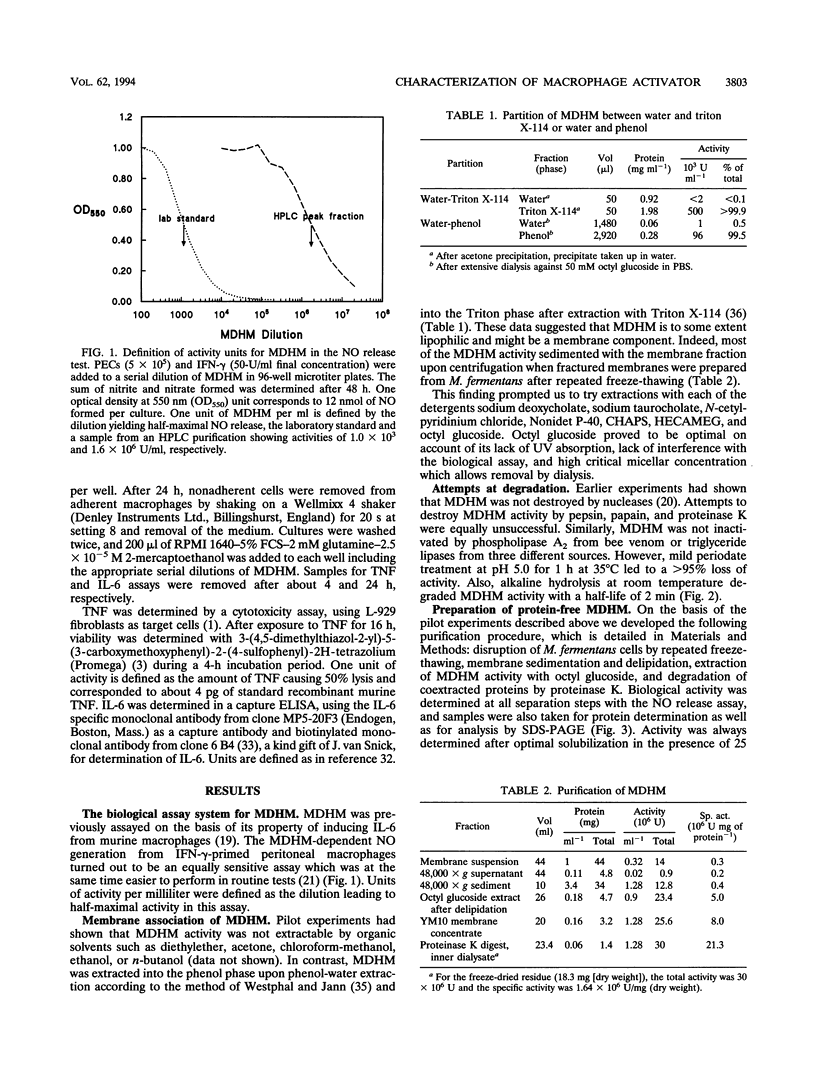
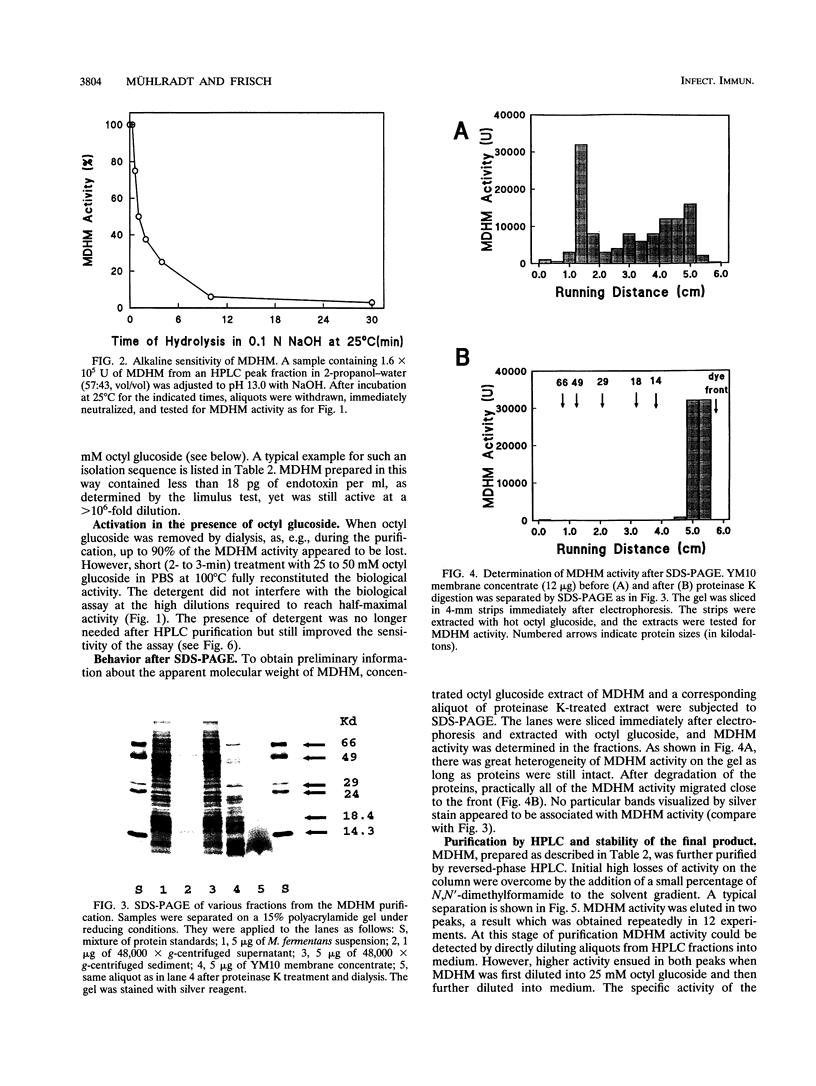
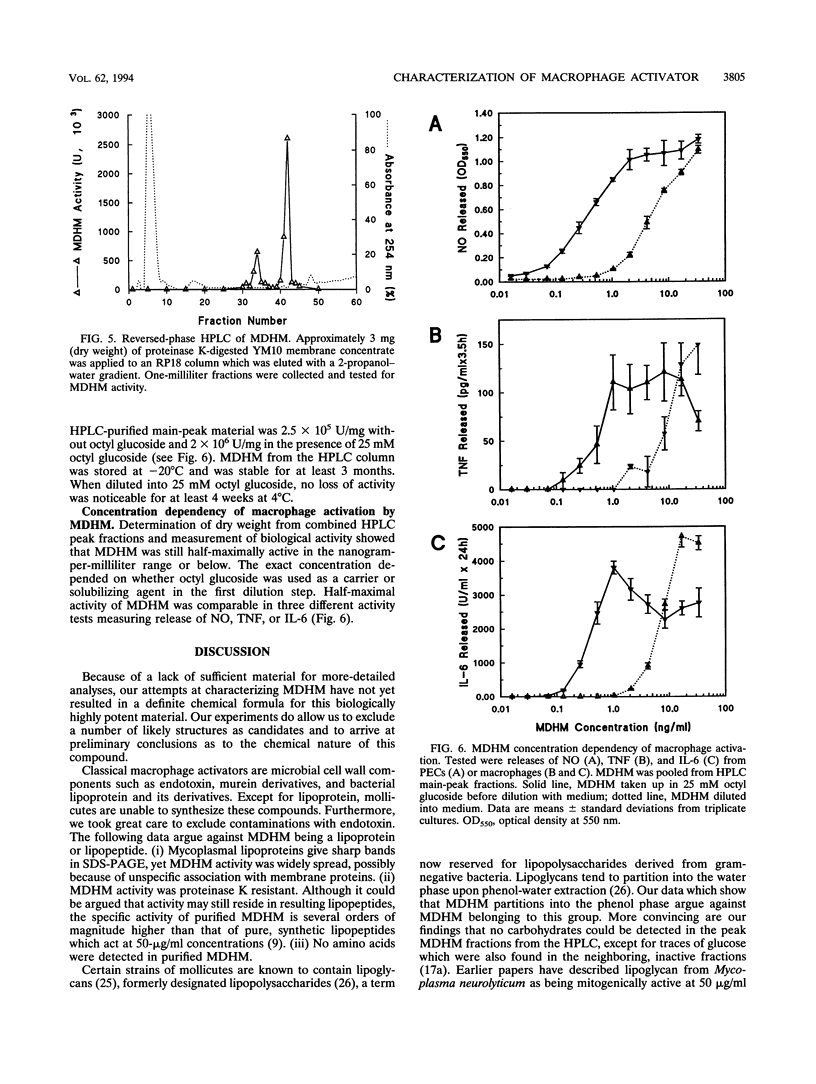
Mycoplasmal products may exert a number of diverse in vitro effects on cells of the immune system. A macrophage-activating substance from Mycoplasma fermentans was described in this laboratory and named mycoplasma-derived high-molecular-weight material (MDHM). Using synthesis of nitric oxide by peritoneal cells from endotoxin low-responder mice as an assay system, MDHM was purified as follows. After freeze-thawing of M. fermentans, MDHM activity was sedimented with the membrane fraction. Membranes were delipidated with chloroform-methanol, and MDHM activity was extracted with octyl glucoside. Coextracted proteins were degraded by proteinase K. MDHM was further purified by reversed-phase high-pressure liquid chromatography and eluted in one major and one minor peak of activity. Neither carbohydrates nor amino acids were found as constituents. MDHM had the following properties: it partitioned into the phenol phase upon phenol-water extraction and into the Triton phase after extraction with Triton X-114. MDHM was not inactivated by either phospholipase A2 or triglyceride lipases. However, mild periodate treatment led to a > 95% loss of activity. Also, alkaline hydrolysis at 25 degrees C completely abolished MDHM activity with a half-life of 2 min. MDHM activity was spread out over a wide molecular weight range upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis of membranes, whereas after proteinase treatment MDHM activity migrated close to the front. These features of MDHM, taken together, speak in favor of an amphiphilic molecule with a lipid moiety carrying fatty acids in ester linkage and a polyol moiety of unknown character. MDHM was active in the nanogram-per-milliliter range, activating macrophages to release nitric oxide, interleukin-6, and tumor necrosis factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Barile M. F., Yoshida H., Roth H. Rheumatoid arthritis: new findings on the failure to isolate or detect mycoplasmas by multiple cultivation or serologic procedures and a review of the literature. Rev Infect Dis. 1991 Jul-Aug;13(4):571–582. doi: 10.1093/clinids/13.4.571. [DOI] [PubMed] [Google Scholar]
- Buttke T. M., McCubrey J. A., Owen T. C. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J Immunol Methods. 1993 Jan 4;157(1-2):233–240. doi: 10.1016/0022-1759(93)90092-l. [DOI] [PubMed] [Google Scholar]
- Chagnon A., Auzanneau G., Moreau X., Othmani S., Roche J. C. Responsabilite de Mycoplasma pneumoniae dans une fièvre prolongée sans pneomopathie. Presse Med. 1986 Nov 15;15(40):2021–2021. [PubMed] [Google Scholar]
- Chowdhury M. I., Koyanagi Y., Kobayashi S., Yamamoto N., Munakata T., Arai S. Mycoplasma and AIDS. Lancet. 1990 Jul 28;336(8709):247–248. doi: 10.1016/0140-6736(90)91773-4. [DOI] [PubMed] [Google Scholar]
- Dietz J. N., Cole B. C. Direct activation of the J774.1 Murine macrophage cell line by mycoplasma arthritidis. Infect Immun. 1982 Aug;37(2):811–819. doi: 10.1128/iai.37.2.811-819.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Gallily R., Salman M., Tarshis M., Rottem S. Mycoplasma fermentans (incognitus strain) induces TNF alpha and IL-1 production by human monocytes and murine macrophages. Immunol Lett. 1992 Sep;34(1):27–30. doi: 10.1016/0165-2478(92)90023-h. [DOI] [PubMed] [Google Scholar]
- Hakkarainen K., Turunen H., Miettinen A., Karppelin M., Kaitila K., Jansson E. Mycoplasmas and arthritis. Ann Rheum Dis. 1992 Oct;51(10):1170–1172. doi: 10.1136/ard.51.10.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschildt S., Wolf B., Lückhoff A., Bessler W. G. Determination of second messengers and protein kinase C in bone marrow derived macrophages stimulated with a bacterial lipopeptide. Mol Immunol. 1990 Jun;27(6):473–479. doi: 10.1016/0161-5890(90)90065-8. [DOI] [PubMed] [Google Scholar]
- Kaplan P. J., Garvey J. S. Mycoplasma neurolyticum membranes: a T-independent antigen in the rat. Immunol Invest. 1986 Mar;15(1):35–53. doi: 10.3109/08820138609042017. [DOI] [PubMed] [Google Scholar]
- Kirchhoff H., Maass C., Runge M., Franz B., Schmidt R., Quentmeier H., Mühlradt P. F. Tetrazolium [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reduction by mycoplasmas. Int J Syst Bacteriol. 1992 Jul;42(3):506–508. doi: 10.1099/00207713-42-3-506. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo S. C., Dawson M. S., Wong D. M., Newton P. B., 3rd, Sonoda M. A., Engler W. F., Wang R. Y., Shih J. W., Alter H. J., Wear D. J. Identification of Mycoplasma incognitus infection in patients with AIDS: an immunohistochemical, in situ hybridization and ultrastructural study. Am J Trop Med Hyg. 1989 Nov;41(5):601–616. doi: 10.4269/ajtmh.1989.41.601. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Migliorini P., Corradin G., Corradin S. B. Macrophage NO2- production as a sensitive and rapid assay for the quantitation of murine IFN-gamma. J Immunol Methods. 1991 May 17;139(1):107–114. doi: 10.1016/0022-1759(91)90357-l. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Quentmeier H., Schmitt E. Involvement of interleukin-1 (IL-1), IL-6, IL-2, and IL-4 in generation of cytolytic T cells from thymocytes stimulated by a Mycoplasma fermentans-derived product. Infect Immun. 1991 Nov;59(11):3962–3968. doi: 10.1128/iai.59.11.3962-3968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F., Schade U. MDHM, a macrophage-stimulatory product of Mycoplasma fermentans, leads to in vitro interleukin-1 (IL-1), IL-6, tumor necrosis factor, and prostaglandin production and is pyrogenic in rabbits. Infect Immun. 1991 Nov;59(11):3969–3974. doi: 10.1128/iai.59.11.3969-3974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentmeier H., Schmitt E., Kirchhoff H., Grote W., Mühlradt P. F. Mycoplasma fermentans-derived high-molecular-weight material induces interleukin-6 release in cultures of murine macrophages and human monocytes. Infect Immun. 1990 May;58(5):1273–1280. doi: 10.1128/iai.58.5.1273-1280.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschmeyer D., Thude H., Mühlradt P. F. MDHM, a macrophage-activating product of Mycoplasma fermentans, stimulates murine macrophages to synthesize nitric oxide and become tumoricidal. FEMS Immunol Med Microbiol. 1993 Oct;7(3):223–229. doi: 10.1111/j.1574-695X.1993.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Ruuth E., Praz F. Interactions between mycoplasmas and the immune system. Immunol Rev. 1989 Dec;112:133–160. doi: 10.1111/j.1600-065x.1989.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Seid R. C., Jr, Smith P. F., Guevarra G., Hochstein H. D., Barile M. F. Endotoxin-like activities of mycoplasmal lipopolysaccharides (lipoglycans). Infect Immun. 1980 Sep;29(3):990–994. doi: 10.1128/iai.29.3.990-994.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher T., Yamin A., Rottem S., Gallily R. In vitro induction of tumor necrosis factor alpha, tumor cytolysis, and blast transformation by Spiroplasma membranes. J Natl Cancer Inst. 1990 Jul 4;82(13):1142–1145. doi: 10.1093/jnci/82.13.1142. [DOI] [PubMed] [Google Scholar]
- Smith P. F. Antigenic character of membrane lipoglycans from Mollicutes--a review. Isr J Med Sci. 1987 May;23(5):448–452. [PubMed] [Google Scholar]
- Smith P. F., Langworthy T. A., Mayberry W. R. Distribution and composition of lipopolysaccharides from mycoplasmas. J Bacteriol. 1976 Mar;125(3):916–922. doi: 10.1128/jb.125.3.916-922.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart P. M., Cassell G. H., Woodward J. G. Induction of class II MHC antigen expression in macrophages by Mycoplasma species. J Immunol. 1989 May 15;142(10):3392–3399. [PubMed] [Google Scholar]
- Sugama K., Kuwano K., Furukawa M., Himeno Y., Satoh T., Arai S. Mycoplasmas induce transcription and production of tumor necrosis factor in a monocytic cell line, THP-1, by a protein kinase C-independent pathway. Infect Immun. 1990 Nov;58(11):3564–3567. doi: 10.1128/iai.58.11.3564-3567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takema M., Oka S., Uno K., Nakamura S., Arita H., Tawara K., Inaba K., Muramatsu S. Macrophage-activating factor extracted from mycoplasmas. Cancer Immunol Immunother. 1991;33(1):39–44. doi: 10.1007/BF01742526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Uno K., Takema M., Hidaka S., Tanaka R., Konishi T., Kato T., Nakamura S., Muramatsu S. Induction of antitumor activity in macrophages by mycoplasmas in concert with interferon. Cancer Immunol Immunother. 1990;32(1):22–28. doi: 10.1007/BF01741720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink A., Coulie P. G., Wauters P., Nordan R. P., Van Snick J. B cell growth and differentiation activity of interleukin-HP1 and related murine plasmacytoma growth factors. Synergy with interleukin 1. Eur J Immunol. 1988 Apr;18(4):607–612. doi: 10.1002/eji.1830180418. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Smith P. F., Kahane I. Bacterial lipopolysaccharides and mycoplasmal lipoglycans: a comparison between their abilities to induce macrophage-mediated tumor cell killing and Limulus amebocyte lysate clotting. Biochem Biophys Res Commun. 1980 Nov 28;97(2):493–499. doi: 10.1016/0006-291x(80)90290-9. [DOI] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]